To the editor:
Recently an acquired activating mutation in the gene encoding the non–receptor tyrosine kinase Janus kinase 2 (JAK2V617F) has been identified in a significant number of Philadelphia chromosome–negative myeloproliferative disease (MPD) patients. In particular, this mutation is found frequently in the multilineage disease polycythemia vera (PV).1-3 Sensitive methods for detection of the JAK2V617F allele have allowed investigations of the cell types that carry the mutation, and several groups have used these approaches to examine highly enriched hematopoietic subpopulations. Recent work by Jamieson et al4 demonstrated in several PV patients the presence of JAK2V617F allele in multipotent hematopoietic stem cells (HSCs) and their myeloid-restricted progeny. However, this study did not investigate progenitors and mature cells restricted to the lymphoid lineage. Absence of the JAK2V617F allele in mature lymphoid cells has been reported in some studies,2,5 however recently Ishii et al6 have detected the JAK2V617F allele in T and B lymphocytes in a subset of PV patients. The authors suggest that this may be due to acquisition of the mutation in HSCs in these patients, speculating further that in patients where the JAK2V617F allele was not detected in mature lymphocytes the mutation has arisen in a progenitor restricted to the myeloid lineage, such as the common myeloid progenitor (CMP).
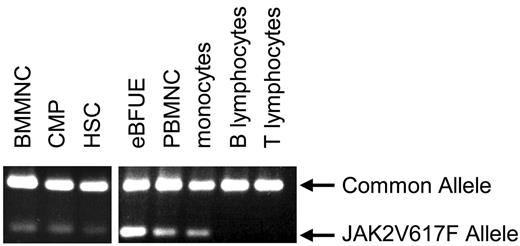
Here, we have used a sensitive technique for specific detection of the JAK2V617F allele1 to monitor the presence of the mutation following flow-cytometric fractionation of progenitor and mature cell populations from bone marrow and peripheral blood of a homozygous JAK2V617F-positive PV patient. We have detected the JAK2V617F allele in bone marrow mononuclear cells (BMMNCs), peripheral blood mononuclear cells (PBMNCs), monocytes, HSCs, CMPs, and EPO-independent blast forming units–erythroid (eBFUEs) but not in the T- or B-lymphocyte fractions (Figure 1) The presence of the mutation in HSCs and CMPs, but not in the mature lymphoid cells, suggests that in this patient the mutation arose in the HSC compartment and was passed on to cells in the CMP fraction, while being lost in the lymphoid lineage. Thus our data are consistent with the finding that the JAK2V617F mutation arises in HSCs in the majority of PV patients.4 The origin of the mutation in the HSC compartment, in a multipotent cell that has self-renewal capacity, is consistent with the maintenance of clonal hematopoiesis. We propose that the JAK2V617F mutation is passed on to the common lymphoid progenitor (CLP) compartment and suggest several alternative explanations for the selective loss of mature lymphoid cells carrying the JAK2V617F allele in the majority of PV patients. First, the mutation may not confer a selective advantage during lymphoid growth and differentiation and may require a significant period of time to reach detectable levels in the mature lymphocyte population. The ability to consistently detect the mutation in lymphocytes in PV patients may therefore depend on the sensitivity of detection methods and the duration of disease. Alternatively, it seems possible that altered properties of CLPs, associated with the presence of the JAK2V617F allele, may result in a lack of mature lymphocytes carrying JAK2V617F in some patients. For example, JAK2V617F may instruct a myeloid lineage choice upon CLPs, mimicking the effect seen when exogenous GM-CSF receptor, activated by GM-CSF, confers myeloid differentiation to CLPs.7,8 It is also possible that JAK2V617F may bias differentiation of CLPs to the myeloid lineage via lympho-myeloid progenitors, which have been reported in humans9 and mice.10 Introduction of the JAK2V617F allele into CLPs from healthy donors will test these possibilities directly.
JAK2V617F detection in progenitor and mature cell subtypes. An allele-specific PCR technique1 was used to detect the JAK2V617F allele in eBFUE, PBMNC, monocytes (CD14+), HSCs (CD34+, CD38−, lin−), and CMP (CD34+, CD38+, lin−) from a JAK2V617F-positive polycythemia vera patient. The mutant allele was not detectable in the T lymphocyte (CD4/8+) or B lymphocyte (CD20+) fractions.
JAK2V617F detection in progenitor and mature cell subtypes. An allele-specific PCR technique1 was used to detect the JAK2V617F allele in eBFUE, PBMNC, monocytes (CD14+), HSCs (CD34+, CD38−, lin−), and CMP (CD34+, CD38+, lin−) from a JAK2V617F-positive polycythemia vera patient. The mutant allele was not detectable in the T lymphocyte (CD4/8+) or B lymphocyte (CD20+) fractions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
This work was supported by a grant from the Myeloproliferative Disorders Foundation and Leukemia Lymphoma Society.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal