Abstract
Juvenile hemochromatosis (JH) is a rare autosomal recessive disorder of iron metabolism, genetically heterogeneous. In JH, symptomatic organ involvement occurs as early as the second decade of life. Heart failure and/or arrhythmias are the most frequent causes of death. Phlebotomy is the safest, most effective, and most economic therapeutic approach in hemochromatosis patients but is not indicated during the treatment of severe congestive heart failure with unstable hemodynamic status. The treatment of iron overload in these prohibitive clinical situations has to be carried out using iron chelators. We report a case of heart failure in the setting of unrecognized juvenile hemochromatosis successfully treated by the simultaneous administration of deferoxamine and deferiprone. To our knowledge, this is the first patient affected by JH treated with combined chelation regimen.
Introduction
Juvenile hemochromatosis (JH) is a rare autosomal recessive disorder of iron metabolism, genetically heterogeneous; most families are related to the recently cloned hemojuvelin gene and a small subset of JH patients was shown to harbor mutations in the HAMP gene encoding hepcidin antimicrobial peptide that modulates intestinal iron absorption. The HAMP gene is located at chromosome 19q13. HAMP mutations are associated with a new type of severe juvenile hemochromatosis not related to chromosome 1q.1-3
Total hepcidin deficiency resulting in increased iron export to plasma characterizes the severe iron overload of JH.
In JH, symptomatic organ involvement occurs as early as the second decade of life. Although liver involvement is a constant feature in genetic hemochromatosis, diabetes, hypogonadotropic hypogonadism, cardiomyopathy, arrhythmias, and heart failure are far more frequent in JH than in the adult-onset form. The progression of this disease is rapid and, left untreated, heart disease may be evident by the age of 20 to 30 years, possibly resulting in death in the absence of heart transplantation. Heart failure and/or arrhythmias are the most frequent causes of death.4,5
We report a case of heart failure in the setting of unrecognized JH successfully treated by the combination of deferoxamine (DFO) and deferiprone (DFP).
Patient, materials, and methods
A 29-year-old man was admitted to the intensive care unit because of cardiac failure. He lived in Albania until 2 months before admission when he came to Italy, to join his brothers and to search for the cause of his infertility. He had been well until a few days before admission when he began to notice fatigue, malaise, nonproductive cough, and progressive dyspnea.
A chest X-ray showed cardiomegaly. A 2-dimensional heart ultrasound demonstrated the presence of a dilatated cardiomyopathy with profound global left ventricular hypokinesis and ejection fraction of 25%. A mild increase of pulmonary arterial pressure was also detected. Shortly after admission, the patient developed atrial fibrillation. He was treated by diuretics, ACE inhibitors, and amiodarone and was started on anticoagulation with intravenous heparin followed by warfarin therapy.
Hematologic findings included a hematocrit level of 0.38 (38%), a hemoglobin concentration of 130 g/L (13 g/dL), a mean corpuscular volume of 91 fL, and a mean corpuscular hemoglobin value of 35 pg. Platelet counts were 44 × 109/L and leukocyte counts were 2.1 × 109/L. Blood tests revealed the following results: total bilirubin, 15.39 μM (0.9 mg/dL; conjugated bilirubin, 1.71 μM [0.1 mg/dL]); aspartate aminotransferase, 132 U/L; alanine aminotransferase, 216 U/L; γ-glutamyltransferase, 79 U/L; alkaline phosphatase, 125 U/L; serum ferritin, 3773 ng/mL (normal, 15 to 250 ng/mL); serum transferrin saturation, 100%; and serum iron, 26.31 μM (147 μg/dL; normal, 10.56-28.28 μM [59-158 μg/dL]). Non–transferrin-bound iron (NTBI), evaluated by chromatographic method,6 was 4.91 μM (healthy individuals always have negative NTBI values). Glucose tolerance was normal.
The patient complained of loss of libido and decreased sexual potency in the past few months. Basal serum testosterone was low (< 0.015 mM; normal values: 0.304-0.936 mM) as were FSH (< 0.1 IU/L [0.1 mU/mL]; normal range, 0.7-11.1 IU/L [0.7-11.1 mU/mL]) and LH (0.1 IU/L [0.1 mU/mL]; normal range, 0.8-7.6 IU/L [0.8-7.6 mU/mL]), showing pituitary hypogonadism. On physical examination there was hepatosplenomegaly. Abdominal ultrasonography showed marked liver enlargement and a portal vein diameter of 14 mm. On a sagittal ultrasonogram, the spleen measured 19.0 cm in the long dimension. No signs of portal hypertension at esophagogastroduodenoscopy were detected.
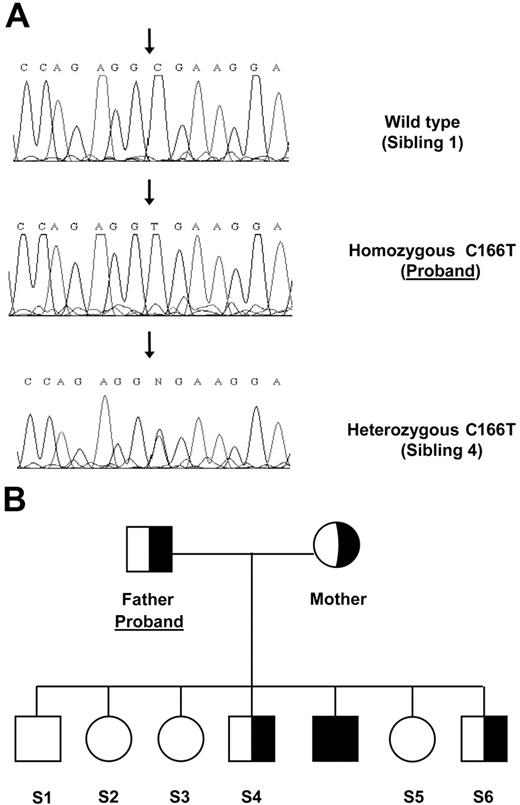
Acquired causes of iron overload were excluded (hemolytic anemias, thalassemia, blood transfusions); thus a genetic hemochromatosis was hypothesized. HFE analysis revealed a wild-type genotype. Hepcidin gene was characterized and a homozygous C→T mutation was identified in exon 3 leading to an arginine substitution at position 56 with stop codon (R56X). No mutations were detected in TFR2, ferroportin, and hemojuvelin genes (Figure 1A).
Family tree and mutation. (A) Sequence chromatographs of the HAMP gene region spanning the C166T mutation (forward sequence show) from the indicated individuals. (B) Pedigree of family carrying the R56X.
Family tree and mutation. (A) Sequence chromatographs of the HAMP gene region spanning the C166T mutation (forward sequence show) from the indicated individuals. (B) Pedigree of family carrying the R56X.
Parents were heterozygous carriers of the hepcidin (HAMP) mutation as were 2 brothers (Figure 1B). All had normal iron parameters.
Because of severe iron overload, the patient was started on intensive iron chelation. Approval was obtained from the ethics committee of the University of Milan institutional review board for these studies. Informed consent was provided in accordance with the Declaration of Helsinki, and the patient was started on combined chelation treatment with DFO and DFP (intravenous DFO 30 mg/kg per day and oral DFP 75 mg/kg per day in 3 administrations).
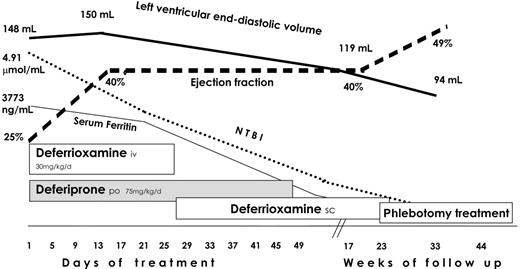
After 3 weeks of intensive chelation, a marked clinical and biochemical improvement was observed: at echocardiography, ventricular diameters normalized and ejection fraction raised to 40%. Anticoagulation was stopped. The chelation regimen was changed by pump subcutaneous infusion of DFO (20 mg/kg per day for 6 d/wk) maintaining oral DFP. Chorionic gonadotropin treatment (2000-3000 U/wk) was started.
Oral DFP was stopped after 2 months and DFO was continued until ferritin serum values decreased to 480 ng/mL (5 months); then phlebotomy treatment was started. The LVEF improved from 25% to 49% during pharmacologic iron chelation, and it remains stable after a follow-up of 10 months (Figure 2). His arrhythmia disappeared and did not recur even after the antiarrhythmic drug was discontinued.
Blood counts became normal except for a persistent mild leukocytopenia and thrombocytopenia (3.20 × 109/L and 82 × 109/L, respectively); liver function tests were normal with an aspartate transaminase of 28 U/L and an alanine transaminase of 30 U/L. Abdomen ultrasonography did not show any changes in hepatosplenomegaly.
At follow-up after 5 months, testosterone concentration was partially restored with a basal serum value of 0.131 nM; then testosterone replacement was started with a return of normal sexual function, a significant improvement in the quality of life, and an important effect on muscle mass and bone mineral density.
When the patient was admitted, heart MRI T2* measurements to assess myocardial iron were not yet available at our center; however, the dark tissue signal of the heart was evaluated at the beginning and at the end of combined intensive chelation, showing significant changes of signal intensity.
Results and discussion
Phlebotomy is the safest, most effective, and most economic therapeutic approach in hemochromatosis patients but is not indicated during the treatment of severe congestive heart failure with unstable hemodynamic status. The treatment of iron overload in these prohibitive clinical situations has to be carried out using iron chelators.
At present, the 3 available iron chelators are deferoxamine mesylate, deferiprone, and deferasirox (ICL670).7
The major component of iron removed in iron-loaded patients by subcutaneous or intravenous DFO is thought to be NTBI iron. The liver is the organ more affected by DFO chelation, whereas other organs such as the heart are also gradually depleted of iron during DFO treatment provided the patients can tolerate higher and continuous administration. The DFO efficacy in preventing early death from iron-induced cardiac disease and in reversing established cardiac disease intravenously, especially when given continuously or have been extensively documented in transfusion-dependent thalassemia patients.8-10 However, cardiac disease continues to occur and remains the most common cause of death in those patients.11 Studies in iron-loaded rat heart cells and in gerbils12 had in the past shown the ability of DFP to remove iron from myocardial cells at concentrations that can be achieved in the circulation. DFP is smaller and more lipophilic than DFO, and therefore it could be more efficient than DFO in accessing intracellular chelatable iron.
Patients with thalassemia major switched to deferiprone therapy had a remarkably lower prevalence of cardiac disease and cardiac death than patients chelated with DFO only.13,14 Moreover, combined therapy with DFO and DFP induced significant improvement in cardiac siderosis and function in those patients.
A number of in vitro and in vivo studies have suggested that the simultaneous use of DFO and DFP is associated with an additive or even synergistic iron excretion in patients with thalassemia major, and that combined therapy could decrease iron overload in patients who had previously been unable to achieve a satisfactory response to deferiprone or desferrioxamine alone.15 The basis for this effect could be explained by the fact that DFP easily enters cells and is subsequently able to transfer the intracellularly chelate iron to DFO in plasma.16
Few data are available on pharmacologic iron chelation in patients with GH.17
In our patient affected by severe type of JH, we obtained the reversal of cardiac complications by DFP and DFO simultaneous administration. The aggressive pharmacologic treatment was essential to induce a regression of myocardial dysfunction in a very short time, which was associated with an improvement in clinical status. To our knowledge, this is the first patient affected by JH treated with combined chelation regimen.
In JH patients with a poor prognosis because of the development of cardiac complications, a combination of intense DFO treatment and oral DFP appears to be rapid, effective, and nontoxic in reversing iron cardiac toxicity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: G.F. and M.D.C. drafted the manuscript; G.F., F.M., and A.B. handled inpatient care; M.D.C. handled outpatient care; and P.D. performed molecular analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal