Abstract
The activity and safety of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid, SAHA) were evaluated in patients with refractory cutaneous T-cell lymphoma (CTCL). Group 1 received vorinostat 400 mg daily, group 2 received vorinostat 300 mg twice daily for 3 days with 4 days rest, and group 3 received vorinostat 300 mg twice daily for 14 days with 7 days rest followed by 200 mg twice daily. Treatment continued until disease progression or intolerable toxicity. The primary objective was to determine the complete and partial response (PR) rate. Time to response (TTR), time to progressive disease (TTP), response duration (DOR), pruritus relief, and safety were determined. Thirty-three patients who had received a median of 5 prior therapies were enrolled. Eight patients achieved a PR, including 7 with advanced disease and 4 with Sézary syndrome. The median TTR, DOR, and TTP for responders were 11.9, 15.1, and 30.2 weeks, respectively. Fourteen of 31 evaluable patients had pruritus relief. The most common drug-related AEs were fatigue, thrombocytopenia, diarrhea, and nausea. The most common grade 3 or 4 drug-related AEs were thrombocytopenia and dehydration. Vorinostat demonstrated activity in heavily pretreated patients with CTCL. The 400 mg daily regimen had the most favorable safety profile and is being further evaluated.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are extranodal non-Hodgkin T-cell lymphomas that appear in skin. The most common variants are mycosis fungoides (MF) and the leukemic, erythrodermic variant, Sézary syndrome (SS).1 Therapeutic choices are based on whether the patient has early (T1-2N0-1M0; IA-IIA) or late (T1-4N0-3M0-1; IIB-IVB) stage disease. Although lesions of patients with early MF are generally well controlled with topical skin-directed therapies or phototherapy, extensive skin or blood involvement, tumors, or nodal disease require systemic therapy with biologic response modifiers, bexarotene, denileukin diftitox, skin radiation, and single or multiagent chemotherapy.2-7 Often a combination of either sequential or concomitant therapies gives a higher rate of response, but patients with advanced disease often relapse, and curative therapy remains elusive.
Alterations in histone-acetylation regulatory enzymes (histone acetylases and histone deacetylases [HDACs]) have been identified in various hematologic and solid malignancies.8,9 These findings raise the possibility that epigenetic modulation of gene transcription can be used therapeutically for cancer. Acetylation of core nucleosomal histones is associated with transcriptional activation presumably due, in part, to increased accessibility of transcription factors to DNA promoter sequences. Inhibition of HDAC activity causes an accumulation of acetylated proteins, including histones.10 Vorinostat (Zolinza [Merck, Whitehouse Station, NJ], suberoylanilide hydroxamic acid, SAHA) is an orally bioavailable inhibitor11 of class I and II HDACs.12 In preclinical studies, vorinostat has been shown to cause accumulation of acetylated histones and to induce cell-cycle arrest and apoptosis in a broad range of cancer cell lines, including CTCL lines.12,13 Vorinostat also showed antitumor activity at in leukemia, lymphoma, and solid-tumor models in vivo.14-18
Phase 1 trials to evaluate the safety and activity of vorinostat were conducted in patients with advanced solid and hematologic malignancies, including CTCLs.11,19-21 Both intravenous and oral formulations were well tolerated. Dose-limiting toxicities observed were gastrointestinal toxicity (nausea, vomiting, or diarrhea), anorexia, dehydration, fatigue, and myelosuppression (thrombocytopenia, neutropenia, or leukopenia).11,19-21 The maximum tolerated doses of oral vorinostat were determined to be 400 mg orally every day or 200 mg orally twice daily as continuous dosing,11 300 mg orally twice daily for 3 consecutive days per week,11 or 200 mg orally twice daily or thrice daily for 14 days followed by 7 days of rest.19 Vorinostat was shown to cause accumulation of acetylated histones in tumor, bone marrow, or peripheral blood cells.11,19-21 Importantly, a broad range of antitumor activity was observed with complete or partial responses in patients with refractory solid and hematologic malignancies, including CTCLs.11,19-21
Both phase 1 and preclinical data supported further investigation of vorinostat as an anticancer treatment. Therefore, we conducted a phase 2 trial to determine the response rate and duration, safety, and tolerability of oral vorinostat in heavily pretreated patients with refractory CTCL.
Patients, materials, and methods
This open-label, nonrandomized sequential cohort, phase 2 trial was approved by the Institutional Review Board of the MD Anderson Cancer Center, and written informed consent was obtained from each patient prior to study enrollment, in accordance with the Declaration of Helsinki.
Patient eligibility
All patients were required to be 18 years or older and to have histologically documented and evaluable CTCLs refractory to or intolerant of conventional therapy. All patients with MF were staged as IVA according to pathologic detection of involved lymph nodes or flow cytometry detection of blood involvement (B2) criteria for SS.22 Stage IVB required biopsy-proven bone marrow or visceral involvement. Patients were also required to have adequate hematologic, hepatic, and renal function; an ECOG (Eastern Cooperative Oncology Group) performance status of 2 or less; and a life expectancy of 3 or more months. At least 3 weeks were required to have passed since prior chemotherapy, major surgery, radiation therapy, or other investigational anticancer therapy, unless patients had rapidly progressive disease (PD). Patients were required to have recovered from toxicities related to these therapies and were not allowed to take oral retinoids, vitamin A, or alternative medicines. Fertile patients agreed to practice effective contraception during the study period, and pregnant or lactating women were excluded. Patients with central nervous system involvement by tumor or clinically significant illness (including active infection, uncontrolled hypertension, symptomatic congestive heart failure, unstable angina pectoris, or myocardial infarction within the past 6 months, or uncontrolled arrhythmia) were excluded.
Study design
Three dose schedules of vorinostat were sequentially evaluated, and patients were assigned to a treatment group according to the dose open for enrollment. The starting doses were chosen because they represented the maximum tolerated doses for continuous or intermittent dosing in phase 1 trials of oral vorinostat.11,19 In group 1, vorinostat treatment was started at 400 mg every day and was modified to the intermittent dosing schedules shown in Table 1. In group 2, patients received vorinostat at 300 mg twice daily for 3 days per week, and the frequency was escalated to 5 days per week if tolerated. In group 3, patients were induced with 300 mg twice daily for 2 weeks, and after a week off they resumed therapy at 200 mg twice daily continuously. If drug-related toxicity of grade 3 or 4 occurred, study drug was withheld until the toxicity resolved to grade 1 or less. Doses were then modified according the schedule shown in Table 1.
Oral vorinostat dosing and dose modification schedules
| Treatment group . | Dosing schedule . | First dose reduction . | Second dose reduction . |
|---|---|---|---|
| 1 | 400 mg every day* | 350 mg every day | 300 mg every day |
| 2 | 300 mg twice daily × 3 d per wk × 4 wk, then 5 d every wk | 250 mg × 3 d/wk | 200 mg twice daily × 3 d/wk |
| 3 | Ind: 300 mg twice daily × 14 d with 7 d rest; mtnc; 200 mg twice daily | 200 mg twice daily | 200 mg twice daily × 5 d/wk |
| Treatment group . | Dosing schedule . | First dose reduction . | Second dose reduction . |
|---|---|---|---|
| 1 | 400 mg every day* | 350 mg every day | 300 mg every day |
| 2 | 300 mg twice daily × 3 d per wk × 4 wk, then 5 d every wk | 250 mg × 3 d/wk | 200 mg twice daily × 3 d/wk |
| 3 | Ind: 300 mg twice daily × 14 d with 7 d rest; mtnc; 200 mg twice daily | 200 mg twice daily | 200 mg twice daily × 5 d/wk |
Ind indicates induction; mtnc, maintenance.
Initial 3 patients treated at 250 mg/m2 per day.
Medications permitted during the study included antiemetics, antidiarrheals, antipyretics, antihistamines, antihypertensive medications, analgesics, antibiotics, blood products, and erythropoietin. Although antihistamines or topical steroids were permitted for patients who had received a stable dose for 2 or more weeks, the dose was not allowed to change or increase while the patient was on study. Bone marrow growth factors were not permitted except at the investigator's discretion for patients who had at least grade 3 hematologic toxicity. Prophylactic treatment for drug-related nonhematologic adverse events of at least grade 2 was permitted after the first dose of vorinostat.
Pretreatment evaluation included a complete history and physical examination; hematologic, biochemical, and coagulation profiles; thyroid function test; pregnancy test (if appropriate); urinalysis; chest X-ray; electrocardiogram (ECG); and computed tomography scan or magnetic resonance imaging for measurable disease. Skin biopsy of index lesions, photographs of skin lesions, measurements of pruritus intensity, and the total body surface area (BSA; MF) or erythroderma skin score (patients with erythrodermic SS)23 were also done at baseline.
Patients were observed for at least 2 hours after their first oral dose of vorinostat in case of an allergic reaction. Patients giving consent had a second skin biopsy at the same site taken 2 hours and 4 and 8 weeks after starting vorinostat. At weeks 1, 2, and 4, patients were evaluated by physical and laboratory examinations, pruritus intensity measurement, and adverse event assessment. Additional tests at week 3 and every 3 weeks thereafter for the study duration included ECGs, half-body global digital photographs, photographs of cutaneous index lesions, skin biopsies of designated index lesions, or other tumor imaging studies if necessary. BSA tumor involvement (MF) and skin score (for patients with erythrodermic SS) were also assessed at each visit, although not mandated by protocol.
Patients requiring more than 2 dose modifications were discontinued from the study, as were patients in whom any drug-induced toxicity did not resolve after a 2-week delay in further treatment. Patients were also discontinued if they experienced PD, withdrew consent, were noncompliant with study procedures, or for any other reason that warranted discontinuation in the investigator's opinion. Patients were allowed to enroll a second time in a different treatment group than their initial enrollment, and 4 were treated at different dosing schedules.
Response, pruritus relief, and toxicity criteria
Responses by a Physician's Global Assessment from baseline were based on improvement in the overall percentage of BSA involved with patches, plaques, or tumors; index cutaneous lesions; lymph nodes; and all other disease manifestations.5 Complete response (CR) was defined as 100% clearing of all findings, and partial response (PR) signified at least 50% improvement in either BSA or skin score with reduction in lymph node or blood involvement, where present. PR and CR required confirmation by repeat assessment at 4 weeks or later, whereas stable disease (SD) was to be confirmed after an 8-week minimum interval. PD was defined as at least a 25% increase in the number or area of clinically abnormal lymph nodes or percentage of BSA, and new pathologically positive node or visceral disease while the patient was taking the study drug, or increases in absolute numbers of CD4+CD26− Sézary cells by flow cytometry. Confirmation by a second consecutive assessment was required to avoid premature discontinuation of patients with a temporary disease flare.
All patients rated the severity of their pruritus on a scale of 0 to 10 at the beginning of each study visit. Those with baseline pruritus scores were included in the analysis. Pruritus relief was defined as a reduction of at least 3 points or complete resolution for at least 4 weeks. Complete resolution of pruritus required a score of 0 for at least 4 continuous weeks of treatment. The National Cancer Institute Common Toxicity Criteria, version 2.0, was used to grade the severity of adverse events.
Correlative studies
Serial 4-mm punch biopsies of the same index lesions were taken at baseline, at 2 hours, and at 4, 8, and 12 weeks after starting vorinostat. Paired biopsy specimens were obtained from the same lesions. Tissues fixed in 4% paraformaldehyde and embedded in paraffin were sectioned and stained with hematoxylin and eosin. Sections were also immunoreacted with the monoclonal mouse antihuman antibodies to thrombospondin-1 (TSP-1) Ab-4 (clone A6.1, 1:500 dilution of 1 mg/mL; Neomarkers, Freemont, CA) and phospho–STAT-3 (p-STAT-3, Ser 727, clone 6E4, 1:800; Cell Signaling Technology, Danvers, MA) and detected by immunohistochemistry with a tyramine-catalyzed signal amplification system (DakoCytomation, Carpinteria, CA). The mouse anti–human CD31 antibody (clone JC704, 345 μg/mL, 1:80; DakoCytomation) was detected by DakoVision+ system followed by hematoxylin staining. The negative controls were performed with purified mouse IgG1 (DakoCytomation). Acetylated histone H4 (lysine 8) was detected by immunohistochemistry using a polyclonal antibody (1:25 dilution; Cell Signaling Technology), followed by a biotinylated secondary antibody (0.5 μg/mL; Jackson Immunoresearch, West Grove, PA) and ABC/DAB (Vector Laboratories, Burlingame, CA), then hematoxylin counterstaining. Stained hematoxylin and eosin as well as immunohistochemistry-stained sections were examined under a Leica DMLB microscope (Leica, Heidelberg, Germany) equipped with a 10× eyepiece and a 10×/0.40 CS HC PL APO objective lens. Images were captured with an Optronics Magnafire digital camera (Meyer Instruments, Houston, TX), and imaging was performed with Optimas version 6.1 software (Media Cybernetics, Silver Spring, MD) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
Statistical analysis
The primary study objective was to determine the response rate for vorinostat administered to patients with advanced, refractory CTCL. The secondary objectives were to determine the response duration, safety, and tolerability of vorinostat in this population. Rigorous sample size and power calculations were not applied, because the primary study objective was not hypothesis testing. The study was designed to have 10 to 14 patients enrolled in each group. All patients who received at least 1 dose of vorinostat were included in the response and safety analyses. If at least 1 response was observed among the first 12 patients treated in a group, the dose was considered worthy of further study. Response rates were calculated along with their 95% confidence intervals using the Exact method (Clopper-Pearson). Median time-to-objective response (TTR) and time to PD (TTP) were estimated by the Kaplan-Meier analysis method. Adverse events were recorded by body system, and the incidence of specific reactions was reported for patients as a whole. Paired t tests were used to compare microvessel density and immunohistochemistry staining intensity between the same paired skin lesional biopsies taken before and after therapy with vorinostat.
Results
Patient population
Thirty-three patients were enrolled in the study, and baseline characteristics are summarized in Table 2. The median age was 67 years, and the majority of the patients were white (76%) and men (55%). Median BSA involvement was 45% (range, 2%-100%) and the median pruritus score was 8 (range, 0-10). Patient characteristics were similar across the 3 treatment dosing groups. Most of the patients (85%) had advanced MF stage IIB or higher at study entry. One third had both erythroderma and ISCL criteria for SS22 and had failed frontline therapy with photopheresis and biologic response modifiers. Twenty patients (61%) had evidence for blood involvement (B1 or B2) by flow cytometry. Fifty-eight percent had clinically abnormal lymph nodes at the time of entry. The patients were refractory and heavily pretreated because they had received a median of 5 prior systemic therapies. Twenty-nine (88%) of the 33 patients had received at least 1 prior chemotherapy regimen.
Patient characteristics and demographics
| Characteristic . | Group 1; n = 13 . | Group 2; n = 11 . | Group 3; n = 9 . | All patients; n = 33 . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 65 | 69 | 67 | 67 |
| Range | 37-82 | 26-80 | 49-78 | 26-82 |
| Male, n (%) | 8 (62) | 5 (45) | 5 (56) | 18 (55) |
| White, n (%) | 10 (77) | 7 (64) | 8 (89) | 25 (76) |
| Black, n (%) | 3 (23) | 4 (36) | 1 (11) | 8 (24) |
| Blood involved by FC, n (%) | 8 (62) | 7 (64) | 5 (56) | 20 (61) |
| Sezary syndrome, n (%) | 3 (23) | 4 (36) | 4 (44) | 11 (33) |
| Lymph node involvement, n (%) | 8 (62) | 7 (64) | 4 (44) | 19 (58) |
| Tumor involvement, n (%) | 2 (15) | 1 (9) | 2 (22) | 5 (15) |
| BSA involvement | ||||
| Median | 54 | 38 | 17 | 45 |
| Range | 18-85 | 2-100 | 2-98 | 2-100 |
| Pruritus scores | ||||
| Median | 10 | 6 | 3 | 8 |
| Range | 3-10 | 1-10 | 0-10 | 0-10 |
| CTCL stage, n (%) | ||||
| IA | 0 (0) | 0 (0) | 1 (11) | 1 (3) |
| IB | 1 (8) | 1 (9) | 1 (11) | 3 (9) |
| IIA | 1 (8) | 0 (0) | 0 (0) | 1 (3) |
| IIB | 2 (15) | 1 (9) | 2 (22) | 5 (15) |
| III | 1 (8) | 2 (18) | 2 (22) | 5 (15) |
| IVA | 5 (38) | 4 (36) | 1 (11) | 10 (30) |
| IVB | 3 (23) | 3 (27) | 2 (22) | 8 (24) |
| Time from CTCL diagnosis, y | ||||
| Median | 3.7 | 3.1 | 2.7 | 3.3 |
| Range | 0.4-17.4 | 1.1-27.2 | 0.2-15.0 | 0.2-27.2 |
| Prior systemic treatments | ||||
| Median | 5 | 5 | 4 | 5 |
| Range | 1-10 | 2-15 | 1-9 | 1-15 |
| Prior chemotherapy, n (%) | 11 (85) | 10 (91) | 8 (89) | 29 (88) |
| Prior bexarotene, n (%) | 9 (69) | 7 (64) | 6 (67) | 22 (67) |
| Prior denileukin diftitox, n (%) | 5 (39) | 6 (55) | 3 (33) | 14 (42) |
| Prior photopheresis*, n (%) | 9 (69) | 5 (45) | 5 (56) | 19 (58) |
| Characteristic . | Group 1; n = 13 . | Group 2; n = 11 . | Group 3; n = 9 . | All patients; n = 33 . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 65 | 69 | 67 | 67 |
| Range | 37-82 | 26-80 | 49-78 | 26-82 |
| Male, n (%) | 8 (62) | 5 (45) | 5 (56) | 18 (55) |
| White, n (%) | 10 (77) | 7 (64) | 8 (89) | 25 (76) |
| Black, n (%) | 3 (23) | 4 (36) | 1 (11) | 8 (24) |
| Blood involved by FC, n (%) | 8 (62) | 7 (64) | 5 (56) | 20 (61) |
| Sezary syndrome, n (%) | 3 (23) | 4 (36) | 4 (44) | 11 (33) |
| Lymph node involvement, n (%) | 8 (62) | 7 (64) | 4 (44) | 19 (58) |
| Tumor involvement, n (%) | 2 (15) | 1 (9) | 2 (22) | 5 (15) |
| BSA involvement | ||||
| Median | 54 | 38 | 17 | 45 |
| Range | 18-85 | 2-100 | 2-98 | 2-100 |
| Pruritus scores | ||||
| Median | 10 | 6 | 3 | 8 |
| Range | 3-10 | 1-10 | 0-10 | 0-10 |
| CTCL stage, n (%) | ||||
| IA | 0 (0) | 0 (0) | 1 (11) | 1 (3) |
| IB | 1 (8) | 1 (9) | 1 (11) | 3 (9) |
| IIA | 1 (8) | 0 (0) | 0 (0) | 1 (3) |
| IIB | 2 (15) | 1 (9) | 2 (22) | 5 (15) |
| III | 1 (8) | 2 (18) | 2 (22) | 5 (15) |
| IVA | 5 (38) | 4 (36) | 1 (11) | 10 (30) |
| IVB | 3 (23) | 3 (27) | 2 (22) | 8 (24) |
| Time from CTCL diagnosis, y | ||||
| Median | 3.7 | 3.1 | 2.7 | 3.3 |
| Range | 0.4-17.4 | 1.1-27.2 | 0.2-15.0 | 0.2-27.2 |
| Prior systemic treatments | ||||
| Median | 5 | 5 | 4 | 5 |
| Range | 1-10 | 2-15 | 1-9 | 1-15 |
| Prior chemotherapy, n (%) | 11 (85) | 10 (91) | 8 (89) | 29 (88) |
| Prior bexarotene, n (%) | 9 (69) | 7 (64) | 6 (67) | 22 (67) |
| Prior denileukin diftitox, n (%) | 5 (39) | 6 (55) | 3 (33) | 14 (42) |
| Prior photopheresis*, n (%) | 9 (69) | 5 (45) | 5 (56) | 19 (58) |
BSA indicates body surface area; CTCL, cutaneous T-cell lymphoma; FC, flow cytometry.
Patients also received a combination of low-dose biologic response modifiers.
Four of the 33 patients participated in 2 different dosing level groups. The data from their first enrollment were used for demographic and efficacy analyses. However, the data from both treatment phases were used in the safety analyses. A summary of drug exposure and patient disposition across groups is shown in Table 3. Group 1 had a longer median duration of treatment (12 weeks) than did groups 2 and 3 (both 7 weeks) because of a lower rate of PD (versus group 2) or dose-limiting toxicities, such as thrombocytopenia (versus group 3), respectively. Ultimately, 25 of 33 patients discontinued because of PD, 1 had SD and discontinued at the discretion of the investigator, and 7 patients discontinued because of an adverse experience.
Drug exposure and patient disposition
| Event . | Group 1; n = 13 . | Group 2; n = 12 . | Group 3; n = 12 . | All patients; n = 37 . |
|---|---|---|---|---|
| Treatment duration, wk | ||||
| Median | 12 | 7 | 7 | 8 |
| Range | 4-36 | 1-67 | 3-36 | 1-67 |
| Discontinuation, n (%) | ||||
| Adverse event | 1 (8) | 4 (33) | 2 (17) | 7 (19) |
| Progressive disease | 10 (77) | 7 (58) | 8 (67) | 25 (68) |
| Other | 1 (8) | 0 (0) | 0 (0) | 1 (3) |
| Withdrew consent | 1 (8) | 1 (8) | 2 (17) | 4 (11) |
| Event . | Group 1; n = 13 . | Group 2; n = 12 . | Group 3; n = 12 . | All patients; n = 37 . |
|---|---|---|---|---|
| Treatment duration, wk | ||||
| Median | 12 | 7 | 7 | 8 |
| Range | 4-36 | 1-67 | 3-36 | 1-67 |
| Discontinuation, n (%) | ||||
| Adverse event | 1 (8) | 4 (33) | 2 (17) | 7 (19) |
| Progressive disease | 10 (77) | 7 (58) | 8 (67) | 25 (68) |
| Other | 1 (8) | 0 (0) | 0 (0) | 1 (3) |
| Withdrew consent | 1 (8) | 1 (8) | 2 (17) | 4 (11) |
Efficacy
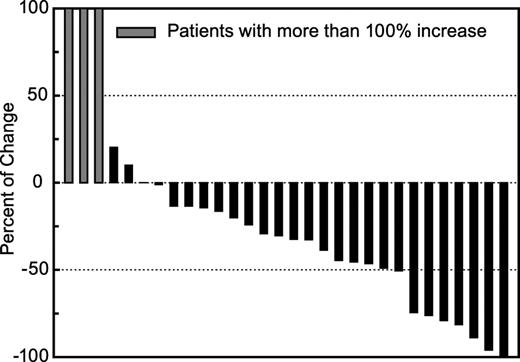
The intent-to-treat response rate for the study was 24.2% (8 of 33 patients) which is analyzed by dose and stage, as shown in Table 4. No CRs were observed. Eight patients achieved a PR of whom 1 had early-stage disease (IA-IIA), and 7 had advanced-stage disease (IIB-IVB), including 4 with SS. In addition to confirmed PRs, additional patients had minor responses with a less than 50% reduction in their BSA involvement with MF or had disease stabilization while on vorinostat (Figure 1). Responses were observed in 5 (23%) of 22 patients who had previously received oral bexarotene. Response rates in dosing group 1 (31%) and group 3 (33%), in which continuous therapy was administered, were higher than in group 2 (9%) in which patients took the drug only 3 of 7 days per week.
Patients with a partial response
| . | Group 1 . | Group 2 . | Group 3 . | All patients . | ||||
|---|---|---|---|---|---|---|---|---|
| Population . | n/n (%) . | 95% CI . | n/n (%) . | 95% CI . | n/n (%) . | 95% CI . | n/n (%) . | 95% CI . |
| All patients | 4/13 (31) | 9.1, 61.4 | 1/11 (9) | 0.2, 41.3 | 3/9 (33) | 7.5, 70.1 | 8/33 (24) | 11.1, 42.3 |
| Stage less than IIB* | 0/2 (0) | 0.0, 84.2 | 0/1 (0) | 0.0, 97.5 | 1/2 (50) | 1.3, 98.7 | 1/5 (20) | 0.5, 71.6 |
| Stage at least IIB† | 4/11 (36) | 10.9, 69.2 | 1/10 (10) | 0.3, 44.5 | 2/7 (29) | 3.7, 71.0 | 7/28 (25) | 10.7, 44.9 |
| Sezary syndrome | 1/3 (33) | 0.8, 90.6 | 1/4 (25) | 0.6, 80.6 | 2/4 (50) | 6.8, 93.2 | 4/11 (36) | 10.9, 69.2 |
| . | Group 1 . | Group 2 . | Group 3 . | All patients . | ||||
|---|---|---|---|---|---|---|---|---|
| Population . | n/n (%) . | 95% CI . | n/n (%) . | 95% CI . | n/n (%) . | 95% CI . | n/n (%) . | 95% CI . |
| All patients | 4/13 (31) | 9.1, 61.4 | 1/11 (9) | 0.2, 41.3 | 3/9 (33) | 7.5, 70.1 | 8/33 (24) | 11.1, 42.3 |
| Stage less than IIB* | 0/2 (0) | 0.0, 84.2 | 0/1 (0) | 0.0, 97.5 | 1/2 (50) | 1.3, 98.7 | 1/5 (20) | 0.5, 71.6 |
| Stage at least IIB† | 4/11 (36) | 10.9, 69.2 | 1/10 (10) | 0.3, 44.5 | 2/7 (29) | 3.7, 71.0 | 7/28 (25) | 10.7, 44.9 |
| Sezary syndrome | 1/3 (33) | 0.8, 90.6 | 1/4 (25) | 0.6, 80.6 | 2/4 (50) | 6.8, 93.2 | 4/11 (36) | 10.9, 69.2 |
CI indicates confidence interval.
Stages IA, IB, and IIA.
Stages IIB, III, IVA, and IVB.
Percentage of change in body surface area (BSA) involvement of CTCL in evaluable patients with mycosis fungoides during vorinostat treatment. ▪ indicates patients with greater than 100% increase in BSA involvement of CTCL.
Percentage of change in body surface area (BSA) involvement of CTCL in evaluable patients with mycosis fungoides during vorinostat treatment. ▪ indicates patients with greater than 100% increase in BSA involvement of CTCL.
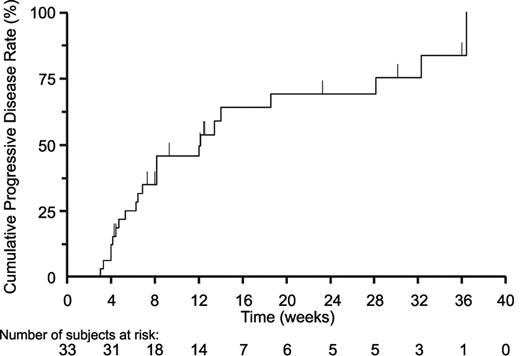
The median time to response and duration of response were 11.9 and 15.1 weeks, respectively. Time to response ranged from 3.6 to 21.9 weeks, and duration of response ranged from 9.4 to 19.4 weeks. Six of the 8 responders discontinued because of PD and the other 2 withdrew consent. Seven of the 33 patients remained on vorinostat treatment for 23 weeks or longer: 4 in group 1, 1 retreated patient in group 2, and 2 in group 3. Median time to progression was 12.1 weeks overall (Figure 2); 5 weeks for nonresponders (n = 14), 12.1 weeks for those with clinical improvement who did not achieve a confirmed greater than 50% objective response (n = 11), and 30.2 weeks for responders (n = 8).
Time to progression during treatment with vorinostat for the overall study population is shown. The median time to progression for all 33 patients based on the Kaplan-Meier estimate was 12.1 weeks.
Time to progression during treatment with vorinostat for the overall study population is shown. The median time to progression for all 33 patients based on the Kaplan-Meier estimate was 12.1 weeks.
A patients with SS in group 1 achieved a rapid response that lasted 10 weeks. This patient withdrew consent but subsequently reenrolled in group two 12 weeks later. The patient had a delayed, durable response with a time to response of 14.9 weeks and a response duration of 55.1 weeks. This response was not counted in the efficacy analysis because it was observed when the patient was treated in a second group. However, the patient was treated with vorinostat for 1.4 years.
Fourteen (45%) of 31 patients with baseline pruritus had symptomatic relief, including 73% in group 1, 18% in group 2, and 44% in group 3. Three patients had complete resolution of pruritus. The rate of pruritus relief among patients with baseline pruritus scores of 3 to 6 or 7 to 10 were 33% and 59%, respectively. Relief typically occurred within the first 4 weeks of therapy and was generally maintained throughout the study. Overall, the mean pruritus score decreased approximately 3 points, and pruritus relief was present, even in patients with SS who did not have a confirmed response.
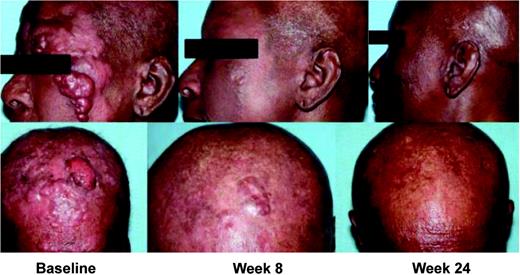
Patients with plaques, tumors, and erythroderma with circulating Sézary cells responded to vorinostat. The patient in Figure 3 had large-cell transformation with fast-growing cutaneous and subcutaneous tumors, bone marrow, and nodal disease (stage IVB) MF. She was heavily pretreated and refractory to combined chemotherapy and had received 6 prior therapies, including total skin electron beam radiation therapy; cyclophosphamide, vincristine, and prednisolone; denileukin diftitox; and bexarotene. She experienced a rapid flattening and disappearance of facial tumors within 4 to 8 weeks of vorinostat therapy.
Partial response observed in a patient with tumor stage IVB CTCL. This patient received 6 prior therapies, including total skin electron beam radiation therapy; cyclophosphamide, vincristine, and prednisolone; denileukin diftitox; and bexarotene. This patient was treated with vorinostat 400 mg every day at the time the response was observed. The CTCL lesions were assessed at baseline and visits at week 8 and week 24.
Partial response observed in a patient with tumor stage IVB CTCL. This patient received 6 prior therapies, including total skin electron beam radiation therapy; cyclophosphamide, vincristine, and prednisolone; denileukin diftitox; and bexarotene. This patient was treated with vorinostat 400 mg every day at the time the response was observed. The CTCL lesions were assessed at baseline and visits at week 8 and week 24.
Safety and toxicity
The most common adverse experiences overall were fatigue (78%), diarrhea (60%), nausea (60%), thrombocytopenia (54%), dysgeusia (51%), and dry mouth (38%). A breakdown of the grade 3 or 4 adverse experiences overall by group is shown in Table 5, and a summary of the drug-related adverse experiences by grade is shown in Table 6. Thrombocytopenia (19%) and dehydration (8%) were the most common drug-related grade 3 or 4 adverse experiences. Although the adverse experience profiles were generally similar between the groups, 5 of 12 patients in group 3 had grade 3 or 4 thrombocytopenia compared with only 1 of 13 patients in group 1 and 1 of 12 patients in group 2.
Grade 3 or 4 adverse experiences by group in more than 1 patient
| Adverse experience . | Group 1; n = 13 . | Group 2; n = 12 . | Group 3; n = 12 . | All patients; n = 37 . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | n . | % . | |
| Thrombocytopenia | 1 | 8 | 1 | 8 | 5 | 42 | 7 | 19 |
| Anemia | 1 | 8 | 2 | 17 | 0 | 0 | 3 | 8 |
| Deep vein thrombosis | 0 | 0 | 3 | 25 | 0 | 0 | 3 | 8 |
| Dehydration | 1 | 8 | 0 | 0 | 2 | 17 | 3 | 8 |
| Pyrexia | 0 | 0 | 3 | 25 | 0 | 0 | 3 | 8 |
| Hypotension | 0 | 0 | 2 | 17 | 0 | 0 | 2 | 5 |
| Pulmonary embolism | 0 | 0 | 2 | 17 | 0 | 0 | 2 | 5 |
| Sepsis | 0 | 0 | 1 | 8 | 1 | 8 | 2 | 5 |
| Adverse experience . | Group 1; n = 13 . | Group 2; n = 12 . | Group 3; n = 12 . | All patients; n = 37 . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | n . | % . | |
| Thrombocytopenia | 1 | 8 | 1 | 8 | 5 | 42 | 7 | 19 |
| Anemia | 1 | 8 | 2 | 17 | 0 | 0 | 3 | 8 |
| Deep vein thrombosis | 0 | 0 | 3 | 25 | 0 | 0 | 3 | 8 |
| Dehydration | 1 | 8 | 0 | 0 | 2 | 17 | 3 | 8 |
| Pyrexia | 0 | 0 | 3 | 25 | 0 | 0 | 3 | 8 |
| Hypotension | 0 | 0 | 2 | 17 | 0 | 0 | 2 | 5 |
| Pulmonary embolism | 0 | 0 | 2 | 17 | 0 | 0 | 2 | 5 |
| Sepsis | 0 | 0 | 1 | 8 | 1 | 8 | 2 | 5 |
Most common drug-related adverse experiences in greater than 10% of patients
| Adverse experience . | Total†, n (%) . | All patients by grade*†, n (%) . | |||
|---|---|---|---|---|---|
| Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||
| Fatigue | 27 (73) | 16 (43) | 10 (27) | 0 | 1 (3) |
| Thrombocytopenia | 20 (54) | 10 (27) | 3 (8) | 4 (11) | 3 (8) |
| Diarrhea | 18 (49) | 14 (38) | 4 (11) | 0 | 0 |
| Nausea | 18 (49) | 15 (41) | 3 (8) | 0 | 0 |
| Dysgeusia | 17 (46) | 13 (35) | 4 (11) | 0 | 0 |
| Dry mouth | 13 (35) | 13 (35) | 0 | 0 | 0 |
| Weight loss | 10 (27) | 9 (24) | 1 (3) | 0 | 0 |
| Vomiting | 9 (24) | 5 (14) | 4 (11) | 0 | 0 |
| Anorexia | 8 (22) | 7 (19) | 1 (3) | 0 | 0 |
| Decreased appetite | 8 (22) | 8 (22) | 0 | 0 | 0 |
| Blood creatinine increase | 6 (16) | 3 (8) | 3 (8) | 0 | 0 |
| Dehydration | 6 (16) | 1 (3) | 2 (5) | 1 (3) | 2 (5) |
| Anemia | 4 (11) | 1 (3) | 2 (5) | 1 (3) | 0 |
| Adverse experience . | Total†, n (%) . | All patients by grade*†, n (%) . | |||
|---|---|---|---|---|---|
| Group 1 . | Group 2 . | Group 3 . | Group 4 . | ||
| Fatigue | 27 (73) | 16 (43) | 10 (27) | 0 | 1 (3) |
| Thrombocytopenia | 20 (54) | 10 (27) | 3 (8) | 4 (11) | 3 (8) |
| Diarrhea | 18 (49) | 14 (38) | 4 (11) | 0 | 0 |
| Nausea | 18 (49) | 15 (41) | 3 (8) | 0 | 0 |
| Dysgeusia | 17 (46) | 13 (35) | 4 (11) | 0 | 0 |
| Dry mouth | 13 (35) | 13 (35) | 0 | 0 | 0 |
| Weight loss | 10 (27) | 9 (24) | 1 (3) | 0 | 0 |
| Vomiting | 9 (24) | 5 (14) | 4 (11) | 0 | 0 |
| Anorexia | 8 (22) | 7 (19) | 1 (3) | 0 | 0 |
| Decreased appetite | 8 (22) | 8 (22) | 0 | 0 | 0 |
| Blood creatinine increase | 6 (16) | 3 (8) | 3 (8) | 0 | 0 |
| Dehydration | 6 (16) | 1 (3) | 2 (5) | 1 (3) | 2 (5) |
| Anemia | 4 (11) | 1 (3) | 2 (5) | 1 (3) | 0 |
INR indicates International normalized ratio.
National Cancer Institute Common Toxicity Criteria, version 2.0.
n = 37.
The most common serious adverse experiences included dehydration (11%), thrombocytopenia (8%), vomiting (8%), anemia (5%), hypotension (5%), infection (5%), nausea (5%), pulmonary embolism (5%), pyrexia (5%), and sepsis (5%). Serious adverse experiences occurred in 37% (14 of 37) of all patients: 3 of 13 (23%) in group 1, 7 of 12 (58%) in group 2, and in 4 of 12 (33%) in group 3. All of the drug-related serious adverse experiences resolved except in 2 patients for which the experiences (dehydration or vomiting) were ongoing at the end of the study. Two patients on study died, respectively, from disease progression and untreated sepsis that was unrelated to vorinostat.
Adverse experiences that resulted in discontinuation of therapy were anemia, drug eruption, fatigue, cutaneous polyneuropathy (tingling), pulmonary embolism, thrombocytopenia, pyrexia, and subdural hematoma. One of the 2 patients with pulmonary emboli had a history of deep venous thrombosis and pulmonary embolism prior to starting vorinostat. The subdural hematoma occurred in a patient who fell while taking warfarin with an INR of 5 and experiencing drug-related thrombocytopenia. INRs were increased in some patients treated with warfarin and decreased in others; however, vorinostat has not been shown to inhibit CYP enzymes at pharmacologically relevant concentrations (< 10 μM). INR should be monitored in patients concurrently receiving vorinostat and warfarin.
The rate of discontinuation because of adverse experiences was lower in group 1 (8%), than in groups 2 (33%) or 3 (17%). Five of the 7 patients requiring discontinuation recovered within a median of 7.5 days. Of the 15 patients who required at least 1 dose interruption because of an adverse experience, 3 were not related to vorinostat, and 10 remained on study therapy. Three patients, including 1 from each group, required dose modification because of adverse experiences (fatigue, thrombocytopenia, or gastrointestinal symptoms and dysgeusia).
Response to vorinostat was associated with decreased dermal lymphocytes and microvessel density, and changes in p-STAT-3 localization
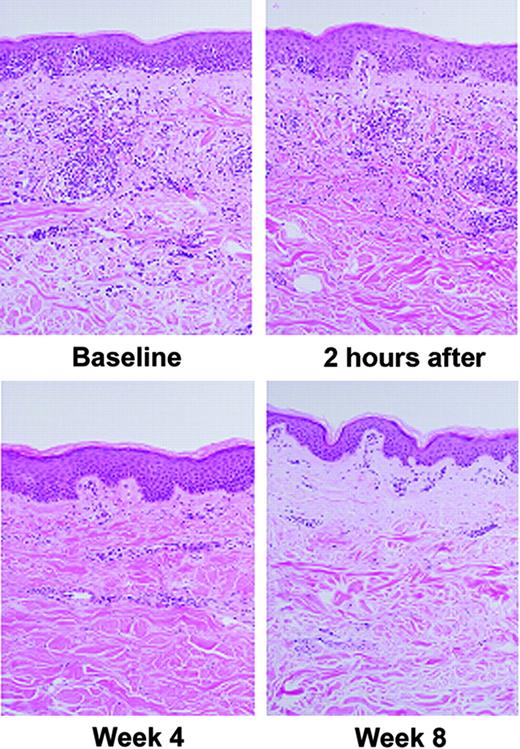
Successful therapy for MF is associated with decreased dermal and epidermal lymphocytes. There were decreased lymphocyte infiltrates after treatment in 9 of 23 paired sets of MF lesions taken at baseline and at 4-week intervals. Lymphocytes were decreased in 39.1% of patients' lesions after 4 weeks on vorinostat therapy (Figure 4). When staining for acetylated histones at baseline was compared with treated, paired lesions, very intense keratinocyte and lymphocyte staining was present at baseline (Figure 5A-B). Because most of the lymphocytes were not present after treatment, we could not correlate increased acetylation with response in these biopsies.
Decreased intensity of dermal lymphocytic infiltrates in paired mycosis fungoides lesions following oral vorinostat treatment: comparison of intensity of lymphocytic infiltrates before and after vorinostat therapy. A decrease in the intensity of the dermal infiltrates was observed after treatment in 9 (39.1%) of 23 sets of lesions after 4 weeks on oral vorinostat therapy. No obvious change in the staining intensity of dermal infiltrates was observed after only 2 hours of treatment.
Decreased intensity of dermal lymphocytic infiltrates in paired mycosis fungoides lesions following oral vorinostat treatment: comparison of intensity of lymphocytic infiltrates before and after vorinostat therapy. A decrease in the intensity of the dermal infiltrates was observed after treatment in 9 (39.1%) of 23 sets of lesions after 4 weeks on oral vorinostat therapy. No obvious change in the staining intensity of dermal infiltrates was observed after only 2 hours of treatment.
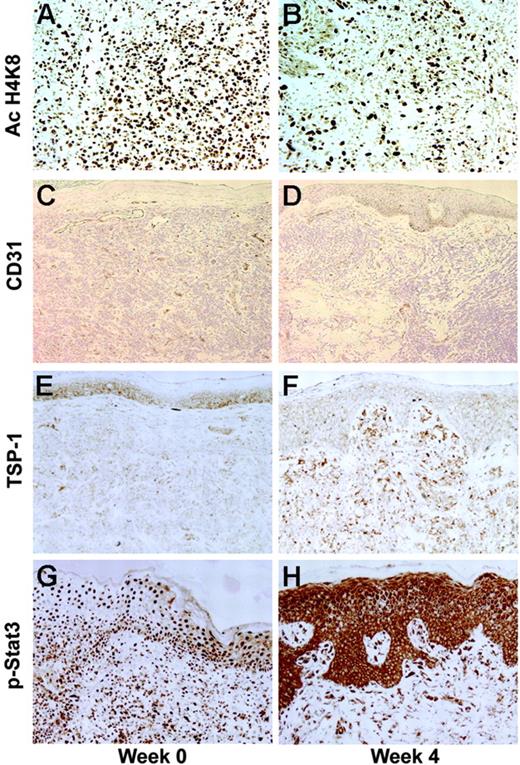
Changes in histone acetylation, microvessel density by CD31+ dermal vessels, thrombospondin-1 (TSP-1) expression, and phospho–STAT-3 (p-STAT-3) localization following 4 weeks of vorinostat treatment. (A) Histone acetylation (Ac H4K8) of keratinocytes and lymphocytes was intense at baseline. (B) After vorinostat treatment, AC H4K8 was less intense because most of the lymphocytes were not present. (C-D) CD31+ dermal vessels were reduced following 4 weeks of therapy in all patients, including responders. (E-F) The antiangiogenic protein TSP-1 was up-regulated following vorinostat treatment, with increased staining in the epidermis and dermal infiltrates. (G-H) p-STAT-3 staining shifted from the nuclei to cytoplasm of keratinocytes and lymphocytes following vorinostat treatment.
Changes in histone acetylation, microvessel density by CD31+ dermal vessels, thrombospondin-1 (TSP-1) expression, and phospho–STAT-3 (p-STAT-3) localization following 4 weeks of vorinostat treatment. (A) Histone acetylation (Ac H4K8) of keratinocytes and lymphocytes was intense at baseline. (B) After vorinostat treatment, AC H4K8 was less intense because most of the lymphocytes were not present. (C-D) CD31+ dermal vessels were reduced following 4 weeks of therapy in all patients, including responders. (E-F) The antiangiogenic protein TSP-1 was up-regulated following vorinostat treatment, with increased staining in the epidermis and dermal infiltrates. (G-H) p-STAT-3 staining shifted from the nuclei to cytoplasm of keratinocytes and lymphocytes following vorinostat treatment.
Of interest, microvessel density based on image analysis of CD31+ dermal vessels was reduced following 4 weeks of therapy in all patients and was significantly lower at week 4 in all patients (P = .011) and in responders (P = .001) (Figure 5C-D). The antiangiogenic protein TSP-1 that was increased 8-fold in a gene array analysis in the HH cell line following vorinostat treatment for 24 hours was also studied (Figure 5E-F). Dermal TSP-1 staining was increased 2 hours after dosing in 4 of 8 patients, including 2 responders. At 4 weeks, dermal staining was increased in 11 (61%) of 18 patients, including 5 responders. At 8 weeks, TSP-1 was increased in the dermis in 6 (35%) of 17 paired lesions, including 4 of 6 responders. In baseline MF lesions, nuclear staining with p-STAT-3 antibody was pronounced in both lymphocytes and keratinocytes (Figure 5G-H). Following 4 weeks of vorinostat treatment, p-STAT-3 staining was noted in the cytoplasm rather than in the nucleus of both keratinocytes and lymphocytes in 9 (81.8%) of 11 patients who experienced a clinical improvement, but in only 3 (18.8%) of 16 patients who did not show a clinical improvement. A shift from nuclear to cytoplasmic p-STAT-3 staining was also observed 2 hours after vorinostat treatment in 4 (36.4%) of 11 paired lesions, in 8 (33.3%) of 24 lesions after 1 to 3 weeks, and in 8 (42.1%) of 19 after 4 to 12 weeks.
Discussion
The primary objective of this single site, open-label phase 2 trial was to determine the response rate to oral vorinostat in patients with the MF or SS variants of CTCL refractory to or intolerant of prior systemic therapy. The response rate to vorinostat was 24%. Eight of 33 patients achieved a PR, and an additional 11 patients had pruritus relief, SD, or both. Therefore, vorinostat was of clinical benefit to 19 (58%) of 33 study patients. Responses to vorinostat were observed in a broad spectrum of the study population, including a patient with early IA refractory MF, patients with advanced CTCL with tumors showing histologic large-cell transformation, as well as those with nodal or blood involvement. Twenty-nine of the patients who were treated in this trial had received previous chemotherapy, and the median number of prior systemic treatments in all groups was 5; thus, the response rate of 24% is noteworthy, given the heavily pretreated and refractory patient population. The response rate was 23% in patients who had previously received bexarotene, suggesting that vorinostat may be non–cross-resistant to retinoid therapy.
Although the small number of patients in each treatment group limits definitive comparisons, the response rates were numerically higher in groups 1 (31%) and 3 (33%) than in group 2 (9%). Because the half-life of vorinostat is short (≤ 2 hours),11 the discontinuous dosing schedule of 3 days on and 4 days off used in group 2 may have tipped the balance toward progression compared with continuous therapy regimens of 400 mg daily (group 1) or high dose (300 mg twice daily for 2 weeks) followed by continuous therapy at 200 mg twice daily (group 3).
Pruritus is the major symptom that significantly affects the quality of life of patients with CTCLs, especially those with SS who experience unrelenting pruritus regardless of treatment.5,24,25 Measurement of pruritus by a visual analog scale is a commonly used tool for assessment of patients' itch intensity in clinical studies. Improvement in pruritus intensity was achieved in 45% of patients who had pruritus at baseline, including 3 patients who had complete resolution of pruritus. Patients with the most intense symptoms (baseline score of 7-10) had a greater reduction in pruritus than those with less intense symptoms (baseline pruritus score ≥ 6). Pruritus relief was rapid and generally maintained throughout the study and was present even in patients who did not meet the criteria for PR. The highest pruritus relief rate was observed in the first treatment group (73%), in which patients were receiving continuous treatment, and lowest in group 2 (18%), in which patients were on intermittent dosing.
Secondary objectives were to determine the duration of response and to evaluate the safety and tolerability of vorinostat in these patients. The median duration of response overall and in patients with stage IIB or higher CTCL was 15.1 weeks (106 days) and ranged from 9.4 to 19.4 weeks (66-136 days). The median duration of response was numerically lowest in group 2 (9.4 weeks [66 days]) and highest in group 1 (16.1 weeks [113 days]). These results are comparable to the median duration of response with oral bexarotene at a dose of 300 mg/m2 per day in the phase 2/3 trial of advanced patients whose response duration was 299 − 180 = 119 days (median time to progression − time to response).5 The median time to progression was more than 7 months among responding patients in this study, which is also similar to that reported with denileukin diftitox at a dose of either 9 or 18 micrograms/kg per day in a phase 3 trial (time from first dose of drug to relapse = 6.9 months).7
Vorinostat was generally well tolerated. The most common adverse experiences were related to gastrointestinal or constitutional symptoms, hematologic abnormalities, or taste disorders. There were no cases of sudden death, clinically significant ECG changes, or cardiac-related severe adverse events. The 2 deaths in patients on study were not related to vorinostat but were the result of disease progression and untreated sepsis. Otherwise, bacterial sepsis was not seen. One potential advantage of vorinostat is the lower incidence of serious infections compared with intravenous therapies which cause myelosuppression and require placement of intravenous catheters.
Two patients experienced pulmonary emboli. One patient had a prior history of deep venous thrombosis and pulmonary embolism with a new event noted in cycle 3. The second patient developed a pulmonary embolism on day 2 of study drug. One patient on warfarin had an elevated INR and thrombocytopenia related to vorinostat and had a subdural hematoma after head trauma with a fall. Although the rate of drug-related adverse experiences was similar across the 3 dosing regimens, the rates of serious adverse experiences and discontinuations because of adverse experiences were lowest in patients on the 400 mg every day schedule. The only drug-related serious adverse events reported in more than 1 patient were dehydration (8%) and thrombocytopenia (8%). Of note, the rate of grade 3 or 4 thrombocytopenia was higher in group 3 and was similar to results observed in a phase 1 trial in patients with hematologic malignancies.21
We investigated the basis for the promising activity of vorinostat in CTCL. The mechanism of action of HDAC inhibition is hypothesized to involve transcription of genes involved in apoptosis, cell-cycle control, and differentiation. HDAC inhibitors such as vorinostat promote the acetylation of other proteins, in addition to histones, including tumor suppressors. MF lesions had unexpectedly high histone H4 acetylation in keratinocyte and T-cell lymphocyte nuclei at baseline, the significance of which is unknown. It was not possible to assess histone acetylation in response to vorinostat treatment because there was a significant decrease in the number of lymphocyte infiltrates that precluded subsequent measurements. In CTCL lines in vitro, vorinostat treatment for 24 hours at 2.5 μM resulted in high levels of acetylation of histones H2B, H3, and H4 and induced T-cell apoptosis that was selective for the malignant cells.13 This suggests that T-cell apoptosis may be an important mechanism for vorinostat action in CTCL patients.
On the basis of studies from our laboratory, vorinostat at clinically significant doses induces malignant T-cell apoptosis, G2 arrest, and p21WAF,1 but does not alter p53, Bax, or Bcl-2 expression in CTCL lines.13 Vorinostat is also known to down-regulate expression of vascular endothelial growth factor (VEGF) in mantle-cell lymphoma,26 and VEGF controls angiogenesis, which is critical for cancer growth and metastasis. TSP-1, a potent inhibitor of angiogenesis, was increased 8-fold by vorinostat in the HH cell line (data not shown); thus, we studied TSP-1 in paired treated skin lesions. Translational studies from patients' paired skin lesions at baseline compared with after treatment (2 hours, 4 weeks, and 8 weeks) suggest possible mechanism(s) of vorinostat's action in CTCL. There were significant decreases in the microvessel density in responding patients' skin lesions at the same time TSP-1 was increased in the dermis of 67% of the specimens. Down-regulation of VEGF, up-regulation of the angiogenesis inhibitor TSP-1, as well as induction of T-cell apoptosis by vorinostat may all participate in resolving MF lymphocyte infiltrates.
CTCL cell lines have also been shown to constitutively express signal transducer and activator of transcription (STAT) proteins that dimerize and become phosphorylated after growth factor stimulation, inducing gene transcription and promoting cellular proliferation.27 An unexpected finding was that after treatment the p-STAT-3 protein localization changed from predominantly nuclear to cytoplasmic in responding patients. In CTCL lines treated with vorinostat, total p-STAT protein levels did not change,13 although p-STAT-3 was reduced in the nuclear fraction and increased in the cytoplasmic compartment following therapy. Vorinostat may inhibit proliferation of CTCL cells by functionally inactivating the STAT proteins which drive cellular proliferation and Th2 cytokine expression. Additional studies exploring the mechanism of action of vorinostat as well as identifying a predictive response signature are ongoing.
In summary, vorinostat provided objective clinical and symptomatic relief with meaningful duration in patients with CTCLs who had received prior therapy. Vorinostat activity was observed in all stages of MF including advanced, refractory patients with large-cell transformation and SS. Of particular interest were the responses observed in 36% of patients with refractory SS. These patients with advanced-stage disease respond poorly to many treatments and are predisposed to frequent infections. The ability to administer vorinostat orally is important for avoidance of frequent line sepsis in this group of patients whose skin is almost always colonized with Staphylococcus aureus.28 Responses were also noted after patients had received bexarotene or combination chemotherapies. The activity seen in this group of patients with refractory CTCL who had failed multiple prior therapies suggests that vorinostat may represent a novel therapeutic option for these patients. Vorinostat was associated with an acceptable safety profile with the most common side effects being fatigue, gastrointestinal symptoms, and thrombocytopenia. The combined efficacy and safety data in 3 separate dosing schedules support further investigation of vorinostat 400 mg daily in patients with CTCLs. This dose and schedule is currently being evaluated in patients with CTCL in a multicenter, phase 2b trial.29
Presented in part at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 200319 ; and the 41st annual meeting of the American Society of Clinical Oncology, Orlando, FL, May 14, 2005.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: Several of the authors (J.H.C., J.F.R., J.L.R., V.M.R., and S.RF.) have declared a financial interest in Merck & Co., Inc. whose potential product was studied in the present work. One of the authors (J.H.C.) has declared a financial interest in a competitor of Merck & Co., Inc. Several of the authors (J.H.C., J.F.R., J.L.R., V.M.R., and S.R.F.) are employed by Merck & Co., Inc. M.D., as principal investigator, received support from Aton/Merck for conducting the clinical trial and NIH K24 CA86815. The remaining authors (R.T., X.N., C.Z., P.H., and C.K.) declare no competing financial interests.
Contribution: M.D. designed research, performed research, analyzed data, and wrote the paper; R.T., X.N., C.Z., P.H., C.K., and J.F.R. performed research, collected data, and analyzed data; J.H.C. designed research, collected data, and analyzed data; J.L.R. analyzed data and wrote the paper; V.M.R. performed research, contributed vital new reagents or analytical tools, and collected data; and S.R.F. designed research, analyzed data, and wrote the paper.
Acknowledgments
This work was supported by research funding from Merck & Co, Inc, the National Institutes of Health (K24) (M.D.), and the Sherry L. Anderson CTCL Research Fund.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal