Abstract
Regulatory T cells (Tregs) are crucial for the induction and maintenance of self-tolerance and are present in peripheral tissues such as skin and gut under normal, noninflamed conditions. We report isolation and expansion of the Treg population resident in normal human skin. Cutaneous Tregs expressed high levels of CD25, L-selectin, GITR, FOXP3, and intracellular CTLA-4, low levels of CD69, and high levels of the skin-homing addressins CLA, CCR4, and CCR6. Skin Tregs suppressed the proliferation of CD25lo T cells from the same skin sample in response to CD3 and CD28 antibodies. Suppression was dependent on cell contact and not affected by neutralizing antibodies to interleukin-10 (IL-10) and transforming growth factor-β (TGF-β). Surprisingly, cutaneous Tregs proliferated in an antigen-independent manner when cultured in contact with dermal fibroblasts and IL-15, conditions similar to those found in chronically inflamed skin. We hypothesize that local proliferation of Tregs may occur within inflamed skin and could serve as a brake for cutaneous inflammation as well as a mechanism for the homeostatic proliferation of natural Tregs that has been observed within intact organisms.
Introduction
The importance of regulatory T cells (Tregs) in the development of self-tolerance was first demonstrated in mice when thymectomy 2 to 4 days after birth was found to prevent the development of CD4+CD25+ T cells, resulting in organ-specific autoimmune disease.1-3 Subsequent findings in humans have confirmed the importance of these cells to immune regulation. Patients with a mutation in FOXP3, a gene crucial for the development of Tregs, develop widespread and fatal autoimmunity.4-6
There are at least 3 separate types of CD4+ Tregs that have been identified in humans and mice. Natural Tregs likely represent a separate T-cell lineage and are released from the thymus as mature, regulatory T cells.7 Induced Tregs develop in the periphery from non-Tregs; these cells are induced to adopt a Treg phenotype by contact with natural Tregs in a process known as infectious tolerance. Natural Tregs can be distinguished from induced Tregs by their constant high expression of CD25 and FOXP3 and by their ability to suppress the proliferation and cytokine production of effector T cells in a manner that is cytokine-independent but requires cell contact. Natural Tregs are known to expand in mice in vivo,8-10 but are anergic to physiologic levels of T-cell receptor (TCR) stimulation and interleukin-2 (IL-2). Human natural Tregs can be expanded from the blood of both humans and mice by use of repeated cycles of intense TCR signaling and costimulation in the presence of high levels of IL-2.11 These conditions provide robust proliferation of natural Tregs in vitro, but it is not clear if these conditions exist within the intact animal and represent the physiologic mechanism for Treg expansion in vivo.
Studies of Tregs have so far focused on cells isolated from blood, but little is known about the Tregs that populate tissues such as skin. We have recently found that there are 1 million memory T cells resident in every square centimeter of normal, noninflamed human skin.12 These cells play a crucial role in immunosurveillance against both infections and malignancies, as shown by the susceptibility of patients with HIV to cutaneous infection and the increased numbers of squamous cell carcinomas observed in organ transplant recipients taking T-cell–immunosuppressive medications. We have recently identified a subset of T cells resident in normal human skin that has a phenotype similar to that of Tregs isolated from the blood. These T cells, present at 1 of the largest interfaces of the body with the external environment, are well placed to play a role in tolerance both to self-antigens and to normal flora. We report here the isolation, expansion, and characterization of natural Tregs from human skin.
Materials and methods
The protocols of this study were performed in accordance with the Declaration of Helsinki, and were approved by the Institutional Review Board of the Partners Human Research Committee (Partners Research Management, Boston, MA).
Skin explant cultures
Normal human skin was obtained from patients undergoing cosmetic surgery procedures. Three-dimensional matrices (Statamatrix, previously named Cellfoam) were obtained from Cell Sciences (Singapore). Explant cultures were established as described.13 Skin was cut into explants approximately 2 mm by 2 mm by 2 mm, and 3 explants were placed on the surface of each matrix. Each matrix was placed into 1 well of a 24-well plate in 2 mL/well of Iscove modified medium (Mediatech, Herndon, VA) with 20% heat-inactivated fetal bovine serum (FBS; Sigma, St Louis, MO), penicillin and streptomycin, and 3.5 μL/L β-mercaptoethanol. Cultures were fed 3 times a week by careful aspiration of 1 mL of culture medium and replacement with fresh medium. Cells were harvested at 3 weeks or at the interval specified. When indicated, IL-15 (20 ng/mL) and/or IL-2 (100 IU/mL) (R&D Systems, Benicia, CA) were added and refreshed with each feeding. For blockade of human leukocyte antigen (HLA)–DR, HLA-DP, HLA-DQ, neutralizing antibodies (clone TU39; BD Pharmingen, San Diego, CA) was added at 20 μg/mL and refreshed at each feeding. Mixed leukocyte reactions (MLRs) to confirm HLA blockade were performed by incubating 2 × 105 peripheral-blood mononuclear cells (PBMCs) from 1 donor with 2 × 105 PBMCs from a second donor in 200 μL of RPMI and 10% fetal calf serum (FCS) in a 98-well plate; samples were performed in triplicate. On day 5, 0.037 MBq (1 μCi) [3H]thymidine was added to each well; on day 5, wells were harvested and incorporation of [3H]thymidine was assayed as described in “Assay for Treg activity.”
Flow cytometry studies
Flow cytometry analysis of T cells was performed using directly conjugated monoclonal antibodies (mAbs). CD3, CD25, CD69, and CCR4 antibodies were obtained from BD Biosciences (San Diego, CA); CLA, CCR6, HLA-DR, DP, DQ antibodies and CTLA-4 antibodies were purchased from BD Pharmingen; l-selectin antibody was purchased from BD Pharmingen (Miami, FL). GITR (110416) antibody was purchased from R&D Systems, CD104 antibody (HML-1) was purchased from Biodesign International (Saco, ME), and a FOXP3 (PCH101) staining kit was purchased from eBioscience (San Diego, CA); staining was performed per manufacturer's instructions. Analysis of flow cytometry samples was performed on Becton Dickinson FACScan or FACSCanto instruments (San Jose, CA), and data were analyzed with CellQuest or FACSDiva software (BD Pharmingen). For analysis of FOXP3 expression after stimulation of skin T cells, T cells were cultured on fibroblast monolayers for 1 week in the presence of IL-15 (20 ng/mL) and IL-2 (100 IU/mL), then analyzed for FOXP3 expression.
Assay for Treg activity
T cells were isolated from explant cultures of normal human skin cultured in the presence of IL-2 (100 U/mL) and IL-15 (20 ng/mL) as described in “Skin explant cultures.” Cells were harvested at 21 days. CD4+ CD25hiCD69lo and CD25lo populations were separated by staining with anti-CD4–PE-Cy5, anti-CD69–FITC, and anti-CD25–PE, followed by sorting on a FACSAria cell sorter (BD Pharmingen). Treg assays were performed as described previously.14 Irradiated (30 Gy “3000 rad”) T-cell–depleted PBMCs prepared from an unrelated blood donor were used as accessory cells. Skin CD4+CD25lo T cells (2500 cells/well) were used as T responder (Tresp) cells. In combined CD25hi+lo wells, 1250 CD4+CD25hi cells were added to 2500 CD25lo T cells (1:2 Treg/Tresp-cell ratio). Cells were cultured for 5 days in a final volume of 200 μL RPMI-1640 medium with l-glutamine supplemented with 5 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin (GIBCO, Carlsbad, CA), 1 mM sodium pyruvate, nonessential amino acids (Mediatech, Herndon, VA), and 5% human AB serum (Fisher Scientific, Hampton, NH) in the presence of accessory cells (25 000 cells/well) in U-bottom 96-well plates (Corning, Acton, MA). When indicated, the cells were stimulated with 1 ng/mL soluble anti-CD3 (HIT3a) plus 100 ng/mL soluble anti-CD28. All well cultures were performed in triplicate. After 5 days of culture, 0.037 MBq (1 μCi) [3H]thymidine was added to each well. The cells were harvested after 16 hours, and radioactivity was measured using a liquid scintillation counter (Wallac “PerkinElmer”, Wellesley, MA). The results are expressed as the mean counts per minute (cpm) plus or minus SEM of triplicate wells. For assays including neutralizing antibodies, 5 μg/mL of anti–IL-10 (clone 23728; R&D Systems) and/or anti–transforming growth factor-β (TGF-β) (clone 1D11; R&D Systems) was added to each well on days 1, 3, and 5. For transwell experiments, 0.4-μm pore 6.5-mm diameter transwells (Costar) were used to separate Treg and Tresp cells in 24-well plate culture wells; cell numbers and media volume were scaled up 3.5-fold. For cell proliferation assays (Figure 5A), 2500 cells/well were incubated for 5 days with indicated cytokines or stimulatory antibodies; 0.037 MBq (1 μCi) [3H]thymidine was added to each well on day 5, and cells were harvested as described in “Assay for Treg activity” on day 6. No accessory cells were used.
Culture of skin T cells on fibroblast monolayers
Fibroblasts were isolated from human skin and cultured on 24-well plates until monolayers formed as described.13 After addition of T cells, medium was changed to Iscove/20% FCS as described in “Skin explant cultures,” and where noted, IL-2 and/or IL-15 were included and refreshed with feedings. Cultures were fed as described for explant cultures. For CFSE labeling, T cells were labeled with 0.5 μmol CFSE (Invitrogen) per manufacturer's directions and cultured for 1 week on fibroblast monolayers with indicated cytokines. For transwell experiments, 0.4-μm pore 6.5-mm diameter transwells (Costar) were used to separate fibroblasts and T cells.
Immunofluorescence studies
Samples of normal human skin obtained after plastic surgery procedures were embedded in OCT and snap-frozen in liquid nitrogen. Frozen sections (5-μm thickness) were cut, allowed to dry, and stored at −80°C until use. Sections were fixed in acetone for 5 minutes, air dried, and rehydrated in phosphate-buffered saline (PBS), blocked with a 20 μg/mL solution of human immunoglobulin G (IgG) for 15 minutes, then incubated with a mixture of anti-CD3–PE (1:50; BD Pharmingen) and anti-FOXP3–FITC (1:5; clone PCH101) for 30 minutes at room temperature. Sections were washed 3 times by incubating for 5 minutes in PBS/1% BSA. Prolong antifade mounting medium (Invitrogen) was added, and the sections were examined by fluorescence microscopy and photographed immediately. To evaluate the percentage of FOXP3+ T cells, 20 high-power fields (hpf's; 40×) were photographed. The number of CD3+ and FOXP3+ T cells in each field was then counted and used to calculate the percentage of Tregs. Sections were photographed using a Nikon Eclipse 6600 microscope (Nikon, Tokyo, Japan) equipped with a Nikon 40×/0.75 Plan Fluor objective lens. Images were captured with a SPOT RT model 2.3.1 camera (Diagnostic Instruments, Sterling Heights, MI) and were acquired with SPOT 4.0.9 software (Diagnostic Instruments).
Studies on blood lymphocytes
T cells were isolated from blood as described12 and labeled with anti-CLA, anti-CD3, and anti-CD25 mAbs. CD25hi T cells were isolated by high-speed cell sorting on a FACSAria instrument by gating on the highest 5% of CD25-expressing T cells. These cells were further divided into CLA+ and CLA− fractions. CD25lo T cells were isolated by sorting for the lowest 10% of CD25 expression. Cells were labeled with CFSE and cultured on fibroblast monolayers with the indicated cytokines as described in “Culture of skin T cells on fibroblast monolayers.”
Results
Isolation and expansion of Tregs from skin explant cultures
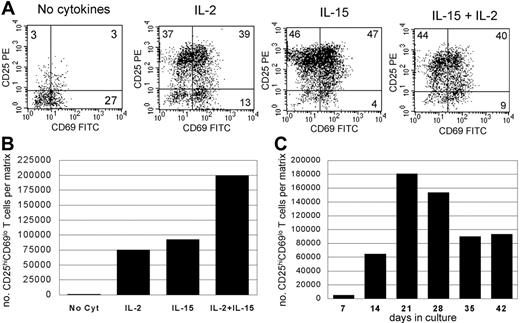
We have recently reported efficient isolation of skin-resident T cells from skin using cultures of skin explants.13 Explant cultures maintained without exogenous cytokines allowed isolation of nonexpanded skin-resident T cells, whereas inclusion of IL-2 and IL-15 in explant cultures produced a 5- to 10-fold expansion of T cells without significant loss of diversity or expression of skin-homing addressins.12,13 Most T cells isolated from explant cultures treated with IL-2 and IL-15 expressed high levels of CD25 (Figure 1A). CD25 is expressed by both Tregs and by T cells that are activated and undergoing proliferation, a fact that can make it difficult to discriminate between Tregs and “contaminating” populations of activated T cells. However, recently activated T cells can be identified by their expression of the activation antigen CD69. A significant population of T cells were CD4+CD25hiCD69lo, similar to Tregs isolated from blood. CD25hiCD69lo T cells were most prevalent when explant cultures were maintained in a combination of IL-2 and IL-15 (Figure 1A), and production of CD25hiCD69lo cells was maximal at 3 weeks of explant culture (Figure 1C). Substantial numbers of CD25hiCD69lo T cells could be isolated from small samples of normal human skin. A mean of 160 400 CD25hiCD69lo T cells were isolated from the equivalent of a 4-mm punch biopsy of normal skin (SD, 33 216; n = 5 different skin donors).
Significant numbers of CD25hiCD69lo skin-resident T cells are isolated from skin explants cultured in IL-2 and IL-15. (A) Skin from a single donor was cultured for 3 weeks on 3D matrices with or without exogenous IL-2 (100 IU/mL) and/or IL-15 (20 ng/mL). Large populations of CD25hiCD69lo T cells were observed in IL-15– and IL-2–treated cultures. (B) Explants cultured in both IL-2 and IL-15 produced the largest absolute number of CD25hiCD69lo T cells. (C) Explants cultured in IL-2 and IL-15 using skin from a second donor; maximal production of CD25hiCD69lo skin-resident T cells occurred at 21 days of culture. Experiments using skin from 5 different donors produced similar results. Numbers indicate the percentage of cells in each quadrant.
Significant numbers of CD25hiCD69lo skin-resident T cells are isolated from skin explants cultured in IL-2 and IL-15. (A) Skin from a single donor was cultured for 3 weeks on 3D matrices with or without exogenous IL-2 (100 IU/mL) and/or IL-15 (20 ng/mL). Large populations of CD25hiCD69lo T cells were observed in IL-15– and IL-2–treated cultures. (B) Explants cultured in both IL-2 and IL-15 produced the largest absolute number of CD25hiCD69lo T cells. (C) Explants cultured in IL-2 and IL-15 using skin from a second donor; maximal production of CD25hiCD69lo skin-resident T cells occurred at 21 days of culture. Experiments using skin from 5 different donors produced similar results. Numbers indicate the percentage of cells in each quadrant.
Skin CD25hiCD69lo T cells contain a population of functional natural Tregs
FOXP3 is 1 of the best markers for Treg function and is expressed at a high and constant level by natural Tregs.3,15,16 Mice and humans with mutations of the FOXP3 gene develop lethal autoimmune disease.17-22 Moreover, ectopic expression of Foxp3 in naive mouse T cells forces them to adopt a regulatory phenotype, suggesting that FOXP3 may be 1 of the master switches for Treg function.23 We found that between 5% and 10% of T cells from explant cultures without cytokines expressed CD25 and FOXP3 (Figure 2A; mean, 7.4% total cells; SD, 1.8%; n = 3). Significantly larger numbers of CD25hi FOXP3+ T cells were isolated from explant cultures treated with IL-15 and IL-2 (Figure 2A; mean, 24% of total cells; SD, 9.9%; n = 5). FOXP3+ cutaneous T cells were almost universally CD25+CD69−, as reported for Tregs isolated from blood.3 FOXP3+ skin-resident T cells were universally CD25+, but they could not be selectively identified as a population that expressed the very highest levels of CD25, as has been reported for human Tregs from blood.24
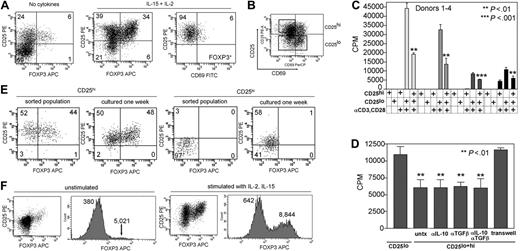
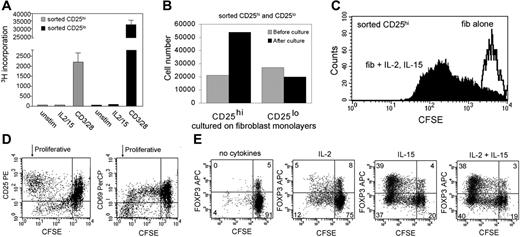
CD25hiCD69lo T cells isolated from skin contain a population of natural regulatory T cells. (A) Skin-resident T cells isolated from skin cultured in IL-2 and IL-15 contained increased numbers of FOXP3+ T cells that also expressed high levels of CD25 and low levels of CD69. (B) Skin-resident T cells isolated from explant cultures were sorted into CD25hiCD69lo and CD25lo populations. (C) CD25hiCD69lo T cells (CD25hi) were anergic to stimulation with soluble anti-CD3 and anti-CD28 antibodies (αCD3, CD28) and suppressed the proliferation of CD25lo T cells (CD25lo) isolated from the same sample of skin. (D) Suppression was not affected by neutralizing antibodies to IL-10 (αIL-10) and/or TGF-β (αTGF-β) but was dependent upon cell-cell contact. Suppression was prevented by separation of the CD25hi and CD25lo T-cell populations by a 0.4-μm pore membrane (transwell). (E) A subpopulation of sorted CD25hi T cells retain high expression of CD25 and FOXP3 after 1 week of culture on fibroblast monolayers in the presence of IL-2 and IL-15. Sorted CD25lo cells lack FOXP3+ T cells and FOXP3+ T cells do not develop after 1 week of culture under the same conditions. (F) FOXP3 was up-regulated in both Treg and non-Tregs with cell activation, but this did not obscure identification of Tregs. T cells isolated from skin were examined for FOXP3 expression before and after stimulation with IL-2 and IL-15. Dotplots demonstrate that a clear population of Tregs was discernible under both conditions. The mean fluorescent intensities for each peak are shown on the histograms. FOXP3 expression increased in both groups with stimulation, but the Treg population remained separated from non-Tregs by at least a log increase in FOXP3 staining intensity. For scatterplots, numbers indicate the percentage of cells in each quadrant. For bar graphs, error bars indicate standard deviation.
CD25hiCD69lo T cells isolated from skin contain a population of natural regulatory T cells. (A) Skin-resident T cells isolated from skin cultured in IL-2 and IL-15 contained increased numbers of FOXP3+ T cells that also expressed high levels of CD25 and low levels of CD69. (B) Skin-resident T cells isolated from explant cultures were sorted into CD25hiCD69lo and CD25lo populations. (C) CD25hiCD69lo T cells (CD25hi) were anergic to stimulation with soluble anti-CD3 and anti-CD28 antibodies (αCD3, CD28) and suppressed the proliferation of CD25lo T cells (CD25lo) isolated from the same sample of skin. (D) Suppression was not affected by neutralizing antibodies to IL-10 (αIL-10) and/or TGF-β (αTGF-β) but was dependent upon cell-cell contact. Suppression was prevented by separation of the CD25hi and CD25lo T-cell populations by a 0.4-μm pore membrane (transwell). (E) A subpopulation of sorted CD25hi T cells retain high expression of CD25 and FOXP3 after 1 week of culture on fibroblast monolayers in the presence of IL-2 and IL-15. Sorted CD25lo cells lack FOXP3+ T cells and FOXP3+ T cells do not develop after 1 week of culture under the same conditions. (F) FOXP3 was up-regulated in both Treg and non-Tregs with cell activation, but this did not obscure identification of Tregs. T cells isolated from skin were examined for FOXP3 expression before and after stimulation with IL-2 and IL-15. Dotplots demonstrate that a clear population of Tregs was discernible under both conditions. The mean fluorescent intensities for each peak are shown on the histograms. FOXP3 expression increased in both groups with stimulation, but the Treg population remained separated from non-Tregs by at least a log increase in FOXP3 staining intensity. For scatterplots, numbers indicate the percentage of cells in each quadrant. For bar graphs, error bars indicate standard deviation.
To determine whether the CD4+CD25hiCD69lo T cells we isolated from skin were functional Tregs, we isolated these cells by flow sorting (Figure 2B) and tested them for the ability to suppress the proliferation of CD25lo T cells isolated from the same sample of skin. CD25hiCD69lo T cells were largely anergic to stimulation with soluble CD3 and CD28 at levels that induced robust proliferation of the CD25lo subset (Figure 2C). However, inclusion of CD25hiCD69lo T cells significantly reduced the proliferation of CD25lo cells. The level of suppression varied from donor to donor (mean, 49% suppression; SD, 8.3%; n = 4) but was statistically significant in all donors tested. Suppression was unaffected by neutralizing antibodies to IL-10 and TGF-β but was abrogated when CD25hi and CD25lo cells were physically separated, demonstrating that suppression was cell-contact dependent (Figure 2D).
When we examined the sorted population of CD25hiCD69lo T cells used in our suppression assays, we found that they actually contained a mixed population of CD25hi FOXP3+ Tregs and FOXP3− non-Tregs. Natural Tregs can be distinguished from induced CD4+ Tregs (Tr1 and Th4) by their constant and high expression of CD25. We found that the FOXP3+ T cells within the sorted CD25hiCD69lo population maintained high expression of CD25 after 1 week in culture with dermal fibroblasts, IL-2, and IL-15, providing further evidence that these cells are natural Tregs (Figure 2E). The FOXP3+ population also remained CD69lo after 1 week in culture (data not shown).
We found increased numbers of Tregs in explant cultures maintained in IL-2 and IL-15, suggesting that either FOXP3+ T cells were proliferating, or there was conversion of FOXP3− non-Tregs into FOXP3+ Tregs under our culture conditions. To address this question, we isolated a CD25lo population of T cells via cell sorting that lacked FOXP3+ T cells (Figure 2E). We cultured these cells on fibroblast monolayers in the presence of IL-2 and IL-15 for 1 week. A subset of these cells did develop CD25 expression, but there was no induction of FOXP3 to the levels seen in Tregs, demonstrating that our culture conditions do not support the development of Tregs from non-Tregs (Figure 2E).
Expression of FOXP3 in human lymphocytes can fluctuate with cell activation. To examine this issue, we studied T cells isolated from skin before and after activation with IL-2 and IL-15. We found that under both unstimulated and stimulated conditions, a clear population of Tregs expressing very high levels of CD25 and FOXP3 were discernible (Figure 2F, dotplots). With cell activation, FOXP3 expression increased in both Tregs and non-Tregs, but this increase was always less than a 2-fold increase in the mean fluorescence intensity (Figure 2F, histograms). Under both conditions, Tregs were separated from non-Tregs by a full log increase in FOXP3 staining intensity. Therefore, very high levels of FOXP3 expression can be used to identify Tregs, even under conditions of cellular activation.
FOXP3+ Tregs are present in normal human skin
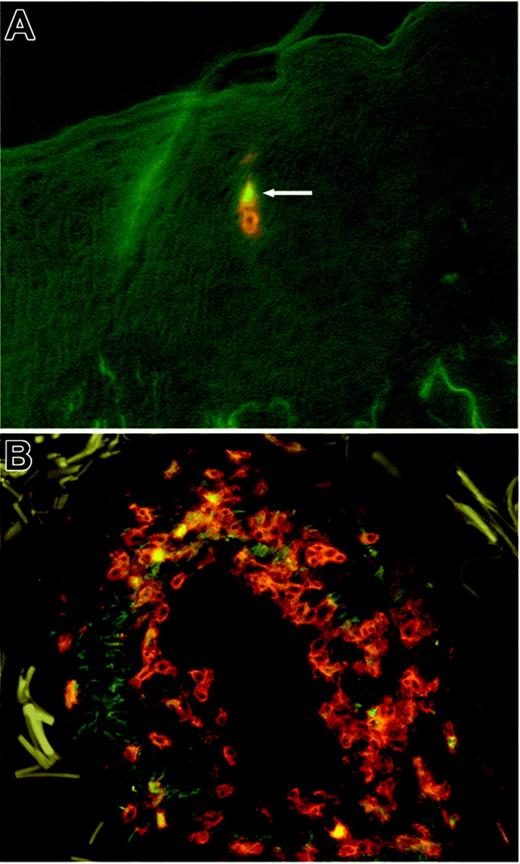
We have used explant cultures to isolate the T cells from normal human skin. This method allows isolation of significant numbers of T cells from normal skin without changes in surface phenotype or functionality.12,13 In order to demonstrate that CD3+FOXP3+ T cells are truly present in normal human skin and were not simply induced by explant culture, we have stained sections of normal human skin for both CD3 and FOXP3. Using 2-color immunofluorescence staining, we were able to clearly identify FOXP3+CD3+ Tregs within the infiltrate of T cells that is normally present within noninflamed human skin (Figure 3) Tregs were dispersed within the skin at the same locations as non-Tregs. Most T cells were found within the dermis, located near appendages and blood vessels. In order to quantify Tregs, we photographed 20 hpf's (40 ×) obtained from 4 different normal-skin donors. The number of CD3+ T cells were counted and compared with the number of FOXP3+CD3+ Tregs within each field. In this manner, we found that 10.8% of T cells from noninflamed skin were FOXP3+ Tregs (SD, 3.15%; n = 20). This percentage was quite similar to that obtained using explant cultures without exogenous cytokines, in agreement with our earlier observation that explant cultures maintained in the absence of exogenous cytokines release a nonexpanded population of skin-resident T cells.12
FOXP3+ Tregs are resident in healthy human skin. Sections of healthy human skin were costained with directly conjugated antibodies to CD3 (red) and FOXP3 (green). FOXP3+ Tregs were clearly visible as cells with red membranes and green nuclei. (A) Two T cells present within the epidermis are shown, 1 regulatory (white arrow) and 1 nonregulatory. In general, Tregs were found in the same locations as non-Tregs, located near blood vessels and appendages within the dermis. (B) A particularly brisk infiltrate of T cells containing Tregs surrounding a blood vessel. Such infiltrates can be characteristic of normal human skin.
FOXP3+ Tregs are resident in healthy human skin. Sections of healthy human skin were costained with directly conjugated antibodies to CD3 (red) and FOXP3 (green). FOXP3+ Tregs were clearly visible as cells with red membranes and green nuclei. (A) Two T cells present within the epidermis are shown, 1 regulatory (white arrow) and 1 nonregulatory. In general, Tregs were found in the same locations as non-Tregs, located near blood vessels and appendages within the dermis. (B) A particularly brisk infiltrate of T cells containing Tregs surrounding a blood vessel. Such infiltrates can be characteristic of normal human skin.
Surface phenotype of skin-resident Tregs
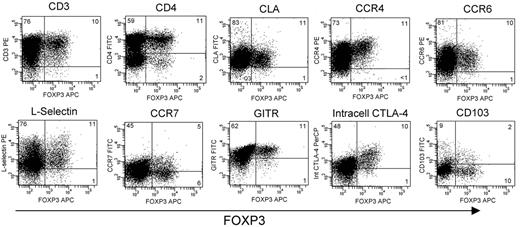
Expression of FOXP3 is 1 of the more specific markers for Treg function, but it is an intracellular antigen that cannot be used to sort viable cells.3 We could not completely separate out confirmed FOXP3+ Tregs from other T cells based only on their CD25hiCD69lo phenotype. Bulk-sorted CD25hiCD69lo T cells did have regulatory activity, as we show above, but this suppression may be mediated by only the FOXP3+ fraction of this population. To selectively study the phenotype of only FOXP3+ cutaneous Tregs, we costained T cells expanded from the skin with FOXP3 and a panel of other markers (Figure 4) FOXP3+ skin T cells were universally CD3+, and most expressed CD4, although a small subpopulation was CD4− and also did not express CD8 (data not shown). FOXP3+ skin Tregs expressed CLA, CCR6, and very high levels of CCR4, consistent with their ability to home to and populate the skin. FOXP3+ skin T cells also expressed l-selectin, GITR, and high levels of intracellular CTLA-4, as previously reported for Tregs isolated from human blood.14,25 Approximately half of FOXP3+ skin Tregs expressed CCR7, similar to the CCR7 expression observed in other skin-resident T cells.12 CD103 is expressed on skin Tregs in mice and underlies their retention within the skin.26 In contrast, we found that most human skin Tregs did not express CD103. Last, the vast majority of T cells isolated from skin expressed high levels of HLA-DR, regardless of FOXP3 expression (data not shown).
Phenotype of FOXP3+ natural Tregs isolated from skin. Skin-resident T cells were obtained from skin samples cultured for 3 weeks in IL-2 and IL-15. Unsorted T-cell populations were costained for FOXP3 and the indicated markers. Experiments from 3 different skin donors produced similar results. Numbers indicate the percentage of cells in each quadrant.
Phenotype of FOXP3+ natural Tregs isolated from skin. Skin-resident T cells were obtained from skin samples cultured for 3 weeks in IL-2 and IL-15. Unsorted T-cell populations were costained for FOXP3 and the indicated markers. Experiments from 3 different skin donors produced similar results. Numbers indicate the percentage of cells in each quadrant.
IL-15 and dermal fibroblasts induce the proliferation of FOXP3+ skin Tregs
We noted a significant increase in the number of CD4+CD25hiFOXP3+ functional Tregs when skin explants were cultured in the presence of IL-2 and IL-15.
Tregs expanded from the skin resembled natural Tregs from the blood in that they expressed high and stable levels of CD25 and FOXP3 and suppressed cellular responses through a cell-contact–dependent mechanism. Natural Tregs develop as a separate T-cell lineage within the thymus.7 It was therefore likely that Tregs were proliferating in culture as opposed to the conversion of T cells from a nonregulatory phenotype to the regulatory phenotype. To determine if cytokines alone were sufficient to induce skin Treg proliferation, we isolated CD4+ CD25hiCD69lo and CD25lo T-cell populations from skin, confirmed that the CD25hiCD69lo population had regulatory activity with T-cell–suppression assays, and then stimulated these cells with IL-2 and IL-15 or with anti-CD3 and anti-CD28 antibodies for 1 week (Figure 5A) IL-2 and IL-15 alone did not induce proliferation of the CD25hi or CD25lo subsets, whereas strong TCR and costimulatory signaling via soluble antibodies induced strong proliferation of the CD25lo subset and a 15-fold lower proliferation of the CD25hi subset.
IL-15 and dermal fibroblasts induce the proliferation of skin-resident FOXP3+ Tregs. (A) CD25hiCD69lo (CD25hi) skin-resident T cells did not proliferate when treated with IL-2 and IL-15 alone (IL2/15) but proliferated at low levels when stimulated with soluble anti-CD3 and anti-CD28 antibodies (CD3/28). CD25lo skin-resident T cells isolated from the same skin sample also did not proliferate when treated with IL-2 and IL-15 alone, but proliferated robustly after treatment with CD3 and CD28 antibodies. Error bars indicate standard deviation. (B) Sorted CD25hi skin-resident T cells with regulatory activity proliferated when cultured on monolayers of dermal fibroblasts for 1 week in the presence of IL-2 and IL-15. (C) Sorted CFSE-labeled CD25hi skin-resident T cells with regulatory activity cultured on fibroblasts without IL-2 and IL-15 did not proliferate (fib alone) but cells cultured on fibroblast monolayers with IL-2 and IL-15 (fib + IL-2, IL-15) did proliferate. (D) Unsorted CFSE-labeled skin-resident T cells cultured on fibroblast monolayers with IL-2 and IL-15 showed preferential expansion of the CD25hiCD69lo subset. (E) IL-15 and culture on dermal fibroblasts is necessary and sufficient to induce preferential expansion of FOXP3+ skin Tregs. Skin-resident T cells isolated from 2-week explant cultures were labeled with CFSE and cultured for 1 week on fibroblast monolayers with the indicated cytokines. CFSE-low cells have undergone proliferation. All results shown have been replicated using T cells from a minimum of 3 different skin donors. Numbers indicate the percentage of cells in each quadrant.
IL-15 and dermal fibroblasts induce the proliferation of skin-resident FOXP3+ Tregs. (A) CD25hiCD69lo (CD25hi) skin-resident T cells did not proliferate when treated with IL-2 and IL-15 alone (IL2/15) but proliferated at low levels when stimulated with soluble anti-CD3 and anti-CD28 antibodies (CD3/28). CD25lo skin-resident T cells isolated from the same skin sample also did not proliferate when treated with IL-2 and IL-15 alone, but proliferated robustly after treatment with CD3 and CD28 antibodies. Error bars indicate standard deviation. (B) Sorted CD25hi skin-resident T cells with regulatory activity proliferated when cultured on monolayers of dermal fibroblasts for 1 week in the presence of IL-2 and IL-15. (C) Sorted CFSE-labeled CD25hi skin-resident T cells with regulatory activity cultured on fibroblasts without IL-2 and IL-15 did not proliferate (fib alone) but cells cultured on fibroblast monolayers with IL-2 and IL-15 (fib + IL-2, IL-15) did proliferate. (D) Unsorted CFSE-labeled skin-resident T cells cultured on fibroblast monolayers with IL-2 and IL-15 showed preferential expansion of the CD25hiCD69lo subset. (E) IL-15 and culture on dermal fibroblasts is necessary and sufficient to induce preferential expansion of FOXP3+ skin Tregs. Skin-resident T cells isolated from 2-week explant cultures were labeled with CFSE and cultured for 1 week on fibroblast monolayers with the indicated cytokines. CFSE-low cells have undergone proliferation. All results shown have been replicated using T cells from a minimum of 3 different skin donors. Numbers indicate the percentage of cells in each quadrant.
These findings suggested that additional interaction(s) were required to induce Treg proliferation. During skin explant culture, dermal fibroblasts grow into the 3D culture matrices and produce T-cell chemoattractants that induce skin-resident T cells to migrate out of the skin explants.13 The presence of dermal fibroblasts in these cultures is necessary for the migration of T cells out of the skin, their survival in culture, and their continued expression of skin-homing addressins.13 Given the importance of fibroblast-derived factors to other skin-resident T-cell populations, we hypothesized that fibroblasts may participate in supporting skin Treg division. To examine this, we cultured sorted populations of CD25hi skin T cells with confirmed regulatory activity on dermal fibroblast monolayers in the presence of IL-2 and IL-15. We observed proliferation of the CD25hi Treg subset under these conditions (Figure 5B). Culture of sorted CD25hi T cells on fibroblasts alone, in the absence of IL-2 and IL-15, did not induce cell proliferation, but culture on fibroblast monolayers in the presence of these cytokines did induce cell proliferation (Figure 5C). We next isolated unsorted skin-resident T-cell populations from skin explants grown for 3 weeks in IL-2 and IL-15. Skin Tregs isolated from 3-week explant cultures have already undergone significant cell proliferation (Figure 1B). Nonetheless, when these cells were CFSE labeled and cultured on fibroblasts with IL-2 and IL-15, we observed proliferation of the CD25hiCD69lo subset of T cells present in these cultures (Figure 5D).
The CD25hiCD69lo population of skin T cells contains both FOXP3+ and FOXP3− cells (Figure 2D). To selectively examine the behavior of FOXP3+ cells, we labeled unsorted skin-resident T cells with CFSE and cultured them for a week on fibroblast monolayers in the presence and absence of cytokines (Figure 5E). IL-15 alone induced a strong proliferation of FOXP3+ T cells, such that the percentage of FOXP3+ cells increased from 5% to 43% in 1 week in the experiment shown. The absolute number of FOXP3+ T cells was only slightly higher when both IL-2 and IL-15 were included (8.2 × 105 IL-15 alone vs 8.7 × 105 for the donor shown in Figure 5). Experiments using skin T cells from 3 additional donors have confirmed these results.
Proliferation of skin Tregs requires cell contact with fibroblasts but does not require antigen presentation or costimulation
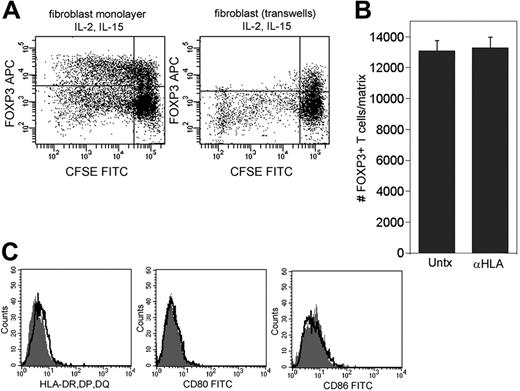
Fibroblasts could support Treg proliferation by the elaboration of soluble factors or by cell-contact interactions with T cells. We cultured skin-resident T cells on fibroblast monolayers in the presence of IL-2 and IL-15 under conditions that either allowed or prevented contact between T cells and dermal fibroblasts. Skin T cells cultured in contact with dermal fibroblasts showed a marked proliferation of FOXP3+ T cells, whereas T cells cultured in transwells above fibroblast monolayers had no detectable proliferation of FOXP3+ T cells (Figure 6A) Thus, cell contact with dermal fibroblasts appeared to be required for the expansion of cutaneous Tregs.
Skin Treg proliferation requires cell contact with fibroblasts but does not require antigen presentation or costimulation. (A) Skin-resident T cells were cultured in IL-2 and IL-15 for 1 week either in contact with dermal fibroblasts (left panel) or separated from the monolayer by a 0.4 μm transwell membrane (right panel). (B) Dermal fibroblasts do not express HLA-DR, DP, or DQ, nor do they express costimulatory molecules CD80 (B7-1) or CD86 (B7-2). Isotype controls (heavy black line) and test antibodies (⊡) histograms are shown. (C) Blockade of HLA-DR, DP, and DQ with neutralizing antibodies did not reduce production of FOXP3+ Tregs from explant cultures. Explant cultures were maintained in IL-2 and IL-15 for 3 weeks; neutralizing antibody was included throughout the culture period and was added with each feeding. Values shown represent the means and SDs of duplicate measurements. Similar results were produced using 2 additional skin donors.
Skin Treg proliferation requires cell contact with fibroblasts but does not require antigen presentation or costimulation. (A) Skin-resident T cells were cultured in IL-2 and IL-15 for 1 week either in contact with dermal fibroblasts (left panel) or separated from the monolayer by a 0.4 μm transwell membrane (right panel). (B) Dermal fibroblasts do not express HLA-DR, DP, or DQ, nor do they express costimulatory molecules CD80 (B7-1) or CD86 (B7-2). Isotype controls (heavy black line) and test antibodies (⊡) histograms are shown. (C) Blockade of HLA-DR, DP, and DQ with neutralizing antibodies did not reduce production of FOXP3+ Tregs from explant cultures. Explant cultures were maintained in IL-2 and IL-15 for 3 weeks; neutralizing antibody was included throughout the culture period and was added with each feeding. Values shown represent the means and SDs of duplicate measurements. Similar results were produced using 2 additional skin donors.
Natural Tregs from the blood proliferate well in vitro only in the presence of high levels of IL-2 and strong CD3 and CD28 cross-linking.11 Although IL-15 induces antigen-independent proliferation of induced Tr1-type Tregs, IL-15–induced proliferation of natural Tregs has never been reported.27 To confirm that signaling through the TCR and costimulatory molecules does not contribute to Treg proliferation in our system, we examined dermal fibroblasts for expression of HLA class II and costimulatory molecules and studied whether blockade of HLA class II with neutralizing antibodies impairs the division of natural Tregs from skin. The normal, unstimulated dermal fibroblasts used in our experiments did not express HLA-DR, DP, or DQ, nor did they express the costimulatory molecules CD80 (B7-1) or CD86 (B7-2) (Figure 6B). Moreover, blockade of HLA-DR, DP, and DQ with neutralizing antibody did not diminish the number of FOXP3+ Tregs expanded from human skin in the presence of dermal fibroblasts, IL-2, and IL-15. Blockade was confirmed by successful inhibition of MLRs using neutralizing HLA-DR, DP, and DQ antibody (see Figure S1, available on the Blood website; click on the Supplemental Figure link at the top of the online article).
IL-15 and dermal fibroblasts induce proliferation of Tregs from blood
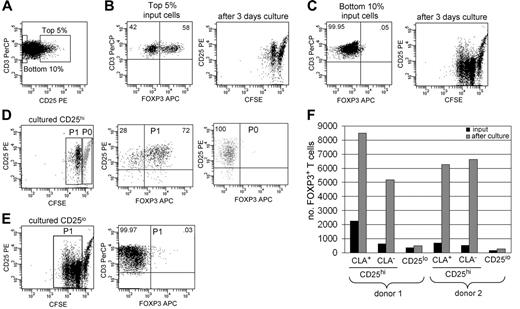
The observed proliferation of skin-derived natural Tregs could be either an intrinsic characteristic of Tregs or could represent a behavior manifested only by T cells isolated from the skin. To examine this question, we isolated FOXP3+ Tregs from the blood and assayed for proliferation of these cells after culture on fibroblast monolayers in the presence of IL-15. An enriched population of FOXP3+ Tregs was isolated by cell sorting for the highest 5% of CD25-expressing T cells, and a FOXP3− population was isolated by sorting for the lowest 10% of CD25 expression (Figure 7A-C) A subset of CD25hi T cells proliferated when cultured for 3 days on fibroblast monolayers with IL-15, and most of the proliferative cells were FOXP3+ Tregs (Figure 7B,D). Interestingly, all FOXP3+ T cells proliferated under these conditions, although only 1 cell division was evident on day 3. CD25lo cells proliferated to a greater extent than CD25hi cells but did not up-regulate FOXP3 to the levels seen in Tregs (Figure 7C,E). This suggests that increased numbers of FOXP3+ cells result from Treg proliferation, not conversion of FOXP3− cells into FOXP3+ cells.
Blood Tregs and non-Tregs both proliferate when cultured with fibroblasts and IL-15. (A) T cells were isolated from the blood, and the highest 5% and lowest 10% of CD25 expressers were isolated by cell sorting and labeled with CFSE. (B) CD25hi T cells contained 58% FOXP3+ Tregs, and a subset of these cells proliferated when cultured for 3 days with IL-15 and fibroblasts. (C) CD25lo T cells contained less than 1% FOXP3+ Tregs; many of these cells proliferated when cultured with IL-15 and dermal fibroblasts. (D) Most CD25hi proliferative cells (P1) were FOXP3+ Tregs, whereas nonproliferative T cells (P0) were purely non-Tregs. (E) Nonregulatory FOXP3− T cells proliferated but did not up-regulate FOXP3 to levels seen in regulatory T cells. For scatterplots, numbers indicate the percentage of cells in each quadrant. (F) Both CLA+ and CLA− blood Tregs expand when cultured with fibroblasts, IL-2, and IL-15. T cells were isolated as above, and CD25hi T cells were further separated into CLA+ and CLA− subsets. The number of FOXP3+ T cells before and after 1 week of culture with fibroblasts, IL-15, and IL-2 are shown.
Blood Tregs and non-Tregs both proliferate when cultured with fibroblasts and IL-15. (A) T cells were isolated from the blood, and the highest 5% and lowest 10% of CD25 expressers were isolated by cell sorting and labeled with CFSE. (B) CD25hi T cells contained 58% FOXP3+ Tregs, and a subset of these cells proliferated when cultured for 3 days with IL-15 and fibroblasts. (C) CD25lo T cells contained less than 1% FOXP3+ Tregs; many of these cells proliferated when cultured with IL-15 and dermal fibroblasts. (D) Most CD25hi proliferative cells (P1) were FOXP3+ Tregs, whereas nonproliferative T cells (P0) were purely non-Tregs. (E) Nonregulatory FOXP3− T cells proliferated but did not up-regulate FOXP3 to levels seen in regulatory T cells. For scatterplots, numbers indicate the percentage of cells in each quadrant. (F) Both CLA+ and CLA− blood Tregs expand when cultured with fibroblasts, IL-2, and IL-15. T cells were isolated as above, and CD25hi T cells were further separated into CLA+ and CLA− subsets. The number of FOXP3+ T cells before and after 1 week of culture with fibroblasts, IL-15, and IL-2 are shown.
To determine if cutaneous (CLA+) and noncutaneous (CLA−) Tregs both proliferate under these conditions, we further sorted CD25hi cells into CLA+ and CLA− subsets and assayed for proliferation. CLA+ and CLA− Tregs both proliferated when cultured with IL-15 and fibroblasts, but optimal proliferation was observed when IL-2 was also included (Figure 7F).
Discussion
We report the isolation, expansion, and characterization of natural Tregs from human skin. Tregs expanded from skin maintained their regulatory function and high expression of skin-homing addressins. Cutaneous natural Tregs proliferated in an antigen-independent fashion when cultured with IL-15 and dermal fibroblasts. To our knowledge, this is the first demonstration of cytokine-driven, antigen-independent proliferation of this important T-cell subset.
Tregs isolated from skin were similar in some respects to their counterparts in blood, but there were also significant differences. Skin-resident Tregs expressed CD25, FOXP3, GITR, L-selectin, and high levels of intracellular CTLA-4. Skin Tregs suppressed the proliferation of autologous CD25lo T cells isolated from the same skin sample and were largely anergic to physiologic levels of IL-2 and TCR stimulation, as reported for Tregs from blood. However, skin-resident Tregs were distinctive in their universal high expression of the skin-homing addressins CLA and CCR4. Only 50% of skin-resident Tregs expressed CCR7, and expression levels were low. However, this may simply reflect their status as skin-resident T cells. CCR7 is up-regulated by tissue-resident T cells when they leave the tissues and enter efferent lymphatics.28,29 In mice, CCR7-deficient T cells are retained within the skin.29 CCR7 is therefore an inducible receptor that is up-regulated on T cells exiting tissues and entering the lymphatics. All skin Tregs expressed high levels of L-selectin and likely express CCR7 once they exit the skin. This L-selectin/CCR7 expression together with the high expression of skin-homing addressins suggests that these cells can traffic to both the lymph nodes and the skin and may play a role in modulating immune responses at both sites.
Natural Tregs are notoriously anergic when stimulated with TCR and costimulatory signals at levels sufficient to drive robust proliferation of non-Tregs.30 We found similar results with skin-resident T cells; skin Tregs proliferated 15-fold less than CD25lo T cells from the same skin sample when stimulated with soluble anti-CD3 and anti-CD28 antibodies (Figures 2B, 5C). Natural Tregs from human blood can be efficiently expanded in vitro, but this requires repeated cycles of intense TCR and costimulation provided by artificial antigen-presenting cells in combination with high-dose (300 U/mL) IL-2.11 Although these and similar methods are extremely valuable for producing large populations of Tregs, it is not clear that such extreme stimulatory conditions exist within a healthy individual. A significant proportion of Tregs recognize autoantigens, and presentation of autoantigens in the presence of strong costimulation (as is needed for in vitro Treg expansion) would not be expected to occur in healthy individuals.31,32 However, natural Tregs proliferate very efficiently in vivo, by mechanisms that are not entirely clear.8-10
We have found that natural Tregs isolated from skin proliferate efficiently when in contact with dermal fibroblasts in the presence of IL-15. Although the addition of IL-2 slightly increased Treg expansion, it was not required. Tregs did not proliferate when treated with IL-2 and IL-15 in the absence of fibroblasts, and fibroblast contact alone did not induce proliferation. However, the combination of IL-15 and contact with dermal fibroblasts reproducibly induced the preferential expansion of FOXP3+ cutaneous Tregs. It is important to note that the decreased proliferation of non-Tregs under these conditions could result from either a lower intrinsic proliferative response or suppression of these cells by Tregs in mixed cultures. Intriguingly, this Treg proliferation was antigen independent and did not require costimulation. Thus, while it appears that skin-resident Tregs are relatively anergic to antigen-specific stimulation, they appear to be remarkably responsive to non–antigen-dependent proliferation, at least under these conditions.
In addition to its effects on natural Tregs, IL-15 supports antigen-independent proliferation of induced Tr1 Tregs isolated from blood27 and CD8+ memory T cells.33,34 However, proliferation of these cell types does not require cell contact with fibroblasts.
The culture conditions we describe are similar to the microenvironment that exists within inflamed skin. Chronic stimulation of dermal fibroblasts with tumor necrosis factor-α (TNF-α) induces these cells to express cell-surface IL-15 that enhances the proliferation of activated T cells.35 In skin biopsies from patients with the chronic inflammatory skin disease discoid lupus erythematosus, dermal fibroblasts express membrane-bound IL-15, whereas fibroblasts from healthy skin do not.35 IL-15 is also produced by keratinocytes under conditions of chronic inflammation.36 Although IL-2 is not absolutely required for the expansion of Tregs, it is likely to be produced in skin lesions in which T cells are undergoing activation and proliferation within the dermis, as seen in psoriasis and other inflammatory skin diseases.37
There are many T cells resident in normal skin under resting conditions. We have found that there are approximately 1 million memory T cells in each square centimeter of normal human skin.12 As a result of the large skin surface area, there are nearly 2-fold more T cells resident in normal skin than are present in the entire circulation. Most of these T cells are present in the dermis, close to or in contact with dermal fibroblasts. Thus, skin inflammation brings together skin-resident T cells and IL-15–producing fibroblasts, conditions we have found to support natural Treg proliferation in vivo.
We hypothesize that conditions present in chronically inflamed skin support the expansion of natural Tregs present in the dermis. This could have 2 main effects. First, expansion of Tregs within the skin may serve as a brake for cutaneous inflammation. The skin is subject to frequent injury and antigenic challenges from both dangerous and benign microorganisms. Numerous studies have shown that suppression by Tregs is effective when the TCR stimulus is of low to moderate intensity, as would be seen with autoantigens, local injury, and perhaps normal flora. However, Tregs cannot suppress immune responses involving very strong TCR signaling, such as a memory response to a dangerous infectious pathogen.38,39 Local proliferation of Tregs within inflamed skin may allow the resolution of low-level inflammation within the skin and lead to tolerance of autoantigens exposed during injury and to normal flora. However, it is unlikely to suppress responses to infectious and dangerous pathogens such as Staphylococcus aureus. Given this hypothesis, it is tempting to speculate that individuals prone to chronic inflammatory skin diseases such as psoriasis and atopic dermatitis may have fewer or less effective natural Tregs resident within the skin.
Second, the proliferation of Tregs at sites of low-level skin inflammation may be a mechanism for the homeostatic expansion of these cells. Tregs transferred into mice undergo efficient homeostatic proliferation.8-10 We have found that IL-15 and dermal fibroblasts, both of which are present at sites of skin inflammation, support the proliferation of skin-resident Tregs. If fibroblasts from other tissues can also support IL-15–induced Treg proliferation, then expansion of Tregs within peripheral tissues such as the skin, gut, and lung may be an important mechanism for the proliferation of these cells within intact organisms.
Skin-resident Tregs proliferate as well or better than non-Tregs, whereas blood Tregs proliferate less well than nonregulatory cells. Also, IL-2 did not enhance skin Treg proliferation but did increase the proliferation of Tregs from the blood. These are important reminders that there are significant differences between the behavior of T cells isolated from blood and those isolated from peripheral tissues such as the skin. For example, T cells resident in many peripheral tissues express the activation antigen CD69 despite the fact that they are not undergoing proliferation.40,41 The partially activated state of these cells is consistent with our recent findings that skin-resident T cells require much lower levels of CD3 stimulation to induce proliferation and that these cells are very resistant to apoptosis, even in the absence of exogenous cytokines (data not shown). Most studies characterizing T-cell responses have been performed on T cells isolated from blood, because they are easily accessible. However, most T-cell effector functions occur within tissues, and study of tissue-resident T cells is likely to provide important insights into T-cell behavior in intact organisms.
Several significant questions are raised by our findings. It will be important to determine what molecules mediate the cell-contact–dependent interaction required for Treg proliferation. Possible candidates include the integrins, such as ICAM-1 and VLA-4, and the hyaluronan receptor CD44.42 However, elucidating the nature of cell-contact–dependent interactions is not always straightforward. Natural Tregs suppress T-cell proliferation of other T cells via a cell-contact mechanism, but despite a great deal of study, the molecular nature of this interaction remains only partially characterized.43 Second, it will be important to determine if the mechanism we describe also supports the proliferation of natural Tregs from other tissues.
In summary, we have isolated and characterized natural Tregs present in normal human skin. These cells express robust levels of FOXP3 and skin-homing addressins, and they suppress the proliferation of non-Tregs isolated from the same sample of skin. Cutaneous Tregs expand when cultured in contact with dermal fibroblasts in the presence of IL-15. This antigen-independent proliferation may be physiologically relevant in that intracutaneous expansion of Tregs in inflamed skin may serve as a brake for cutaneous inflammation and could be 1 mechanism for the homeostatic proliferation of these cells.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest statement: The authors declare they have no conflicts of interest.
Contribution: R.A.C. performed the experiments and prepared the manuscript; T.S.K. provided advice on experimental approaches and assisted in editing the manuscript.
Dr Thomas Cochran of the Boston Center for Plastic Surgery and Dr Elof Eriksson of Brigham and Women's Hospital generously provided adult human skin samples. Dr Kazuki Hirahara provided technical advice regarding Treg assays. Dr Clare Baecher-Allan kindly provided our department with the details of the functional assays for human Treg function that she developed.
This work was supported by National Institutes of Health grants 1K08AI060890-01A1, a Clinical Career Development Award grant from the Dermatology Foundation, and a Translational Research Award from the Leukemia and Lymphoma Foundation (R.A.C.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal