Abstract
The Vitamins and Thrombosis (VITRO) study investigated the effect of homocysteine lowering by daily supplementation of B vitamins on the risk reduction of deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients between 20 to 80 years old with a first objectively confirmed proximal DVT or PE in the absence of major risk factors and a homocysteine concentration above the 75th percentile of a reference group were asked to participate (hyperhomocysteinemic group). A similar study was conducted in a random sample of patients with a homocysteine below the 75th percentile of the reference group (normohomocysteinemic group). After informed consent was obtained, patients were randomized to daily multivitamin supplementation (5 mg folic acid, 50 mg pyridoxine, and 0.4 mg cyanocobalamin) or placebo and were followed for 2.5 years. End points were objectively diagnosed recurrent DVT or PE. A total of 701 patients were enrolled (360 in the hyperhomocysteinemic and 341 in the normohomocysteinemic group). The number of recurrent events of venous thrombosis was 43 of 353 in the vitamin group (54/1000 py) and 50 of 348 in the placebo group (64/1000 py). The hazard ratio associated with vitamin treatment was 0.84 (95% CI, 0.56-1.26): 1.14 (95% CI, 0.65-1.98) in the hyperhomocysteinemic group and 0.58 (95% CI, 0.31-1.07) in the normohomocysteinemic group. The results of our study do not show that homocysteine lowering by B vitamin supplementation prevents recurrent venous thrombosis. This trial was registered at www.clinicaltrials.gov as #NCT00314990.
Introduction
Plasma homocysteine levels are associated with an increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE). To date, 24 case-control studies have been published with an overall relative risk for venous thrombosis of 1.60 (95% CI, 1.10-2.34) for a 5-μM higher measured homocysteine level.1 Moreover, 3 prospective studies showed an overall relative risk for venous thrombosis of 1.27 (95% CI, 1.01-1.59) for a 5-μM higher measured homocysteine level.1 Recent meta-analyses on the effect of the MTHFR 677TT genotype on cardiovascular disease2 and venous thrombosis1,3 showed a modest increase in risk, supporting a hypothesis that homocysteine levels are causally related to thrombotic risk.
Elevated homocysteine levels can be easily treated with B vitamin supplementation (folic acid, vitamin B6, and vitamin B12). Daily use of folic acid gives a 25% reduction in homocysteine levels even at low doses of 0.5 mg.4,5 The question is whether lowering of homocysteine levels by use of B vitamin supplementation also lowers the risk for venous thrombosis.
In the Vitamins and Thrombosis (VITRO) study, the primary aim was to investigate the effect of a combination preparation of 5 mg folic acid, 50 mg pyridoxine, and 0.4 mg cyanocobalamin in the secondary prevention of DVT and PE in patients with a first event of venous thrombosis and hyperhomocysteinemia in a randomized, double-blind, and placebo-controlled setting. A secondary aim was to study the effect of vitamin supplementation in patients with a first event of venous thrombosis and a “normal” homocysteine concentration in an identical setting.6
Patients, materials, and methods
Study participants
Patients were selected through anticoagulation clinics in The Netherlands. Anticoagulation clinics monitor the anticoagulant treatment of virtually all patients in well-defined geographic areas. Participating anticoagulation clinics asked all patients with a first venous thrombosis to donate a blood sample for homocysteine determination. Patients with a homocysteine plasma concentration in the top quartile of its distribution in the general population (homocysteine ≥ 12.6 μM)7 and who met the entry criteria formed the hyperhomocysteinemic group. Enrollment started in March 1996. Because the rate of inclusion was lower than expected, the trial was extended in 1998 with the Thrombosis Centers of Milan and Vienna, which included patients with homocysteine levels above the 75th percentile based on reference population of the respective countries (homocysteine ≥ 10.6 μM in Milano and homocysteine ≥ 8.5 μM in women and ≥ 10.4 μM in men in Vienna). The latest patient was included in May 2001. Parallel to the study in the hyperhomocysteinemic group we performed a study in the normohomocysteinemic group, which was done only in The Netherlands. During the study there was no folate fortification in these 3 countries.
For all patients who consented to donating blood for homocysteine measurement information was retrieved from the general practitioner or specialist of the patients about the diagnosis and circumstances in which patients developed their thrombosis. Patients were eligible when they had objectively confirmed proximal DVT or PE in absence of major risk factors (major surgery, known malignant disease, pregnancy and puerperium, or immobility for > 3 weeks), were between 20 and 80 years old at time of diagnosis, and were without obligatory use of vitamin B. When patients met all entry criteria, they were asked to give their informed consent in accordance with the current revision (2000) of the Declaration of Helsinki.
Randomization and intervention
Eligible patients were randomized to receive a high-dose multivitamin daily or an identical-appearing placebo. The high-dose multivitamin capsule contained 5 mg folic acid, 0.4 mg cyanocobalamin, and 50 mg pyridoxine. The randomization was performed with 4 and 6 random permuted blocks, stratified by homocysteine status (hyperhomocysteinemia versus normohomocysteinemia), sex, and anticoagulation clinic or study center. The study medication was based on an earlier study on the homocysteine lowering effects of B vitamins.5 The medication was tested for stability for the duration of the trial through determination of the vitamin contents of the vitamin capsules. The ranges found during 42 months were 0.4 to 0.5 mg/capsule for cobalamin, 48.3 to 59.1 mg/capsule for pyridoxine, and 5.1 to 7.0 mg/capsule for folic acid. Placebos were made for this trial and capsules were identical for both placebo and vitamins.
Duration of treatment and follow-up was intended for 2.5 years. Participants were seen (after overnight fasting) at the start of the study (before randomization) and 3, 6, and 24 months after randomization. At each visit, blood was collected for determination of homocysteine concentration. Patients received their study medication at these follow-up visits or by mail every 3 or 6 months. The participants started with their study medication as soon as they were randomized, that is, within the period of anticoagulant treatment to achieve the homocysteine-lowering effect in the vitamin group before the cessation of anticoagulant treatment. Compliance of the drugs was monitored by measuring homocysteine levels.
End points
The primary end point of the study was recurrent symptomatic DVT or recurrent PE. This end point was defined as the decision of the treating physician to restart anticoagulant medication. The treating physician was not informed about study medication or homocysteine concentration. Because it might be difficult to make an accurate diagnosis of recurrent DVT or PE because of residual thrombi, we provided a tool for the treating physicians to make the diagnosis of recurrent DVT more accurate. In patients with a DVT of the leg, compression ultrasonography (CUS) was done 3 months after the thrombotic event. If a residue of the old thrombus was seen on CUS, the CUS was repeated 6 months and, if necessary, 12 months after the thrombosis. In patients with a PE, CUS of both legs was performed to exclude a DVT. The ultrasonographies were performed in one hospital or institution in every participating center. The results of these tests were noted in a “patient passport,” a booklet that patients were instructed to take with them if they visited their physician with symptoms of a recurrent thrombosis. By using this passport, data on residual thrombosis were available, even if the patient visited another hospital with complaints of recurrent thrombosis. The recommended definition of “recurrent DVT” was when a previously normal or normalized venous segment could not be compressed with CUS or when there was an increment in the diameter of residual thrombus of 4 mm.8,9 The diagnosis of recurrent PE was according to standard clinical practice.
Laboratory measurements
To screen patients with first-time venous thrombosis for hyperhomocysteinemia, homocysteine has to be measured in a large number of patients. To avoid homocysteine increase after blood sampling, we used blood collection tubes with acidic citrate as anticoagulant.10 After entering the intervention study blood was taken at 0, 3, 6, and 24 months after start of the study. This blood was collected after an overnight fast in EDTA tubes, directly placed on ice, and centrifuged within 1 hour. The total homocysteine concentration in EDTA-plasma was measured in one central laboratory (Laboratory of Pediatrics and Neurology, Nijmegen, The Netherlands) by an automated high-performance liquid chromatography method with reverse-phase and fluorescent detection (Gilson 232-401 sample processor [Gilson, Middleton, CT], Spectra Physics 8800 solvent delivery system, and Spectra Physics LC 304 fluorometer [Spectra Physics, San Jose, CA]), essentially according to the method by Fiskerstrand et al,11 with modifications.12
Study size
Sample size was calculated for the hyperhomocysteinemic group: with α = 0.05 and β = 0.2 and with an expected recurrence rate of 20% in patients with idiopathic thrombosis (based on the study of Eichinger et al13) and hyperhomocysteinemia in 2.5 years, and a 50% risk reduction due to the vitamin therapy (based on a relative risk of > 2 for hyperhomocysteinemia7,13 and the assumption that 90% of those with homocysteine levels above the 90th percentile could be reduced to less than the 90th percentile with multivitamin treatment),5 155 patients in each treatment group were required in the hyperhomocysteinemic group.6 It was decided to randomize the same number of patients in the normohomocysteinemic group. So the intended total sample size was 620.
Statistics
We compared the high-dose vitamin group and the placebo group with respect to age, sex, type of first event (DVT versus PE), and initial homocysteine levels for both the hyperhomocysteinemic patients and normohomocysteinemic patients, respectively.
Relative risk estimates (hazard ratios) and their 95% confidence intervals (CIs) were calculated with a Cox proportional hazard model to assess the effects of high-dose multivitamin supplementation. Variables included in the model were treatment regimen (vitamin versus placebo) and the variables on which the randomization was stratified, that is, sex, anticoagulation clinic, and initial homocysteine levels (hyperhomocysteinemic or normohomocysteinemic).
The primary analysis was an intention-to-treat analysis starting at the day of randomization and a follow-up of 2.5 years. We did an on-treatment analysis with restriction of the observation time to the time that patients had reported to take their capsules. A second on-treatment analysis was performed by stratifying the homocysteine reduction in 3 categories (> 50% reduction, 50%-0% reduction, and no reduction in homocysteine level) and calculating the hazard ratio for the first 2 categories compared to no homocysteine reduction.
Because the treatment regimen started while patients were on anticoagulation treatment (which has a great influence on the risk of recurrence), we also did an analysis without taking into account the recurrences that occurred before 2 months after cessation of anticoagulation. In all these 3 models the data of randomization (and start of the treatment regimen) was the starting time in the Cox model. Finally, we used a Cox model with the date of cessation of anticoagulation as starting time.
To assess the role of baseline homocysteine levels as a risk predictor of recurrent events, we also performed a Cox model with homocysteine as continuous variable (in μM) and age, sex, and study medication as covariates.
Results
The participating anticoagulation clinics screened 4382 patients (Figure 1). Of these patients, 2000 had a homocysteine plasma concentration equal to or more than 12.6 μM (75th percentile in a general Dutch population). Of these 2000 patients, 1522 did not meet the entry criteria and 153 refused to participate. The remaining hyperhomocysteinemic patients (n = 325) were randomized within the hyperhomocysteinemic group. The Thrombosis Centers of Milan and Vienna included an additional 35 patients with homocysteine levels above the 75th percentile based on the reference population of the centers. Of the 4382 patients screened in The Netherlands, 2382 had homocysteine values below 12.6 μM. A total of 1886 patients did not meet the entry criteria or were randomly excluded and 155 refused to participate. A total of 341 patients were randomized within the normohomocysteinemic group.
The baseline characteristics for the vitamin and placebo groups according to their homocysteine level (hyperhomocysteinemic and normohomocysteinemic) are shown in Table 1. The differences in homocysteine levels between the hyperhomocysteinemic and the normohomocysteinemic group based on the homocysteine measurement at time of screening remained high at the start of the treatment study. The hyperhomocysteinemic group was slightly older than the normohomocysteinemic group and included more men, due to the use of a uniform cut-off value. However, the vitamin and placebo groups were similar in both the hyperhomocysteinemic and normohomocysteinemic groups.
Baseline characteristics
| Variable . | Hyperhomocysteinemic group; n = 360 . | Normohomocysteinemic group; n = 341 . | ||
|---|---|---|---|---|
| Multivitamin; n = 177 . | Placebo; n = 183 . | Multivitamin; n = 176 . | Placebo; n = 165 . | |
| Sex, M/F (%) | 103/74 (58/42) | 105/78 (57/43) | 80/96 (45/55) | 74/91 (45/55) |
| Median age, y (range) | 56.4 (18.1-79.9) | 57.2 (17.9-79.8) | 48.2 (20.2-75.5) | 46.3 (19.1-78.5) |
| Type of first event, no. (%) | ||||
| DVT | 119 (67) | 126 (69) | 97 (55) | 100 (61) |
| PE | 43 (24) | 40 (22) | 60 (34) | 51 (31) |
| Both | 15 (8) | 17 (9) | 19 (11) | 14 (8) |
| Median duration of anticoagulation after randomization, mo (range) | 1.6 (0-30) | 1.8 (0-30) | 1.5 (0-18) | 1.6 (0-30) |
| Geometric mean baseline homocysteine level, μM | 15.1 | 15.9 | 9.0 | 9.0 |
| 95% CI | 14.3-16.0 | 14.9-17.0 | 8.7-9.3 | 8.7-9.3 |
| Range | 6.3-84.8 | 7.4-108.3 | 4.0-23.0 | 4.0-15.5 |
| Geometric mean homocysteine level after 3 mo, μM | 8.5 | 15.6 | 6.5 | 9.7 |
| 95% CI | 8.1-8.9 | 14.5-16.8 | 6.2-6.7 | 9.4-10.1 |
| Range | 4.1-21.3 | 6.0-91.7 | 2.9-11.6 | 5.4-25.6 |
| Variable . | Hyperhomocysteinemic group; n = 360 . | Normohomocysteinemic group; n = 341 . | ||
|---|---|---|---|---|
| Multivitamin; n = 177 . | Placebo; n = 183 . | Multivitamin; n = 176 . | Placebo; n = 165 . | |
| Sex, M/F (%) | 103/74 (58/42) | 105/78 (57/43) | 80/96 (45/55) | 74/91 (45/55) |
| Median age, y (range) | 56.4 (18.1-79.9) | 57.2 (17.9-79.8) | 48.2 (20.2-75.5) | 46.3 (19.1-78.5) |
| Type of first event, no. (%) | ||||
| DVT | 119 (67) | 126 (69) | 97 (55) | 100 (61) |
| PE | 43 (24) | 40 (22) | 60 (34) | 51 (31) |
| Both | 15 (8) | 17 (9) | 19 (11) | 14 (8) |
| Median duration of anticoagulation after randomization, mo (range) | 1.6 (0-30) | 1.8 (0-30) | 1.5 (0-18) | 1.6 (0-30) |
| Geometric mean baseline homocysteine level, μM | 15.1 | 15.9 | 9.0 | 9.0 |
| 95% CI | 14.3-16.0 | 14.9-17.0 | 8.7-9.3 | 8.7-9.3 |
| Range | 6.3-84.8 | 7.4-108.3 | 4.0-23.0 | 4.0-15.5 |
| Geometric mean homocysteine level after 3 mo, μM | 8.5 | 15.6 | 6.5 | 9.7 |
| 95% CI | 8.1-8.9 | 14.5-16.8 | 6.2-6.7 | 9.4-10.1 |
| Range | 4.1-21.3 | 6.0-91.7 | 2.9-11.6 | 5.4-25.6 |
We analyzed the effect of the vitamin/placebo treatment 3 months after start of the intervention. These data demonstrated no effect of placebo on the homocysteine values, whereas a 46% reduction of homocysteine values could be demonstrated in the hyperhomocysteinemic group and a 33% reduction was observed in the normohomocysteinemic group.
During the course of the study, 43 of 353 (12.2%) patients suffered from a recurrent event of venous thrombosis in the multivitamin group and 50 of 348 (14.3%) patients had a recurrent venous thrombosis in the placebo group.
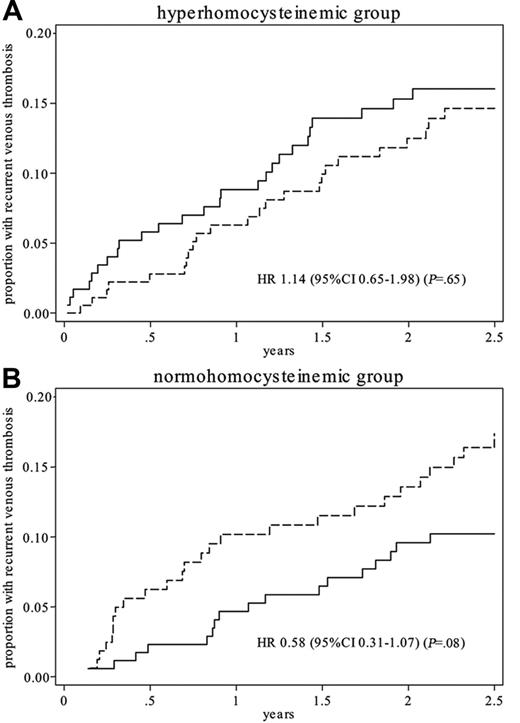
Figure 2 shows the recurrent thrombosis cumulative incidence curves of patients treated with multivitamins versus those treated with placebo. The overall hazard ratio was 0.84 (95% CI, 0.56-1.26), that is, a risk reduction of 16% (95% CI, −26 to 44). The hazard ratio associated with vitamin supplementation was 1.14 (95% CI, 0.65-1.98) in the hyperhomocysteinemic group and 0.58 (95% CI, 0.31-1.07) in the normohomocysteinemic group. The hazard ratio for men versus women was 1.6 (95% CI, 1.05-2.45). There was no significant effect for the other covariates.
Recurrent thrombosis cumulative incidence. Recurrent thrombosis cumulative incidence in patients treated with multivitamin (solid line) or placebo (dashed line) in a hyperhomocysteinemic and a normohomocysteinemic group.
Recurrent thrombosis cumulative incidence. Recurrent thrombosis cumulative incidence in patients treated with multivitamin (solid line) or placebo (dashed line) in a hyperhomocysteinemic and a normohomocysteinemic group.
The results of the on-treatment analysis were similar to the intention-to-treat analysis (Table 2) However, when we stratified the homocysteine reduction into 3 categories (> 50% reduction, 50%-0% reduction, and no reduction in homocysteine level), we found a hazard ratio of 0.82 (95% CI, 0.51-1.32) for a 50% to 0% reduction and 0.43 (95% CI, 0.15-1.24) for a 50% or greater reduction in homocysteine compared to no reduction.
Incidences and hazard ratios for recurrent venous thrombosis
| . | Vitamin, N/py (ir %) . | Placebo, N/py (ir %) . | HR vitamin versus placebo* . |
|---|---|---|---|
| Intention-to-treat analysis | |||
| Hyperhomocysteinemic group | 26/387 (6.7) | 24/403 (6.0) | 1.14 (0.65-1.98) |
| Normohomocysteinemic group | 17/412 (4.1) | 26/373 (7.0) | 0.58 (0.32-1.08) |
| Overall | 43/799 (5.4) | 50/776 (6.4) | 0.84 (0.56-1.26) |
| On-treatment analysis | |||
| Hyperhomocysteinemic group | 24/338 (7.1) | 22/344 (6.4) | 1.13 (0.63-2.02) |
| Normohomocysteinemic group | 16/363 (4.4) | 22/337 (6.5) | 0.65 (0.34-1.24) |
| Overall | 40/702 (5.7) | 44/682 (6.4) | 0.88 (0.57-1.36) |
| Intention-to-treat analysis with exclusion of early recurrences† | |||
| Hyperhomocysteinemic group | 17/387 (4.4) | 21/403 (5.2) | 0.84 (0.44-1.60) |
| Normohomocysteinemic group | 15/412 (3.6) | 20/373 (5.4) | 0.66 (0.34-1.30) |
| Overall | 32/799 (4.0) | 41/776 (5.3) | 0.76 (0.48-1.21) |
| Intention-to-treat analysis beginning after cessation of anticoagulation | |||
| Hyperhomocysteinemic group | 23/338 (6.8) | 24/347 (6.9) | 0.98 (0.55-1.74) |
| Normohomocysteinemic group | 17/379 (4.5) | 24/338 (7.1) | 0.62 (0.33-1.15) |
| Overall | 40/717 (5.6) | 48/685 (7.0) | 0.80 (0.52-1.21) |
| . | Vitamin, N/py (ir %) . | Placebo, N/py (ir %) . | HR vitamin versus placebo* . |
|---|---|---|---|
| Intention-to-treat analysis | |||
| Hyperhomocysteinemic group | 26/387 (6.7) | 24/403 (6.0) | 1.14 (0.65-1.98) |
| Normohomocysteinemic group | 17/412 (4.1) | 26/373 (7.0) | 0.58 (0.32-1.08) |
| Overall | 43/799 (5.4) | 50/776 (6.4) | 0.84 (0.56-1.26) |
| On-treatment analysis | |||
| Hyperhomocysteinemic group | 24/338 (7.1) | 22/344 (6.4) | 1.13 (0.63-2.02) |
| Normohomocysteinemic group | 16/363 (4.4) | 22/337 (6.5) | 0.65 (0.34-1.24) |
| Overall | 40/702 (5.7) | 44/682 (6.4) | 0.88 (0.57-1.36) |
| Intention-to-treat analysis with exclusion of early recurrences† | |||
| Hyperhomocysteinemic group | 17/387 (4.4) | 21/403 (5.2) | 0.84 (0.44-1.60) |
| Normohomocysteinemic group | 15/412 (3.6) | 20/373 (5.4) | 0.66 (0.34-1.30) |
| Overall | 32/799 (4.0) | 41/776 (5.3) | 0.76 (0.48-1.21) |
| Intention-to-treat analysis beginning after cessation of anticoagulation | |||
| Hyperhomocysteinemic group | 23/338 (6.8) | 24/347 (6.9) | 0.98 (0.55-1.74) |
| Normohomocysteinemic group | 17/379 (4.5) | 24/338 (7.1) | 0.62 (0.33-1.15) |
| Overall | 40/717 (5.6) | 48/685 (7.0) | 0.80 (0.52-1.21) |
N indicates the number of recurrences; py, person years; ir, annual incidence in percent.
HR is hazard ratio (95% CI), adjusted for study center, sex, and hyperhomocysteinemia or normohomocysteinemia.
Early recurrences are recurrences before 2 months after cessation of anticoagulation.
Because the treatment regimen started while patients were on anticoagulation treatment (which has a great influence on the risk of recurrence), we also did an analysis after exclusion of early recurrences (during anticoagulant treatment or within the first 2 months after cessation of anticoagulant treatment; Table 2). This subgroup analysis gave similar risk estimates for the hyperhomocysteinemic and normohomocysteinemic groups. This was also seen in the fourth analysis in which we took the date of cessation of anticoagulation as starting time in the Cox model. Although the duration of anticoagulant treatment was similar for the various groups, there was a relatively high number of early recurrences in the hyperhomocysteinemic vitamin group (9 events) compared with the hyperhomocysteinemic placebo group (3 events). In contrast, in the normohomocysteinemic group early recurrences occurred more often in the placebo group (6 events) than in the vitamin group (2 events).
To assess the role of baseline homocysteine levels as a risk predictor of recurrent events we performed a Cox model with homocysteine as continuous variable (in μM) and age, sex, and study medication as covariates. The hazard ratio for recurrence associated with a 5 μM higher measured homocysteine level at baseline was 1.13 (95% CI, 1.05-1.20). This effect was similar in the placebo group as in the vitamin group. The hazard ratio for a homocysteine concentration above the 90th percentile (20.1 μM) was 1.8 (95% CI, 1.1-3.2). Homocysteine levels were not associated with early recurrences.
Discussion
Our study is the first clinical trial on the effect of B vitamins in the prevention of recurrent venous thrombosis. Our study shows that supplementation with B vitamins lowers homocysteine values, but it does not show a risk reduction in recurrent venous thrombosis. Homocysteine level at baseline is a modest risk factor for recurrent events.
The results of our trial showed a difference in effect in the hyperhomocysteinemic group compared to the normohomocysteinemic group. This difference in effect was contrary to what was expected and could not be biologically explained. Therefore, we looked for possible explanations for this finding. One explanation is that there is an uneven distribution of early recurrences during or shortly after discontinuation of anticoagulation. These recurrences might be explained by other risk factors (such as cancer) or may be the result of a rebound phenomenon.14 In fact, these early recurrences were not associated with basal homocysteine levels (as were the recurrences during follow-up), so the uneven distribution over the various treatment groups could be attributed to chance. When we excluded early recurrences, the overall risk estimate became 0.76, and the effects in the hyperhomocysteinemic and normohomocysteinemic groups were quite similar. The same occurs after taking the date of anticoagulant cessation as the starting point for the survival analysis. Although these are post-hoc analyses, they support that the overall estimate of 0.84 (95% CI, 0.56-1.26) is the best summary of the study, despite an initial heterogeneity of effect.
The domain of our trial was idiopathic venous thrombosis. We had very strict inclusion criteria (objectively confirmed first event of proximal DVT or PE in absence of major risk factors [major surgery, known malignant disease, pregnancy and puerperium, or immobility for > 3 weeks], between 20 and 80 years old at time of diagnosis, and without obligatory use of B vitamins). Most of the patients were not eligible because thrombosis occurred after surgery or patients were older than 80 years, had cancer, or had a recurrent event. For these reasons many patients had to be screened to include the required number of patients for this study.
An important point in clinical trials with B vitamins is the difference achieved in homocysteine concentration in the vitamin group and the placebo group. This difference was small in a trial in stroke patients in North America.15 In the design of our study we opted for a strong homocysteine-lowering effect, which was found in a schedule with 5 mg folate, 0.4 mg vitamin B12, and 50 mg vitamin B6.5 Furthermore, our study was done in an area without food fortification with folate.
Therefore, a strong difference in median homocysteine level between high-dose multivitamin and placebo of 6.3 μM (42%) in the hyperhomocysteinemic group and 2.9 μM (30%) in the normohomocysteinemic group was found.
An on-treatment analysis based on the percentage of reduction showed a trend to a risk reduction in subjects with the highest reduction in homocysteine. This finding stresses the importance of adequate homocysteine reduction in clinical trials with B vitamins. The dose-response relationship also gives some indication that our trial does not completely exclude an effect of vitamin supplementation to prevent recurrent venous thrombosis.
Our study was designed in 1995. For the sample size calculation, we assumed a risk reduction of 50% that was based on earlier case-control studies and especially on a cohort study in patients with first-time venous thrombosis with a relative risk of 2.7 for recurrent thrombosis in patients in the top-quartile of the homocysteine distribution.13 Findings from others, after the start of this trial, indicated less strong effects of hyperhomocysteinemia on the risk of first thrombosis. In a recent meta-analysis we found a relative risk for venous thrombosis between 1.27 in prospective and 1.60 in retrospective studies for a 5-μM increase in homocysteine.1 On the basis of a meta-analysis of MTHFR 677TT genotype the risk associated with a 3-μM increase in homocysteine levels was 16%.1,3 So, the main conclusion of our study is that vitamin supplementation for treatment of hyperhomocysteinemia does not result in an apparent decrease in incidence of recurrent events. A second conclusion is that our study has not enough power to detect or rule out a modest risk reduction of 10% to 20% that is expected now on the basis of prospective and genetic studies. However, the question is whether such a modest risk reduction is clinically relevant because the associated numbers needed to treat are large (75-150 for 1 year of supplementation).
In the field of arterial vascular disease, 12 studies on the effect of vitamin treatment on vascular disease are initiated16 of which 3 are published now.15,17,18 None of these trials showed a beneficial effect of vitamin supplementation on the incidence of recurrent vascular events. It should be noted that in these trials vitamin supplementation was added to standard treatment that included generally platelet aggregation inhibitors, cholesterol-lowering drugs, and antihypertensive medication, which is not a standard treatment after an event of venous thrombosis. Therefore, the effect of vitamin supplementation might be different in a trial in patients with venous thrombosis compared to trials in cardiovascular patients.
Our study was a secondary prevention study. This implies that the risk of recurrent venous thrombosis was the subject of study, which might be different from the risks of first-time venous thrombosis. This is clearly demonstrated by the observation that factor V Leiden, which is a strong risk factor for first-time venous thrombosis, is not or only weakly associated with recurrent venous thrombosis.19 Two prospective studies have been published on the risk for a recurrent event of venous thrombosis associated with hyperhomocysteinemia. In the first study, elevated homocysteine levels (above the 75th percentile) were associated with a 2.7-fold increase in risk.13 In the second study, no increased risk was found (hazard ratio 0.9; 95% CI, 0.5-1.6).19 In our study baseline homocysteine concentration is a predictor of recurrent venous thrombosis. However, the relative risk is lower than the risk for first-time venous thrombosis.1
One of the problems with secondary prevention studies in venous thrombosis is the diagnosis of a recurrent event. It could be difficult to distinguish between a recurrent event and the persistence of a residual thrombus (especially in DVT). To facilitate uniform diagnosis within the study we did repeated ultrasound examinations after the first event and provided a “patient passport” with information for the treating physician. There was, however, no central validation of the diagnosis, which is a potential limitation of the study. We opted for the decision of the treating physician to restart anticoagulant treatment as a defined end point, which is, in fact, the most clinical relevant parameter.
Several explanations can be given to the observation that baseline homocysteine levels are predictive for a recurrent event but homocysteine lowering does not result in a decrease in incidence. First, it might be a matter of insufficient power of our study to detect small effects. This explanation is supported by the post-hoc analyses and the dose-response relationship. Second, it can be explained by another factor that is related to homocysteine but is not affected by vitamin supplementation. However, based on current knowledge there is no evidence to treat patients with venous thrombosis with B vitamins to prevent recurrent events.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: M.d.H. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; M.d.H., H.J.B., W.B.J.G., F.R.R., and G.M.J.B. created the study concept and design; H.P.J.W., M.C., and S.E. acquired the data; M.d.H., F.R.R., and G.M.J.B. analyzed and interpreted the data; M.d.H., H.P.J.W., and G.M.J.B. drafted the manuscript; H.J.B., W.B.J.G., M.C, S.E, and F.R.R. revised the manuscript for important intellectual content; M.d.H. and F.R.R. provided statistical expertise; and W.B.J.G. and G.M.J.B. obtained funding.
Acknowledgments
We thank the staff and personnel of the Anticoagulation Centers of Rotterdam (Dr P. H. Trienekens), The Hague (Dr E. van Meegen), Leiden (Dr F. J. M. van der Meer), Amsterdam (Dr M.G. H. Remkes), Delft (M. Addicks), Utrecht (Dr J. de Vries-Goldschmeding), Amersfoort (Dr M. M. H. Kramer), Centro Emofilia e Trombosi, IRCCS Ospedale Maggiore, University of Milano, Italy (M. L. Zighetti), Centro Emostasi e Trombosi, Azienda Istituti Ospitalieri, Cremona, Italy (S. Testa), and Centro Trombosi, Istituto Clinico Humanitas, Milano, Italy (L. Rota), for all their work in recruiting the patients. We thank Marie-Louise Brantberger and Wilma van Spronsen for their help in the conduct of the study and Dr G. E. Th. Ferguson, pharmacist, and Dr P. P. H. Le Brun, pharmacist, for the randomization procedure and the distribution of the capsules.
This work was supported by The Netherlands Heart Foundation (NHS 94.141, NHS 99.055). M.dH. is recipient of a VENI-grant from the Netherlands Foundation of Scientific Research. The funding sources had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. The vitamin and placebo capsules were supplied by Astra/Viatris.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal