Abstract
Immune tolerance to self-antigens is established during lymphocyte differentiation in the thymus, but a simple means to induce antigen-specific tolerance in the thymus is still elusive. We show here that intrathymic injection of a lentiviral vector expressing the hemagglutinin antigen (HA) in TCR-HA transgenic mice resulted in negative selection of HA-specific effector T cells and sustained positive selection of HA-specific regulatory T cells (Tregs). This positive selection increased the number of HA-specific Tregs 10-fold and was comparable with the one observed in TCR-HA transgenic mice crossed with transgenic mice expressing HA under the control of the insulin promoter (Ins-HA). HA expression by radioresistant thymic epithelial cells was sufficient to drive Treg generation. Intrathymic injection of the lentiviral vector also resulted in an enrichment of HA-specific Tregs in peripheral lymphoid organs, which prevented diabetes induced in Ins-HA mice by transfer of HA-specific effector T cells. In this model, HA-specific Tregs inhibited effector T-cell division in pancreatic lymph nodes. Finally, we show that intrathymic injection of a lentiviral vector expressing preproinsulin-2 could reduce the occurrence of spontaneous diabetes in nonobese diabetic mice. Intrathymic gene transfer using lentiviral vectors thus offers new means to manipulate antigen-specific tolerance.
Introduction
At least 2 mechanisms are involved in the thymus to establish a self-tolerant although not self-ignorant immune system: negative selection of overtly self-reactive effector T cells and positive selection of a unique subset of self-reactive regulatory CD4+ T cells (Tregs) expressing the α-chain of the interleukin-2 receptor (CD25) and the forkhead helix-winged transcription factor foxp3.1 Injection in the thymus of organs, cells, peptide, or whole antigen, either under native or virally encoded forms, has been successful in the manipulation of T-cell–mediated immunologic tolerance.2 Seminal findings were that allogeneic grafts of pancreatic islets had a better survival when grafted in the thymus,3 and provided long-term protection against spontaneous autoimmune diabetes if grafted at birth.4,5 Other investigators have used intrathymic delivery of adenoviral vectors to establish immune tolerance to viral antigens for ensuing gene therapy protocols.6 It was later reported that intrathymic injection in neonates established long-term tolerance to adenoviral antigens with no need for prior lymphocyte depletion.7 To induce T-cell–mediated tolerance, other investigators have relied on the expression of foreign MHC class I8 or MHC class II alleles,9 or preproinsulin,10 on antigen-presenting cells after injection of autologous gene-modified hematopoietic stem cells. However, these studies did not formally demonstrate the involvement of the thymus in the tolerance process. The implication of the thymus has been documented in 2 recent studies related to the prevention of autoimmune responses following bone marrow transplantation.11,12 In particular, it has been shown that thymic deletion of effector T cells correlated with diabetes protection in NOD mice reconstituted with gene-modified autologous hematopoietic stem cells (HSCs).12
Besides negative selection (deletion) of overtly self-reactive T cells, a second major mechanism of central tolerance involves positive selection of CD4+CD25+foxp3+ Tregs. The importance of Tregs for prevention of autoimmunity is perhaps best exemplified by the widespread autoimmune symptoms seen in patients deficient for FOXP3.13 In the thymus, CD4+CD25+ cells are detected during the fetal period in humans14 and during the perinatal period in mice.15,16 The regulatory function of Tregs is acquired during thymic selection, as shown by the ability of isolated CD4+CD25+ thymocytes to suppress lymphocyte proliferation.17 In most studies, expression of the nominal antigen in the thymus led to increased frequency and/or number of specific Tregs in T-cell receptor (TCR) transgenic mice, suggesting an “instructive” role for the antigen in Treg lineage commitment.18-25 Other studies have suggested that intrathymic expression of the nominal antigen enriches for Tregs only because they better survived the deletion process.26,27 In any case, these studies provided the rationale to modulate T-cell–mediated tolerance either by enhancing deletion of autoreactive T cells or by inducing a greater number of antigen-specific Tregs by “forcing” expression of a given antigen in the thymus. A simple, efficient, and safe method to directly manipulate tolerance induction in the thymus would then be most valuable. We previously demonstrated stable transduction of thymic stromal cells in vivo by intrathymic injection of lentiviral vectors.28 We now report that this procedure allows the selection of antigen-specific Tregs in TCR transgenic mice and is capable of preventing autoimmune diabetes in NOD mice. These results open new perspectives for the manipulation of antigen-specific tolerance in the thymus.
Materials and methods
Mice
Heterozygous mice transgenic for the hemagglutinin antigen (HA) and the insulin promoter (Ins-HA)29 or homozygous for a TCR specific for the HA 111-119 epitope (SFERFEIFPK) presented by I-Ed molecules30 on a BALB/c background were bred in our own animal facility. NOD/Ltj mice, originally obtained from Charles River Laboratories (Wilmington, MA), were bred at the animal facility of the University of Medicine of La Pitié-Sâlpétrière. All animals were kept under specific pathogen-free conditions and manipulated according to European council directive 86/609/EEC.
Intrathymic injections
Mice were anesthetized with 30 mg/kg ketamine (Imalgene; Merial, Toulouse, France) and 24 mg/kg xylazine (Rompun; Bayer, Puteaux, France). Briefly, the thymus was made visible after opening the thoracic cage, and 10 to 20 μL lentiviral stock was injected using a 300-μL syringe (Terumo, Tokyo, Japan). Whole thymuses were excised from injected mice and placed in PBS 1 × in a 6-well plate. Pictures of the whole organ were taken with the 20×/0.4 PL objective of a CK-40 inverted UV microscope plugged into the U-RFLT 50 alimentation source (Olympus France, Rungis, France). Pictures were acquired using the software of the DP-11 numeric camera integrated in the system. Images were processed for contrast and luminosity using Adobe Photoshop (Adobe Systems, San Jose, CA).
Lentiviral vectors
Construction of the LvHA vector has been described in detail elsewhere.28 For the construction of the lentiviral encoding Ppins2, total pancreatic mRNA from a BALB/c mouse was reverse transcribed as cDNA. A polymerase chain reaction (PCR) amplifying the Ppins2 mRNA and incorporating the necessary cloning sites was designed. The Ppins2 cDNA was subsequently cloned in the LvPGK-GFP vector. We used internal ribosomal entry site (IRES) from the GTX homeodomain protein31 (kind gift of Dr V. Mauro, Scripps Research Institute, La Jolla, CA) to obtain the LvPGK-Ppins2-IRESGTX-eGFP lentiviral vector hereafter referred to as LvPpins2. Details on the cloning procedure are available on request. Lentiviral vector stocks were produced by calcium phosphate transfection of 3 plasmids encoding the eGFP- or HA-expressing vectors, the vesicular stomatitis virus envelope, and the packaging proteins in 293T cells as described.32 Supernatants were concentrated by ultrafiltration using Centricon-plus columns (Millipore, Billerica, MA) and kept at –80°C until use. Viral titers were evaluated by measuring the frequency of eGFP+ 293T cells at different dilutions for the LvGFP and LvPpins2 vectors or by measuring the amount of p24 in the supernatant with a p24 ELISA (Zeptometrix, Buffalo, NY) for the LvHA vector. Only viral stocks with titers above 107 infectious particles per milliliter (> 100 ng/mL p24) were used for intrathymic injections. Upon infection of 293T cells with the LvPpins2 vector, the amount of insulin produced in the supernatant was measured by ELISA (Alpco, Salem, NH) and was found to be of 1 μg insulin per milliliter per million cells per 24 hours.
Flow cytometry analysis
Cells from teased organs were labeled in PBS containing 3% fetal calf serum at room temperature for 15 to 30 minutes in the dark under continuous agitation. The following monoclonal antibodies (mAbs) were used for phenotypic analysis: allophycocyanin (APC)– or peridinin-chlorophyll-protein (PercP)–labeled anti-CD4 (RM4-5), fluorescein isothiocyanate (FITC)–conjugated anti-CD8 (clone 53.6.7), phycoerythrin (PE)– or APC-labeled anti-CD25, and PE-labeled CD24, CD69, and CD45 mAbs (all from BD Pharmingen, San Diego, CA). Labeling with the anticlonotypic mAb (clone 6.5) specific to TCR-HA was revealed by a biotin anti–rat IgG2b mAb and streptavidin-CyChrome (BD Pharmingen) or streptavidin-PE-Cyanin7 (Caltag Laboratories, Burlingame, CA). The foxp3-PE mAb was used according to the manufacturer's instructions (eBioscience, San Diego, CA). Isotype-matched irrelevant mAbs (BD Pharmingen) were used as controls. At least 100 000 events were collected on a FACScalibur or a FACSAria (Becton Dickinson, San Jose, CA). Data were analyzed using FlowJo (TreeStar, Ashland, OR) with slight modifications of the original compensations matrix established during acquisition.
Cell purification
After mechanical dissociation, cells from spleen and peripheral LN were sequentially incubated with saturating amounts of biotin-labeled anti-CD25 mAb (7D4; BD Pharmingen) and streptavidin microbeads (Miltenyi Biotec, Bergish Gladbach, Germany) for 30 minutes on ice, followed by 2 rounds of LS columns for magnetic cell separation (Miltenyi Biotec), according to the manufacturer's instructions. All steps were performed in PBS with 3% fetal calf serum. The purity of sorted CD4+CD25+ cells was 75% to 85%. For dendritic cell (DC) purification, spleens from Balb/c mice were digested with liberase (1.67 Wünsh U/mL; Roche, Meylan, France) and DNAse (0.1 mg/mL; Roche) diluted in LPS-low RPMI (InVitrogen, Cergy Pontoise, France) in a 37°C, 5% CO2 incubator for 30 minutes to 1 hour. Dissociated splenocytes were filtered and washed in LPS-free 1 × PBS (InVitrogen). Cells were incubated with anti-CD11c microbeads (Miltenyi Biotec) for 30 minutes on ice, followed by magnetic separation using LS columns (Miltenyi Biotec). The purity of CD11c+ cells was superior to 95%.
Cell transfer and diabetes monitoring
To induce diabetes in transgenic Ins-HA mice, 7.5 × 105 CD25– cells from spleen and LN of TCR-HA transgenic mice were given intravenous injections, with or without 5 × 105 purified CD4+CD25+ cells, into 3-Gy irradiated 6-week-old Ins-HA transgenic mice. Then, mice were immunized by intravenous injection of splenic DCs, matured after overnight culture, and pulsed with the HA111-119 peptide. Blood glucose levels were monitored every other day using a glucometer (LifeScan France, Issy-les-Moulineaux, France). Mice were considered diabetic if 2 consecutive readings were above 13.88 mM (250 mg/dL). To evaluate the effect of HA-specific Tregs on effector cell division, 1.5 × 106 purified CD25– cells of TCR-HA-Thy1.1 mice were transferred intravenously in homozygote Ins-HA mice together with 1.5 × 106 purified CD25+ cells of TCR-HA-Thy1.2 mice. Prior to transfer, effector CD25– cells were labeled with 2.5 μM carboxy-fluorescein diacetate, succinimidyl ester (CFSE; Sigma-Aldrich, Lyons, France).
Statistical analysis
Two-tailed unpaired t test with 95% confidence intervals and Kaplan-Meier survival curve comparisons were performed using GraphPad Prism version 4.0 for Macintosh (GraphPad Software, San Diego, CA). Mean values were considered statistically different if the P value was below .05.
Results
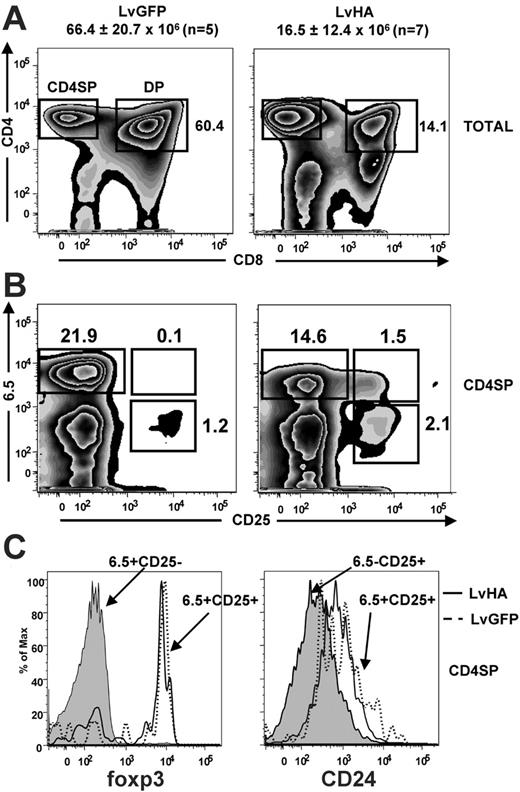
We first studied the impact of HA expression on T-cell differentiation in TCR transgenic mice specific for the HA111-119 peptide (TCR-HA) presented by the MHC class II I-Ed molecule.30 For this, we injected HA-encoding (LvHA) or GFP-expressing (LvGFP) lentiviral vectors (Figure 1) in the thymus of TCR-HA mice and analyzed the representation of HA-specific CD25– and CD25+ cells identified with the 6.5 clonotypic mAb. One week after LvHA injection, we observed a 4-fold reduction in total numbers of thymocytes of the LvHA-injected mice (16.5 ± 12.4 × 106,n = 7) compared with LvGFP-injected mice (66.4 ± 20.7 × 106, n = 5) or to noninjected control mice (data not shown), indicating negative selection of HA-specific thymocytes. Of interest, a massive reduction of CD4+CD8+ cells (DP) was observed in 4 of 7 LvHA-injected compared with LvGFP-injected mice (Figure 2A), indicating that this subset was affected by negative selection. In the CD4+CD8– single-positive (CD4SP) subset, 6.5+CD25– cells were also affected by negative selection as attested by a slight reduction in their frequency and a down-modulation of their transgenic TCR (Figure 2B), as previously noted in TCR-HA transgenic animals.28,33 This reduction in 6.5+CD25– cells in mice injected with the LvHA vector was accompanied by a striking enrichment of 6.5+CD25+ cells compared with mice injected with a control vector (LvGFP) (Figure 2B) or to noninjected mice (data not shown). In both LvGFP- and LvHA-injected mice, these 6.5+CD25+ CD4SP thymocytes expressed high levels of the transcription factor foxp3, indicating that they have acquired a Treg phenotype (Figure 2C). These 6.5+CD25+ CD4SP cells displayed a higher expression of CD24 (heat stable antigen, HSA) than 6.5–CD25+ CD4SP cells (Figure 2C), indicating that they are not mature T cells that would have re-entered the thymus from the periphery.34 Of note, the low expression of CD24 by CD25+ CD4SP cells of the thymus has been previously noticed.17 Altogether, our phenotypic analysis of LvHA-injected mice indicates that expression of the cognate antigen induces negative selection of HA-specific thymocytes and concomitant positive selection of HA-specific regulatory T cells.
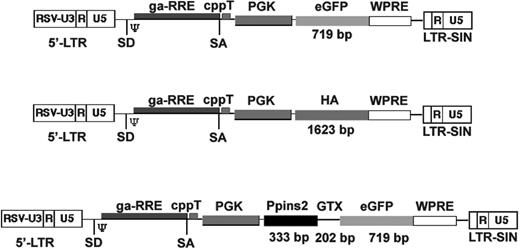
Schematic representation of the lentiviral vectors used in this study (not to scale). Schematic structure of the lentiviral vectors expressing eGFP (LvGFP), hemagglutinin (LvHA), or the murine preproinsulin-2 (LvPpins2). The size of the inserts is indicated on the figure. RSV-U3 indicates U3 promoter/enhancer of the Rous sarcoma virus; SD, splice donor; SA, splice acceptor; psi (Ψ), packaging signal sequence; ga-RRE, truncated gag gene with the rev responsive element; cppT, central polypurine tract; PGK, phospho-glycerate kinase promoter; WPRE, woodchuck hepatitis virus regulatory element; and LTR-SIN, self-inactivating long terminal repeat.
Schematic representation of the lentiviral vectors used in this study (not to scale). Schematic structure of the lentiviral vectors expressing eGFP (LvGFP), hemagglutinin (LvHA), or the murine preproinsulin-2 (LvPpins2). The size of the inserts is indicated on the figure. RSV-U3 indicates U3 promoter/enhancer of the Rous sarcoma virus; SD, splice donor; SA, splice acceptor; psi (Ψ), packaging signal sequence; ga-RRE, truncated gag gene with the rev responsive element; cppT, central polypurine tract; PGK, phospho-glycerate kinase promoter; WPRE, woodchuck hepatitis virus regulatory element; and LTR-SIN, self-inactivating long terminal repeat.
Impact of intrathymic injection of an HA-encoding lentiviral vector in TCR-HA transgenic mice. (A) Representative staining showing CD4/CD8 expression in total cells of the thymus (TOTAL) 6 days after intrathymic injection of GFP-expressing (LvGFP) or HA-expressing (LvHA) lentiviral vectors in TCR-HA mice. Absolute numbers of total cells in injected thymus ± SD are indicated above the panels. Gates defining CD4+CD8– single-positive (CD4SP) and CD4+CD8+ double-positive thymocytes are shown. Numbers indicated on the dot plot represent frequencies of DP cells and are representative of 4 of 7 LvHA-injected mice obtained in 3 independent experiments. (B) Expression of the HA-specific (6.5) TCR and of CD25 in gated thymic CD4+CD8– cells (CD4SP) in the same conditions. Indicated are the frequencies of cells falling within each quadrant. Results are representative of 3 independent experiments. (C, left) Representative profile of foxp3 expression in 6.5+CD25– CD4SP cells (gray histogram) and 6.5+CD25+ CD4SP cells of an LvGFP-injected (dotted line) or an LvHA-injected (solid line) mouse. (C, right) Representative CD24 expression within 6.5–CD25+ CD4SP cells (gray histogram) and 6.5+CD25+ CD4SP cells of an LvGFP-injected (dotted line) or an LvHA-injected (solid line) mouse. Similar profiles were seen in 3 other mice. Total thymus was stained with CD4, CD8, 6.5, CD25, and foxp3 or CD24 mAbs.
Impact of intrathymic injection of an HA-encoding lentiviral vector in TCR-HA transgenic mice. (A) Representative staining showing CD4/CD8 expression in total cells of the thymus (TOTAL) 6 days after intrathymic injection of GFP-expressing (LvGFP) or HA-expressing (LvHA) lentiviral vectors in TCR-HA mice. Absolute numbers of total cells in injected thymus ± SD are indicated above the panels. Gates defining CD4+CD8– single-positive (CD4SP) and CD4+CD8+ double-positive thymocytes are shown. Numbers indicated on the dot plot represent frequencies of DP cells and are representative of 4 of 7 LvHA-injected mice obtained in 3 independent experiments. (B) Expression of the HA-specific (6.5) TCR and of CD25 in gated thymic CD4+CD8– cells (CD4SP) in the same conditions. Indicated are the frequencies of cells falling within each quadrant. Results are representative of 3 independent experiments. (C, left) Representative profile of foxp3 expression in 6.5+CD25– CD4SP cells (gray histogram) and 6.5+CD25+ CD4SP cells of an LvGFP-injected (dotted line) or an LvHA-injected (solid line) mouse. (C, right) Representative CD24 expression within 6.5–CD25+ CD4SP cells (gray histogram) and 6.5+CD25+ CD4SP cells of an LvGFP-injected (dotted line) or an LvHA-injected (solid line) mouse. Similar profiles were seen in 3 other mice. Total thymus was stained with CD4, CD8, 6.5, CD25, and foxp3 or CD24 mAbs.
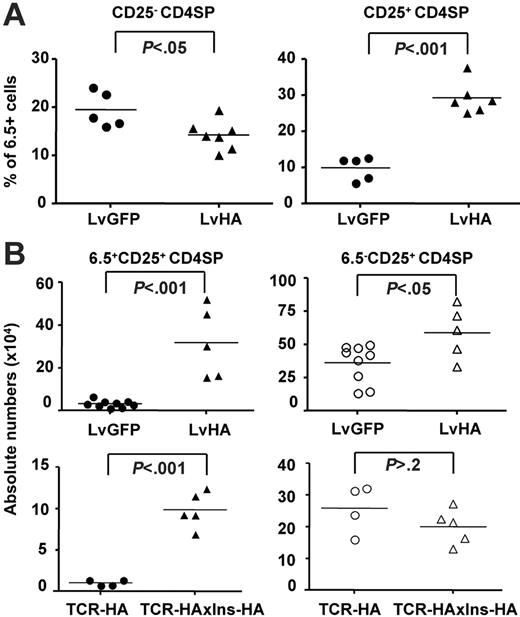
Overall, percentages of HA-specific 6.5+ cells within the major CD25– fraction of CD4SP cells decreased by 2- to 2.5-fold, whereas the percentage of 6.5+ cells within the minor CD25+ CD4SP thymocytes increased by 2- to 10-fold within one week after injection (Figure 3A). To address whether positive selection of HA-specific Tregs could be a long-lasting phenomenon after intrathymic injection, we determined their absolute numbers in the thymus 1 to 2 months after injection (Figure 3B). We also observed increased percentages of 6.5+CD25+ cells in the CD4SP thymocyte subset of LvHA-injected mice compared with LvGFP-injected mice (data not shown), translating into a 10-fold increase in their absolute number. Of note, the mean number of 6.5+CD4+CD25+ T cells in LvHA-injected mice reached the mean number of total CD4+CD25+ T cells in LvGFP-injected mice (Figure 3B) or in nontreated mice (not shown), indicating that the cognate antigen had a major effect on the positive selection of CD4+CD25+ cells. Deletion of 6.5+CD25– CD4SP thymocytes was no longer observed (data not shown).
Injection of the LvHA vector in the thymus of TCR-HA mice increases percentages and numbers of HA-specific Tregs. (A) Shown are the frequencies of HA-specific (6.5+) CD25+ within CD4SP cells (right panel) and CD25– CD4SP cells (left panel) 1 to 2 weeks after intrathymic injection of LvHA or LvGFP lentiviral vectors in TCR-HA mice. Each dot represents individual mice from 3 independent experiments. (B, top panels) Absolute numbers (× 104) of 6.5+CD25+ cells (closed symbols) and 6.5–CD25+ cells (open symbols) within CD4SP thymocytes in TCR-HA transgenic mice 1 to 2 months after injection of a GFP-expressing (LvGFP) or HA-expressing (LvHA) lentiviral vector. (B, bottom panels) Absolute numbers (× 104) of 6.5+CD25+ cells (closed symbols) and 6.5–CD25+ cells (open symbols) within CD4SP thymocytes in TCR-HA or TCR-HAxIns-HA transgenic mice. Absolute numbers of cells were calculated by multiplying the total viable cell count, as determined by trypan blue exclusion, with the frequencies of cells in each population of interest. Each dot represents a value from individual mice in 2 separate experiments. The P values were calculated using unpaired t test.
Injection of the LvHA vector in the thymus of TCR-HA mice increases percentages and numbers of HA-specific Tregs. (A) Shown are the frequencies of HA-specific (6.5+) CD25+ within CD4SP cells (right panel) and CD25– CD4SP cells (left panel) 1 to 2 weeks after intrathymic injection of LvHA or LvGFP lentiviral vectors in TCR-HA mice. Each dot represents individual mice from 3 independent experiments. (B, top panels) Absolute numbers (× 104) of 6.5+CD25+ cells (closed symbols) and 6.5–CD25+ cells (open symbols) within CD4SP thymocytes in TCR-HA transgenic mice 1 to 2 months after injection of a GFP-expressing (LvGFP) or HA-expressing (LvHA) lentiviral vector. (B, bottom panels) Absolute numbers (× 104) of 6.5+CD25+ cells (closed symbols) and 6.5–CD25+ cells (open symbols) within CD4SP thymocytes in TCR-HA or TCR-HAxIns-HA transgenic mice. Absolute numbers of cells were calculated by multiplying the total viable cell count, as determined by trypan blue exclusion, with the frequencies of cells in each population of interest. Each dot represents a value from individual mice in 2 separate experiments. The P values were calculated using unpaired t test.
We also analyzed the numbers of 6.5+ cells in TCR-HA mice crossed with transgenic mice expressing HA under the control of the insulin promoter (Ins-HA), which is likely expressed at low levels in the thymus, similar to the endogenous insulin.35 In agreement with the results obtained in LvHA-injected mice, we observed elevated numbers of 6.5+ cells in CD25+ CD4SP thymic cells of TCR-HAxIns-HA mice (Figure 3B). The increase in 6.5+CD25+ cell numbers observed in double-transgenic mice as well as in LvHA-injected mice was of 8 -to 10-fold in CD4SP thymocytes. In contrast, compared with the controls, the numbers of 6.5–CD25+ CD4SP T cells were increased less than 2-fold in LvHA-injected TCR-HA mice, and not increased at all in double-transgenic TCR-HAxIns-HA mice. Collectively, our results show that intrathymic expression of the cognate antigen leads to the augmentation of frequencies and numbers of HA-specific Tregs in mice that had never previously been exposed to the antigen.
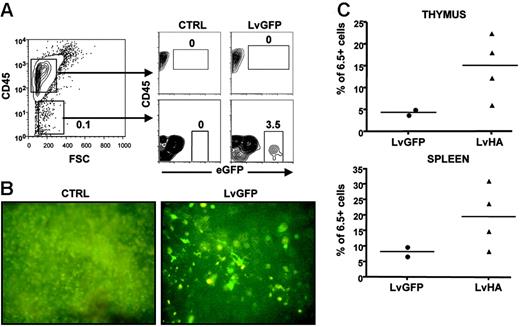
We then assessed which cells could be responsible for Treg selection upon intrathymic injection of the LvHA vector. A first indication was provided by cytometry analysis showing that eGFP+ could readily be observed in CD45– but not in CD45+ cells after injection of an LvGFP-expressing vector (Figure 4A). Thus, cells of epithelial rather than hematopoietic origin appear as the main target of vesicular stomatitis virus (VSV)–pseudotyped lentiviral vectors after intrathymic injection, as we previously suggested.28 To directly assess the role of epithelial cells in Treg selection, we first injected LvGFP or LvHA vectors in the thymus of TCR-HA mice. One month after, mice were lethally irradiated and received a transplant of T-cell–depleted bone marrow from TCR-HA mice. In mice injected with the LvGFP vector, thymic cells expressing eGFP were readily detectable one month after irradiation, showing that cells initially transduced by the LvGFP vector were radio resistant (Figure 4B). In 3 of 4 mice injected with the LvHA vector, we observed elevated percentages of HA-specific cells within CD4+CD25+ thymocytes and splenocytes compared with LvGFP-treated mice (Figure 4C) or with noninjected control mice (data not shown). Collectively, these results indicate that expression of HA by radioresistant epithelial thymic cells was sufficient for the selection of HA-specific CD25+ CD4SP cells.
Thymic CD45– radioresistant cells are sufficient for positive selection of HA-specific Tregs following lentiviral vector injection. (A, left) Shown is a representative CD45 versus forward side scatter (FSC) staining of total cells of a thymus from a TCR-HA mouse after collagenase and Dnase I digestion. (A, right) Evaluation of eGFP expression in CD45+ and CD45– cells after injection of PBS (CTRL) or LvGFP vector. Similar result was obtained in another mouse. (B) Direct visualization of radioresistant transduced cells in the thymus of 9 Gy–irradiated TCR-HA mice irradiated 4 weeks after injection of PBS (CTRL) or of the eGFP-expressing vector (LvGFP). Whole thymuses were examined for eGFP fluorescence directly under a UV microscope in PBS 1 × 6 weeks after irradiation and T-cell–depleted bone marrow transfer from TCR-HA mice. Original magnification: ×40. (C) Frequencies of HA-specific cells within CD4SP CD25+ thymocytes or CD4+CD25+ splenocytes in TCR-HA mice injected with the control eGFP-expressing vector (LvGFP) or the HA-expressing vector (LvHA) 4 weeks prior to irradiation. Analysis was performed either 4 or 6 weeks after T-cell–depleted bone marrow transfer in a single experiment.
Thymic CD45– radioresistant cells are sufficient for positive selection of HA-specific Tregs following lentiviral vector injection. (A, left) Shown is a representative CD45 versus forward side scatter (FSC) staining of total cells of a thymus from a TCR-HA mouse after collagenase and Dnase I digestion. (A, right) Evaluation of eGFP expression in CD45+ and CD45– cells after injection of PBS (CTRL) or LvGFP vector. Similar result was obtained in another mouse. (B) Direct visualization of radioresistant transduced cells in the thymus of 9 Gy–irradiated TCR-HA mice irradiated 4 weeks after injection of PBS (CTRL) or of the eGFP-expressing vector (LvGFP). Whole thymuses were examined for eGFP fluorescence directly under a UV microscope in PBS 1 × 6 weeks after irradiation and T-cell–depleted bone marrow transfer from TCR-HA mice. Original magnification: ×40. (C) Frequencies of HA-specific cells within CD4SP CD25+ thymocytes or CD4+CD25+ splenocytes in TCR-HA mice injected with the control eGFP-expressing vector (LvGFP) or the HA-expressing vector (LvHA) 4 weeks prior to irradiation. Analysis was performed either 4 or 6 weeks after T-cell–depleted bone marrow transfer in a single experiment.
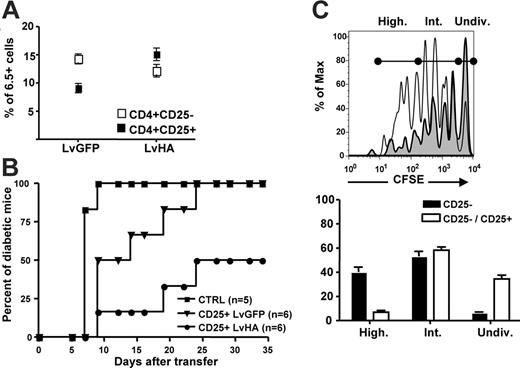
We next assessed whether intrathymic injection of the LvHA vector would lead to enrichment in HA-specific Tregs in peripheral organs. Frequencies of HA-specific Tregs were indeed increased 1 to 2 months after intrathymic injection of the LvHA vector in TCR-HA mice (Figure 5A). To test their in vivo regulatory potential, we compared the ability of CD25+ cells from LvGFP- or LvHA-injected mice to prevent and/or delay experimental diabetes induced in Ins-HA transgenic mice. We first sublethally irradiated Ins-HA mice and then injected them with effector CD25– cells from TCR-HA mice. Mice were subsequently immunized with HA-pulsed dendritic cells. In this setting, 100% of Ins-HA mice became diabetic 10 days after cell transfer (Figure 5B). The injection of 5 × 105 CD25+ cells from TCR-HA mice injected with the control LvGFP vector led to a delayed disease progression, but all mice eventually became diabetic. At the same dose, CD25+ cells from LvHA-injected TCR-HA mice prevented diabetes in 50% of the mice, indicating that functional HA-specific Tregs were enriched in LvHA-injected mice compared with LvGFP-injected mice. To investigate the mechanism by which HA-specific Tregs inhibit effector T cells in this model, we cotransferred CFSE-labeled Thy1.1+ CD25– cells and Thy1.2+ CD25+ T cells from congenic TCR-HA mice into Ins-HA mice. Four days after injection, CFSE loss in Thy1.1+ cells was observed in pancreatic but not in other lymph nodes (data not shown). The frequency of effector CD25– cells that had undergone multiple divisions was reduced by 6-fold in the presence of Tregs (Figure 5C).
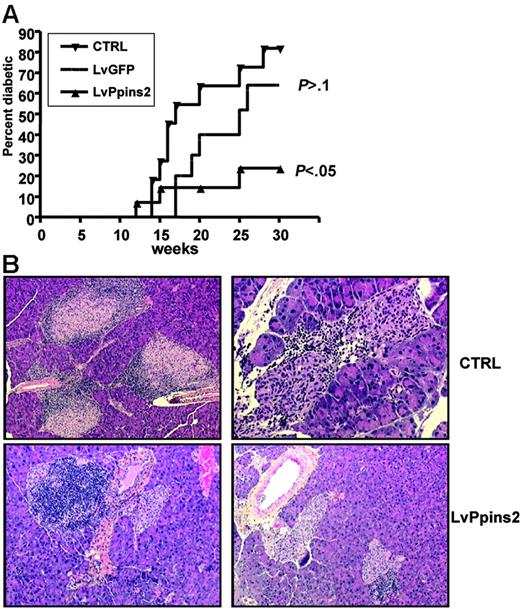
We next asked whether intrathymic injection of a lentiviral vector expressing an autoantigen would restore “broken” tolerance in a context of a fully diversified, polyclonal T-cell repertoire. In NOD mice, clinical diabetes occurs spontaneously after 10 to 15 weeks of age and affects 60% to 80% of female mice at 25 weeks of age. Transgenic expression of preproinsulin-2 (Ppins2) under the control of an MHC class II promoter can prevent diabetes in the majority of NOD mice36,37 ; and Ppins2-deficient NOD mice have an accelerated diabetes compared with NOD mice.38 We thus turned to NOD mice to test the potential of Ppins2 to prevent and/or delay diabetes. We injected a Ppins2-expressing lentiviral vector (Figure 1) in the thymus of NOD females aged from 4 to 8 weeks. NOD mice injected with the LvGFP vector had similar diabetes occurrence than unmanipulated NOD mice of our colony (Figure 6A). This indicates that intrathymic injection per se did not significantly affect the autoimmune process. In contrast, there was a significant reduction in diabetes incidence in mice injected with the Ppins2-expressing vector (Figure 6A), indicating that the treatment had an impact on diabetes development in prediabetic NOD mice. These results correlated with histologic analysis of the pancreas from treated and untreated mice (Figure 6B). In untreated NOD mice with normal glycemia, 22 of the 37 islets counted were heavily infiltrated with leucocytes, indicating severe insulitis. In contrast, in LvPpins2-treated NOD mice with normal glycemia, only 7 of 32 beta-islets were infiltrated with leucocytes. Thus, injection of the Ppins2-expressing vector in the thymus of NOD mice appears to regulate clinical diabetes by partially preventing insulitis.
Prevention of experimental diabetes in Ins-HA mice by CD25+ cells from LvHA-injected TCR-HA mice. (A) Percentages of 6.5+ cells within CD4+CD25– (open symbols) and CD4+CD25+ (closed symbols) splenocytes determined 1 to 2 months after injection of the GFP-expressing (LvGFP) or of the HA-expressing (LvHA) lentiviral vector. (B) Percentages of diabetic Ins-HA mice upon cotransfer of 5 × 105 purified CD25+ peripheral T cells from TCR-HA transgenic mice injected in the thymus 10 days earlier with GFP-expressing (CD25+ LvGFP) or HA-expressing (CD25+ LvHA) lentiviral vectors. One-hundred percent of the mice became diabetic at day 10 without cotransfer of CD4+CD25+ cells (CTRL). Data were obtained with 5 or 6 mice per group in 2 separate experiments. (C) (Top panel) Overlay histograms of CFSE labeling of Thy1.1+ cells 4 days after transfer of CD25– Thy1.1+ cells alone (solid line) or cotransfer of CD25– Thy1.1+ cells and CD25+ Thy1.2+ cells (gray histogram) in pancreatic lymph nodes of Ins-HA mice. (Bottom panel) Statistic representation of the percentages of cells falling within each population of undivided CFSE+ (Undiv), intermediately divided (Int), or highly divided (High) cells defined as shown on the right panel. Results are from 3 mice per group in a single experiment. Not depicted are similar results obtained in 2 other experiments.
Prevention of experimental diabetes in Ins-HA mice by CD25+ cells from LvHA-injected TCR-HA mice. (A) Percentages of 6.5+ cells within CD4+CD25– (open symbols) and CD4+CD25+ (closed symbols) splenocytes determined 1 to 2 months after injection of the GFP-expressing (LvGFP) or of the HA-expressing (LvHA) lentiviral vector. (B) Percentages of diabetic Ins-HA mice upon cotransfer of 5 × 105 purified CD25+ peripheral T cells from TCR-HA transgenic mice injected in the thymus 10 days earlier with GFP-expressing (CD25+ LvGFP) or HA-expressing (CD25+ LvHA) lentiviral vectors. One-hundred percent of the mice became diabetic at day 10 without cotransfer of CD4+CD25+ cells (CTRL). Data were obtained with 5 or 6 mice per group in 2 separate experiments. (C) (Top panel) Overlay histograms of CFSE labeling of Thy1.1+ cells 4 days after transfer of CD25– Thy1.1+ cells alone (solid line) or cotransfer of CD25– Thy1.1+ cells and CD25+ Thy1.2+ cells (gray histogram) in pancreatic lymph nodes of Ins-HA mice. (Bottom panel) Statistic representation of the percentages of cells falling within each population of undivided CFSE+ (Undiv), intermediately divided (Int), or highly divided (High) cells defined as shown on the right panel. Results are from 3 mice per group in a single experiment. Not depicted are similar results obtained in 2 other experiments.
Discussion
We investigated the possibility to induce antigen-specific tolerance using intrathymic injection of antigen-expressing lentiviral vectors. We report here that the injection of an HA-expressing vector led to sustained positive selection of HA-specific Tregs. This de novo generation depended on antigen expression by CD45– radioresistant thymic cells, thus of epithelial rather than hematopoietic origin. Although cortical epithelial cells have been described as sufficient for positive selection of Tregs,39 it does not exclude that antigen cross-presentation by thymic DCs could play a role in Treg selection. A similar cross-presentation of thymic antigen expressed by medullary epithelial cells has been recently described as an effective mechanism for negative selection.40 The exact nature of the mechanisms driving Treg generation in the thymus is the subject of intense scrutiny,41 and lentiviral vectors with defined tropism and/or specificity for discrete cell type of the thymic stroma could be of great help to further advance our understanding of this process.
The signal delivered by TCR recognition of a self-peptide–MHC class II molecule complex may instruct uncommitted precursors to differentiate into the Treg lineage or provide a survival/proliferation signal to precommitted Tregs. Indeed, using double-transgenic mice for a specific antigen and for its cognate TCR, increases of antigen-specific Treg absolute numbers in the thymus have been reported with the OVA,24 but not with the HEL or MCC antigens.26,27 Since the frequencies of antigen-specific Tregs were increased in all models, the latter experiments were interpreted as evidence for increased resistance to negative selection rather than positive selection. Our results showing increased absolute numbers of antigen-specific Tregs both in double-transgenic mice and in mice injected with the lentiviral vector are more in favor of positive selection as the likely mechanism contributing to increased frequencies of antigen-specific Tregs upon recognition of the cognate antigen in the thymus.
Prevention of diabetes and insulitis in NOD mice after intrathymic injection of a preproinsulin-2–expressing lentiviral vector. (A) Cumulative diabetes incidence (Kaplan-Meier survival curve) in unmanipulated mice (CTRL, n = 11) or after intrathymic injection of a GFP-expressing (LvGFP, n = 10) or preproinsulin-2–expressing (LvPpins2, n = 14) lentiviral vector. Indicated for LvGFP and LvPpins2 survival curves are the P values calculated with a log-rank test relative to the untreated NOD mice. (B) Sections of formalin-fixed pancreas from 10-week-old untreated nondiabetic mice (top left: original magnification, ×20), 36-week-old LvGFP-treated diabetic mice (top right: original magnification, ×100), and LvPpins-2–treated nondiabetic mice (bottom left and right: original magnification, ×20). Paraffinembedded sections of pancreas from NOD mice were stained with hematoxylin for 5 minutes followed by eosin for 30 seconds to reveal nucleated cells. Images were taken with either a 10×/0.3 PL or a 20×/0.5 FLUOTAR AB objective of a DM RB microscope (Leica Microsystems, Wetzlar, Germany). Image digitization was performed by a DXC930P CDD color video camera (Sony France, Clichy, France) with TRIBVN ICS software v. 1.2.0.9 (TRIBVN, Châtillon, France). Images were processed for contrast and luminosity using Adobe Photoshop CS.
Prevention of diabetes and insulitis in NOD mice after intrathymic injection of a preproinsulin-2–expressing lentiviral vector. (A) Cumulative diabetes incidence (Kaplan-Meier survival curve) in unmanipulated mice (CTRL, n = 11) or after intrathymic injection of a GFP-expressing (LvGFP, n = 10) or preproinsulin-2–expressing (LvPpins2, n = 14) lentiviral vector. Indicated for LvGFP and LvPpins2 survival curves are the P values calculated with a log-rank test relative to the untreated NOD mice. (B) Sections of formalin-fixed pancreas from 10-week-old untreated nondiabetic mice (top left: original magnification, ×20), 36-week-old LvGFP-treated diabetic mice (top right: original magnification, ×100), and LvPpins-2–treated nondiabetic mice (bottom left and right: original magnification, ×20). Paraffinembedded sections of pancreas from NOD mice were stained with hematoxylin for 5 minutes followed by eosin for 30 seconds to reveal nucleated cells. Images were taken with either a 10×/0.3 PL or a 20×/0.5 FLUOTAR AB objective of a DM RB microscope (Leica Microsystems, Wetzlar, Germany). Image digitization was performed by a DXC930P CDD color video camera (Sony France, Clichy, France) with TRIBVN ICS software v. 1.2.0.9 (TRIBVN, Châtillon, France). Images were processed for contrast and luminosity using Adobe Photoshop CS.
The antigen-specific Tregs generated by intrathymic injection of the LvHA vector in TCR-HA transgenic mice efficiently protected Ins-HA mice from diabetes induced after transfer of HA-specific effector T cells. In this model, we show that the pancreatic lymph nodes were the main site for HA presentation in Ins-HA mice, as previously suggested.42 At this site, HA-specific Tregs inhibited effector T-cell proliferation, in agreement with results obtained in BALB/c mice cotransferred with HA-specific effector and Tregs.20 Altogether, this strongly suggests that the lower incidence of diabetes in the presence of an increased number of HA-specific Tregs following intrathymic injection of the LvHA vector is mediated by the inhibition of effector T-cell division in pancreatic lymph nodes.
A number of reports support a prime role for insulin in the etiology of autoimmune diabetes in NOD mice. For instance, oral, subcutaneous, or intranasal administration of insulin or insulin B chain can prevent spontaneous autoimmune diabetes.43 In addition, the introduction of a single mutation that abrogated T-cell reactivity to the immunodominant insulin epitope B:9-23 led to complete prevention of autoimmune diabetes,44 suggesting that B:9-23 is one of the few epitopes able to drive autoimmunity. We thus explore the potential of intrathymic injection of Ppins2-expressing lentiviral vectors to prevent or delay autoimmune diabetes in NOD mice. In NOD mice in which spontaneous diabetes occurred between weeks 15 to 30, a single injection performed at week 8 prevented diabetes in about 50% of the animals. However, in Treg-depleted NOD mice in which diabetes occurred between weeks 10 to 12, a single injection performed at week 10 only slightly delayed diabetes occurrence (data not shown). This suggests that such treatment is more effective in prophylactic rather than therapeutic settings. Nevertheless, the level of protection achieved by prophylactic intrathymic administration of the lentiviral vector was similar to the one provided by transgenic expression of Ppins2 under the invariant chain promoter37 or after DNA immunization.45
However, the cellular mechanisms responsible for the protection conferred by intrathymic injection of the Lvppins2 vector in NOD mice are still unclear. From the results obtained in TCR-HA transgenic mice injected with the LvHA vector, we would speculate that intrathymic injection of the Ppins2-expressing vector would lead to increased numbers of insulin-specific Tregs, which in turn would be responsible for the clinical effect. However, CD25+ T cells from LvPpins2-treated animals did not mediate significant suppression of IFN-γ production by insulin-specific effector T cells (data not shown). Thymic transgene expression also leads to increased negative selection of effector T cells, as observed upon expression of HA in HA-specific transgenic mice (Figure 2). Thus, negative selection may contribute to protection in the LvPpins2-injected NOD mice, as also proposed in an insulin transgenic model.37 At the present time, it is difficult to weigh the relative contributions of these 2 mechanisms since the tools necessary to directly track insulin-specific CD4+ T cells in NOD mice (ie, class II MHC tetramers) are still lacking.
Regardless of the mechanisms, intrathymic injection of lentiviral vectors represents a novel form of antigen delivery for central tolerance induction. This opens new perspectives to restore tolerance in autoimmune diseases, or to induce tolerance to donor HLA alleles or foreign gene products in transplantation or gene therapy settings. In humans, level of insulin expression in the thymus is, after HLA haplotype, the most predictive factor associated with diabetes susceptibility, with lower levels being closely associated with increased susceptibility.46,47 Thus, artificially rising insulin levels in the thymus by intrathymic gene transfer may positively influence the course of the disease in susceptible humans. Lentiviral vectors might represent the ideal vectors to this end. However, numerous efficacy and safety issues, including the consequences of vector integration into thymic cells, will have to be addressed before any clinical development can be envisioned.
Prepublished online as Blood First Edition Paper, June 29, 2006; DOI 10.1182/blood-2006-03-010900.
Supported by grants from the Agence Nationale de la Recherche (appel d'offre 2005 Microbiologie-Immunologie), the UPMC, the Roche Organ Transplantation Research Foundation, and the Association Française contre les Myopathies (S.F. and G.M.).
G.M., S.F., B.L., and M.F. performed experiments; G.M., B.L.S., and D.K. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr H. von Boehmer (Harvard University, Boston, MA) for originally providing the TCR-HA mice. We gratefully acknowledge Fathia Djelti, Elena Litvinova, Lucile Pret, and Fabienne Billiard for their help in several experiments reported herein, as well as Bruno Gouritin and Sylvie Grégoire for multicolor flow cytometry expertise, and Gwenaelle Piriou, Brice Chanudet, and Pierric Parent for taking care of our animal colonies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal