Abstract
Pain and acute chest syndrome (ACS) episodes are 2 of the most common causes of hospitalization in children with sickle cell anemia (SCA). However, very few potentially modifiable risk factors for either condition have been identified. In this prospective infant cohort study, we tested the hypothesis that asthma is associated with an increased incidence rate of pain and ACS episodes. An infant cohort was composed of 291 African American children with hemoglobin SS enrolled in the Cooperative Study for Sickle Cell Disease before age 6 months and followed beyond age 5 years. Asthma was defined by a physician diagnosis, an acute asthma event, or use of prescription asthma medications. The incidence rates of ACS and painful episodes were compared for children with and without asthma. A clinical diagnosis of asthma was made in 17% of the cohort. Asthma was associated with more frequent ACS episodes (0.39 vs 0.20 events per patient year, P < .001) and painful episodes (1.39 vs 0.47 events per patient year, P < .001). In conclusion, in children with SCA, asthma is associated with an increased incidence of sickle cell disease–related morbidity, including ACS and painful episodes.
Introduction
Painful episodes and acute chest syndrome (ACS) are common complications of sickle cell anemia (SCA) and are the 2 leading causes of hospitalization among people with SCA.1,2 ACS affects up to 50% of individuals with SCA and is the leading cause of premature death.2-4 The pathogenesis of ACS is multifactorial and remains unclear. In a large prospective study, several etiologies were identified, but in approximately 50% of episodes, no cause was determined.5 Known risk factors for the development of ACS include younger age, degree of anemia (higher steady-state hemoglobin level), lower hemoglobin F, and higher steady-state white blood cell count.2,6
Asthma is a common chronic disease affecting approximately 15% to 20% of African American children.7-10 Previous retrospective studies suggest a relationship between ACS and asthma among children with SCA. We previously reported a 4-fold higher risk of ACS among children with SCA and a prior doctor diagnosis of asthma within a cohort of children with SCA hospitalized for pain.11 Knight-Madden et al12 reported a 6-fold higher risk of recurrent ACS among children with SCA and a parental report of doctor-diagnosed asthma. Airway obstruction and airway lability have been previously described in patients with SCA, suggesting that asthma may contribute to the pulmonary complications of the disease.13-16 These data suggest that in children with SCA, a diagnosis of asthma may be a risk factor for ACS. This study also seeks to determine whether asthma is also associated with painful episodes, a common complication in SCA that often precedes or occurs concurrently with ACS episodes.
In a cohort of infants established and followed for 20 years by the Cooperative Study of Sickle Cell Disease (CSSCD), we tested the hypothesis that a concurrent diagnosis of asthma in patients with SCA is associated with increased frequency of ACS and painful episodes.
Patients and methods
Patient population
The CSSCD study design has been reported previously.17 Subjects with sickle cell disease were enrolled in this natural history study from 1978 through 1988; follow-up ended in 1998. Subjects were followed prospectively with annual follow-up including a history and physical exam, monitoring of clinical events and, among a subset of subjects, performance of pulmonary function testing. Consent and assent were obtained in accordance with the requirements and guidelines of the human subjects committees at participating clinical centers, including Washington University School of Medicine. Additional approval was obtained from Washington University School of Medicine's Institutional Review Board for analysis of the de-identified CSSCD data held by the National Heart, Lung, and Blood Institute.
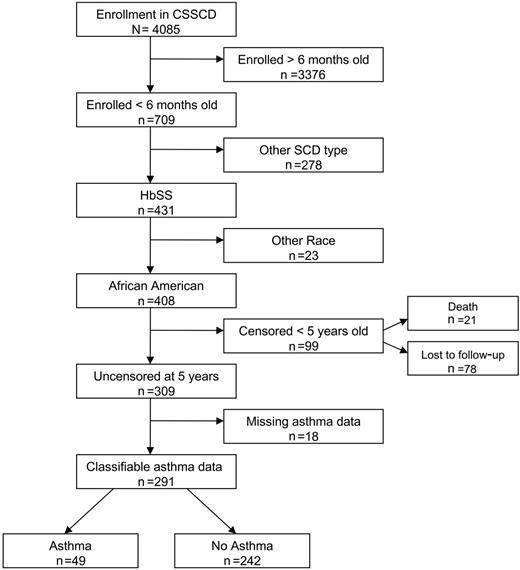
The study cohort was composed of an infant cohort of participants enrolled in the CSSCD. Members of the infant cohort with hemoglobin SS who were self-designated as African American, enrolled in the study before age 6 months, and followed beyond 5 years of age were included in this analysis (Figure 1). The study was limited to African American children due to the small numbers of other ethnic minorities in the cohort and for interpretation of spirometry results based on a reference population with race, sex, age, and height adjustments.18 We excluded patients who had been lost to follow-up before 5 years of age because diagnosis of asthma in that age group is more difficult, and the impact of transient wheezing and remitted asthma on lung function and future morbidity is not established.19,20 Eighteen patients were excluded from the infant cohort because insufficient clinical data were recorded and a history of asthma could not be ascertained. Of the 291 patients included in the cohort, 227 completed pulmonary function testing (PFT) with measurement of forced expiration volume in 1 second (FEV1) and FEV1/forced vital capacity (FVC) (age range, 6-18 years) at least 60 days after any ACS event or pneumonia and at least 1 month after any acute respiratory illness as per study protocol.
Enrollment in the infant sickle cell anemia cohort and eligibility for study cohort.
Enrollment in the infant sickle cell anemia cohort and eligibility for study cohort.
Length of follow-up
The patient years for clinical events including ACS, pain, and transfusion were accrued from date of enrollment until the first of any of the following events: transfer to a non-CSSCD clinic, last required routine CSSCD visit, last special study visit, initiation of chronic blood transfusion therapy, initiation of hydroxyurea, cerebrovascular event, bone marrow transplantation, or death.
Definitions
Acute chest syndrome. An episode of ACS was defined as a new pulmonary infiltrate demonstrable on chest radiograph or perfusion lung scan, or pleuritic chest pain with an abnormal perfusion lung scan, as per study protocol and as previously described in CSSCD studies.2,21
Painful episode. A painful episode was defined, as per CSSCD protocol, as pain in the extremities, back, abdomen, chest, or head for which no explanation other than SCA could be found, lasting at least 2 hours, leading to a clinic visit, and which was not classified as one of the following: skeletal/joint events, ACS, right upper quadrant pain, dactylitis, neurologic events, anemic episodes, febrile illness, and priapism.1
Asthma. A classification of asthma was defined for subjects older than 5 years of age as those who had a clinical diagnosis of asthma recorded during any annual study visit after 5 years of age, who presented with an acute asthma event (determined by ICD-9 codes, 493.xx) during the study period, or who had use of asthma medications (generally bronchodilators) reported on a clinic visit form. Parent report of a clinical diagnosis of asthma was assessed on a history form and also on a pulmonary intake form at the time of pulmonary function testing. The question, “Does the patient currently carry a diagnosis of asthma?” was asked annually. Parent report and physician diagnosis of asthma are standard classifications of asthma in epidemiologic studies of asthma prevalence.7,22-25 An assumption is made that asthma, a chronic condition, is a lifelong illness.26
Lower airway obstruction. Lower airway obstruction (LAO) was determined by a FEV1/FVC ratio below the lower 95% confidence limit for a patient based on age, sex, race, and height18 on the first interpretable spirometry test after 6 years of age. The LAO was classified as mild if the FEV1 was greater than or equal to the lower bound of the 95% confidence interval (CI), and moderate to severe if lower than this value.
Outcome measures and statistical methods
Data analysis was performed in SAS, version 9.1 (SAS Institute, Cary, NC). Demographic parameters, including sex, age at PFT, and length of follow-up were compared between subjects classified by asthma or LAO using t tests and the Fisher exact test. Incidence rates of ACS episodes, painful events, and transfusions were estimated using generalized linear models assuming that counts of events followed a negative binomial distribution with scale parameter estimated by maximum likelihood. Results were confirmed by Poisson regression with a correction for over-dispersion. Times to first ACS and first pain episode were summarized using Kaplan-Meier product-limit estimates and tested by Cox regression. In addition to asthma classification, the models included the following covariates: (1) ACS rate and time to first ACS episode: age and lifetime average hemoglobin concentration, white blood cell count, and percent fetal hemoglobin2 ; and (2) pain episode rate and time to first pain episode: age, sex, and lifetime average hematocrit level and percent fetal hemoglobin.1 Laboratory values (lifetime average hemoglobin, white blood cell count, and percent fetal hemoglobin) were determined based on the average of all values during follow-up, excluding laboratory values during events and percent hemoglobin F before age 2 years. Mean length of hospitalization and requirement for transfusion during ACS events were analyzed in a linear mixed model and a generalized linear model for correlated data, respectively, assuming that repeat observations on a patient were exchangeable. The Fisher exact test was used to test the association between asthma and LAO.
Results
Demographics
A total of 291 African American children with hemoglobin SS were enrolled in the study and followed for a total of 4062 patient years. The cohort consisted of 52% males (150/291) and the mean length of follow-up for clinical events was 11.0 years. In the cohort, 49 children (16.8%) had asthma; 92% were classified by a physician diagnosis and 8% by documentation of an acute asthma event. The average age of asthma classification was 6.4 years and the average number of asthma assessments per patient was 6.9. All patients with an asthma medication recorded also had a physician diagnosis of asthma or an acute asthma event recorded. Sex, age at first asthma assessment, and length of follow-up were not different between those with and without asthma (Table 1).
Demographic features of patients with sickle cell anemia with and without asthma
. | Asthma . | No asthma . | P . |
|---|---|---|---|
| No. patients | 49 | 242 | |
| Sex, no. (%) | .27 | ||
| Male | 29 (59) | 121 (50) | |
| Female | 20 (41) | 121 (50) | |
| Age at entry, mean (range), y | 0.27 (0.29-0.50) | 0.25 (0.22-0.50) | .48 |
| Asthma Dx age, mean (range), y* | 6.2 (5.0-11.7) | 6.4 (5.0-11.6) | .29 |
| Follow-up, mean (range), y† | 11.7 (0-19.0) | 10.9 (0-19.6) | .23 |
| LAO by spirometry, no. (%)‡ | 13 (38) | 9 (7) | < .001 |
. | Asthma . | No asthma . | P . |
|---|---|---|---|
| No. patients | 49 | 242 | |
| Sex, no. (%) | .27 | ||
| Male | 29 (59) | 121 (50) | |
| Female | 20 (41) | 121 (50) | |
| Age at entry, mean (range), y | 0.27 (0.29-0.50) | 0.25 (0.22-0.50) | .48 |
| Asthma Dx age, mean (range), y* | 6.2 (5.0-11.7) | 6.4 (5.0-11.6) | .29 |
| Follow-up, mean (range), y† | 11.7 (0-19.0) | 10.9 (0-19.6) | .23 |
| LAO by spirometry, no. (%)‡ | 13 (38) | 9 (7) | < .001 |
Age is the time when asthma diagnosis was first assessed.
Follow-up is between date of entry and the earliest of transfer away from a study clinic, last routine study visit, last special study visit, initiation of chronic blood transfusion therapy, initiation of hydroxyurea, cerebrovascular event, bone marrow transplantation, or death.
Excluding 64 subjects (13 with asthma, 51 without asthma) with apparent restrictive disease.
Asthma and sickle cell anemia–related morbidity in children
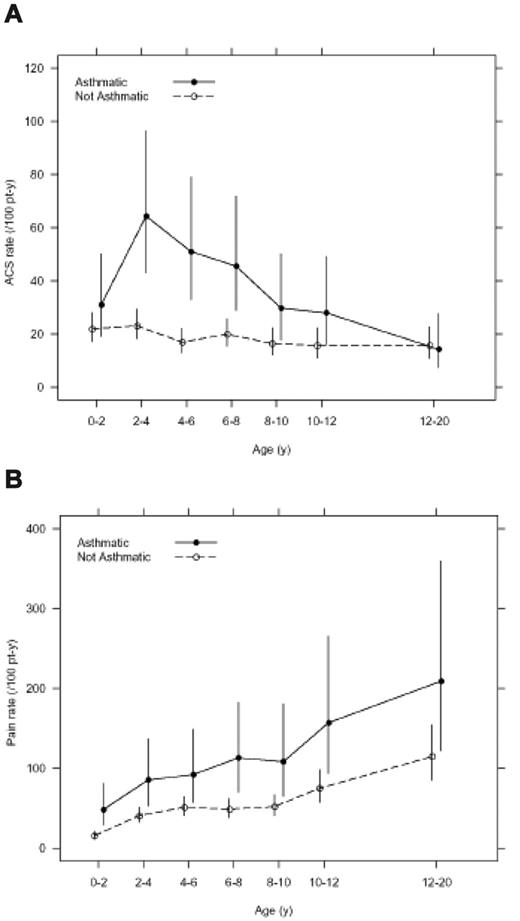
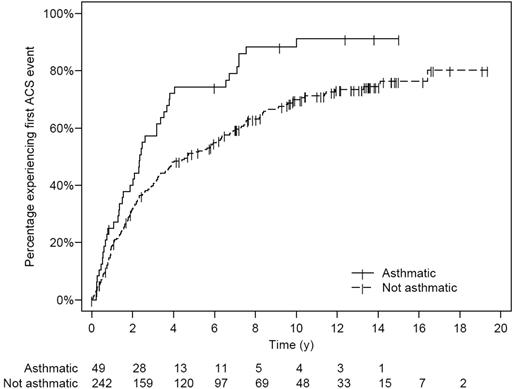
Asthma was associated with an increase in SCA-related morbidity. Children with SCA and a clinical diagnosis of asthma had nearly twice as many episodes of ACS (0.39 episodes per patient year versus 0.20 episodes per patient year, P < .001) and more frequent painful episodes (1.39 episodes per patient year versus 0.47 episodes per patient year, P < .001) when compared with children without asthma even after controlling for previously identified risk factors (Table 2 and Figure 2). Children with SCA and asthma were also younger at the time of their first ACS episode, with a median age of 2.4 years, compared with 4.6 years of age (hazard ratio 1.64, 95% CI 1.13 to 2.39, P = .010; Table 2 and Figure 3), and required more transfusions (1.00 per patient year vs 0.60 per patient year, P = .02) than children with SCA without asthma (Table 2). Comparison of age-specific rates of ACS indicates consistently higher rates of ACS in children with asthma until age 12 years, with the greatest difference at age 2 to 4 years (Figure 2A). Age-specific pain rates were greater in children with asthma when compared with children with SCA without asthma throughout the age range (Figure 2B).
Clinical events in children with sickle cell anemia with and without asthma
. | Asthma . | No asthma . | P . |
|---|---|---|---|
| No. patients | 49 | 242 | |
| Acute chest syndrome (ACS), events per patient year | 0.39 | 0.20 | < .001* |
| Median time to first ACS, y | 2.4 | 4.6 | .01† |
| Pain, no. events per patient year | 1.39 | 0.47 | < .001‡ |
| Transfusion, no. events per patient year | 1.00 | 0.60 | .02 |
. | Asthma . | No asthma . | P . |
|---|---|---|---|
| No. patients | 49 | 242 | |
| Acute chest syndrome (ACS), events per patient year | 0.39 | 0.20 | < .001* |
| Median time to first ACS, y | 2.4 | 4.6 | .01† |
| Pain, no. events per patient year | 1.39 | 0.47 | < .001‡ |
| Transfusion, no. events per patient year | 1.00 | 0.60 | .02 |
Incidence rates compared by negative binomial regression controlling for age at time of asthma diagnosis and lifetime average hemoglobin concentration, white blood cell count, and percent fetal hemoglobin.
Median time to first event estimated by Kaplan-Meier, P value from Cox regression controlling for age at time of asthma diagnosis and lifetime average hemoglobin concentration, white blood cell count, and percent fetal hemoglobin.
Incidence rates compared by negative binomial regression controlling for age at time of asthma diagnosis, sex, and lifetime average hematocrit and percent fetal hemoglobin.
When comparing children with asthma to those without asthma, no significant difference existed between the 2 groups in the age of onset of the first pain episode (median 3.5 years vs 4.7 years of age, hazard ratio 1.35, 95% CI 0.92 to 1.96, P = .12), length of hospitalization for ACS episode (mean 5.4 days vs 6.1 days, P = .14), or transfusion required during an ACS episode (odds 0.38 versus 0.40; odds ratio 1.06, 95% CI 0.71 to 1.59, P = .78).
Relationship between asthma and lower airway obstruction
LAO by spirometry was associated with a clinical diagnosis of asthma. A total of 227 patients in the cohort had spirometry done during the study period. Sixty-four children had evidence of restrictive lung disease and were removed from analyses of LAO because of lack of confirmation with lung volume testing. In the remaining cohort (n = 163), 22 children (9.7%) had LAO; 7 had mild cases and 15 had moderate to severe cases. Thirteen of the 22 children with LAO (59.1%) had a diagnosis of asthma compared with 21 (14.9%) of 141 of those without LAO (odds ratio 8.25, exact 95% CI 3.1 to 21.7, P < .001 by Fisher exact test).
Age-specific incidence of acute chest syndrome (ACS) and pain events classified by clinical asthma status in the infant sickle cell anemia (SCA) cohort. (A) Overall incidence rate of ACS events is higher in children with SCA and asthma (0.39 events per patient year) when compared with children with SCA and without asthma (0.20 events per patient year; P < .001). (B) Overall incidence rate of painful events is higher in children with SCA and asthma (1.39 events per patient year) when compared with children with SCA and without asthma (0.47 events per patient year, P < .001). Line segments are point-wise exact 95% confidence intervals.
Age-specific incidence of acute chest syndrome (ACS) and pain events classified by clinical asthma status in the infant sickle cell anemia (SCA) cohort. (A) Overall incidence rate of ACS events is higher in children with SCA and asthma (0.39 events per patient year) when compared with children with SCA and without asthma (0.20 events per patient year; P < .001). (B) Overall incidence rate of painful events is higher in children with SCA and asthma (1.39 events per patient year) when compared with children with SCA and without asthma (0.47 events per patient year, P < .001). Line segments are point-wise exact 95% confidence intervals.
Discussion
Few modifiable risk factors for pain or ACS have been identified. As reported here, asthma is a significant risk factor for both ACS and painful episodes. This finding confirms previous data that suggest an association between asthma and ACS. Further, a novel finding from this study is the association between pain and asthma. Based on the pathogenesis of asthma and the prevalence of airway obstruction and airway lability, ventilation-perfusion mismatching may result in local tissue hypoxia, promote increased sickling of red blood cells, and initiate an ACS episode or a vaso-occlusive pain episode. This hypothesis is supported by the association between nocturnal hypoxemia and an increased rate of painful episodes in children with SCA.27
Kaplan-Meier plot of time to first ACS event in the sickle cell anemia (SCA) infant cohort. Numbers below the x-axis list the number of children with SCA at risk at each 2-year interval. Exact log-rank, P = .002.
Kaplan-Meier plot of time to first ACS event in the sickle cell anemia (SCA) infant cohort. Numbers below the x-axis list the number of children with SCA at risk at each 2-year interval. Exact log-rank, P = .002.
An association between asthma and ACS may be expected given the significant overlap among symptoms (chest pain, shortness of breath) and clinical findings (fever, cough, increased respiratory effort, oxygen desaturation). We cannot determine definitively if patients with asthma have more episodes of ACS or if patients with multiple ACS episodes are more likely to have a diagnosis of asthma. Regardless of the direction of causality, evidence suggests an important association between asthma, a condition modifiable with medical therapy, and ACS, a significant complication of SCA associated with morbidity and mortality. The diagnosis of asthma as a risk factor for pain was an unexpected finding, particularly given the observation that the definition of pain excluded any patient with a concomitant diagnosis of ACS. Children with asthma are at greater risk of hypoxemia, a known risk factor for pain; however, a cause and effect relationship between asthma exacerbations, concomitant hypoxemia, and subsequent painful episodes could not be evaluated in this study.
In part due to the poor understanding of the etiology and pathogenesis of ACS, few effective therapies have been developed for treatment.28,29 Bernini et al28 showed that a 2-day course of intravenous dexamethasone during an ACS episode had a beneficial effect, including a shorter hospital stay, but was complicated by a high readmission rate when compared with the placebo arm. An observational study has reported that albuterol aerosol treatments may improve the clinical status of patients with ACS.30 For many patients with ACS, therapy is primarily restricted to supportive strategies, often including supplemental oxygen and blood transfusion therapy. The findings of this study suggest that identifying and treating individuals with SCA and asthma with currently available asthma therapies may impact SCA-related morbidity. Additional studies are needed to determine the role of asthma therapy in the treatment of ACS.
Inherent limitations are present in this study. While data were collected prospectively as part of the CSSCD and asthma was assessed multiple times throughout the study, patients were included in the study only if followed beyond 5 years of age. The diagnosis of asthma is more difficult in infants and young children, and the impact of transient wheezing on lung function and morbidity is not established.19,20 The temporal relationship between an asthma exacerbation and ACS or pain episodes cannot be determined because the data collection was not designed to define whether asthma exacerbation occurred simultaneously, sequentially, or separately with either pain or ACS episodes. Children with SCA and asthma with more frequent ACS and painful episodes may represent a more severe phenotype. Additional factors may affect both the degree and characteristics of chest symptoms and sickle cell disease–related morbidity. Nevertheless, the identification of an association between a diagnosis of asthma and both ACS and pain is important. Taken together, these observations strongly suggest that asthma is associated with an increased incidence of SCA-related morbidity.
In summary, we provide further evidence that asthma is a potentially treatable risk factor associated with ACS and painful episodes. Future prospective studies to classify lung disease associated with SCA and determine the effectiveness of asthma management in preventing SCA-related morbidity and mortality are warranted.
Appendix
The following investigators participated in the Cooperative Study of Sickle Cell Disease: R. Johnson, Alta Bates Hospital, Oakland, CA; L. McMahon, Boston City Hospital, Boston, MA; O. Platt, Children's Hospital, Boston, MA; F. Gill and K. Ohene Frempong, Children's Hospital, Philadelphia, PA; G. Bray, J.F. Kelleher, and S. Leikin, Children's Hospital National Medical Center, Washington, DC; E. Vichinsky and B. Lubin, Children's Hospital, Oakland, CA; A. Bank and S. Piomelli, Columbia Presbyterian Hospital, New York, NY; W. Rosse, J. Falletta, and T.R. Kinney, Duke University, Durham, NC; L. Lessin, George Washington University, Washington, DC; J. Smith and Y. Khakoo, Harlem Hospital, New York, NY; R.B. Scott, O. Castro, and C. Reindorf, Howard University, Washington, DC; H. Dosik, S. Diamond, and R. Bellevue, Interfaith Medical Center, Brooklyn, NY; W. Wang and J. Wilimas, LeBonheur Children's Hospital, Memphis, TN; P. Milner, Medical College of Georgia, Augusta, GA; A. Brown, S. Miller, R. Rieder, and P. Gillette, State University of New York Downstate Medical Center, Brooklyn, NY; W. Lande, S. Embury, and W. Mentzer, San Francisco General Hospital, San Francisco, CA; D. Wethers and R. Grover, St Luke's-Roosevelt Medical Center, New York, NY; M. Koshy and N. Talishy, University of Illinois, Chicago, IL; C. Pegelow and P. Klug, University of Miami, Miami, FL; M. Steinberg, University of Mississippi, Jackson, MS; A. Kraus, University of Tennessee, Memphis, TN; C. Dampier, Wyler Children's Hospital, Chicago, IL; H. Pearson and A.K. Ritchey, Yale University, New Haven, CT; S. McKinlay, D. Gallagher, and D. Brambilla, New England Research Institute, Watertown, MA; and M. Gaston and C. Reid, National Heart, Lung, and Blood Institute, Bethesda, MD.
Prepublished online as Blood First Edition Paper, May 11, 2006; DOI 10.1182/blood-2006-01-011072.
Supported in part by the Doris Duke Charitable Foundation, New York, NY (J.H.B., M.R.D.), and by the National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, MD, contract N01-HB-47 110, RO1 HL079 937 (M.R.D., R.C.S.), T32 HL07 873 (J.H.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The Cooperative Study of Sickle Cell Disease was conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with site investigators. This manuscript was not prepared in collaboration with investigators of the Cooperative Study of Sickle Cell Disease and does not necessarily reflect the opinions or views of the Cooperative Study of Sickle Cell Disease or the National Heart, Lung, and Blood Institute. We would like to thank Wayne Morgan, Julio Fontan, Susan Redline, and Avijit Datta for the helpful comments and review of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal