Abstract
The complex molecular mechanisms that drive endothelial cell movement and the formation of new vessels are poorly understood and require further investigation. Eph receptor tyrosine kinases and their membrane-anchored ephrin ligands regulate cell movements mostly by cell–cell contact, whereas the G-protein–coupled receptor CXCR4 and its unique SDF-1 chemokine ligand regulate cell movement mostly through soluble gradients. By using biochemical and functional approaches, we investigated how ephrinB and SDF-1 orchestrate endothelial cell movement and morphogenesis into capillary-like structures. We describe how endogenous EphB2 and EphB4 signaling are required for the formation of extracellular matrix–dependent capillary-like structures in primary human endothelial cells. We further demonstrate that EphB2 and EphB4 activation enhance SDF-1–induced signaling and chemotaxis that are also required for extracellular matrix–dependent endothelial cell clustering. These results support a model in which SDF-1 gradients first promote endothelial cell clustering and then EphB2 and EphB4 critically contribute to subsequent cell movement and alignment into cord-like structures. This study reveals a requirement for endogenous Eph signaling in endothelial cell morphogenic processes, uncovers a novel link between EphB forward signaling and SDF-1–induced signaling, and demonstrates a mechanism for cooperative regulation of endothelial cell movement.
Introduction
The Eph (erythropoietin-producing hepatocellular carcinoma) receptors comprise a large family of tyrosine kinases known to participate in the development of the vascular and nervous systems.1 The family of Eph receptors consists of 14 members divided into A and B subclasses, distinguished on the basis of preferential affinity for glycosylphosphatidylinositol (GPI)–linked (A-class) or transmembrane (B-class) ephrin ligands. Within each A and B subclass, Eph receptor–ephrin ligand interactions are promiscuous, though the binding affinities differ considerably.2,3 For example, EphB4 preferentially binds ephrin B2 and shows only weak binding to ephrinB1 and ephrin B3. Exceptions to class-restricted interactions include ephrin A5, which can bind to EphB2 at high concentrations,2,4 and ephrin B ligands, which can bind to EphA4.2,3,5
Knockout mice lacking ephrin B2 or EphB4 and a proportion of mice lacking EphB2 and EphB3 displayed severe defects in the embryonic vascular system and died at the embryo stage.6-8 Mural cell–specific inactivation of ephrin B2 results in perinatal death, and mice display edema and extensive hemorrhaging in the skin.9 After birth, EphB receptors and ephrin B ligands continue to be expressed by endothelial cells from various tissues; EphB2 and ephrin B2 display some selectivity for the arterial endothelium, whereas EphB4 is expressed in arterial and venous endothelium.10 Functional assays in vitro have provided evidence that several EphB receptors and ephrin B ligands are active in human endothelial cells. Soluble dimeric forms of ephrin B ligands and EphB receptors, including Ephrin B1-Fc, ephrin B2-Fc, EphB4-Fc, and EphB1-Fc, promoted the growth, sprouting, migration, or assembly of capillary-like structures by various human endothelial cells,6,11-16 presumably by activating forward or reverse signaling. However, ephrin B2-Fc also produced repulsive signals and inhibited endothelial cell sprouting while activating EphB4 forward signaling,15 and it reduced VEGF-induced endothelial cell migration and growth.17
One important feature of Eph receptors and ephrin ligands is that they interact through cell–cell contact because they are bound to the plasma membrane, and they activate bidirectional intracellular signaling emanating from both the receptor (forward signaling) and the ligand (reverse signaling).18-20 When appropriately activated, EphB receptor and ephrin B ligands become phosphorylated on tyrosine residues in their cytoplasmic domain and form complexes with various adaptor proteins, including PDZ and Src homology 2 (SH2) domain–containing proteins, resulting in crosstalk between Eph signaling and other signaling pathways.1,20-23 One example of crosstalk involves ephrin B reverse signaling and the G-protein–coupled receptor CXCR4 signaling in primary cerebellar granule cells through the adaptor PDZ-RGS3 protein, resulting in functional inactivation of CXCR4 and failure to migrate in response to SDF-1.24
CXCR4 and its unique ligand, SDF-1, are required for the normal development of the cardiovascular, hematopoietic, and nervous systems.25-29 Mice with targeted deletions of CXCR4 or SDF-1 die in utero and display similar defects in the ventricular septum of the heart, the cerebellum, and the vasculature supplying the gastrointestinal tract.25,27,29,30 Postnatally, vascular endothelial cells constitutively express CXCR4, and some cells also constitutively express and secrete SDF-1.31-33 Functional studies in vitro and in vivo have demonstrated that SDF-1/CXCR4 plays critical roles in the regulation of endothelial cell branching morphogenesis and angiogenesis.33-36
In this study, we have explored the possibility that ephrin B/EphB and SDF-1/CXCR4 may coordinately regulate endothelial cell movement and other functions. We report that endogenous EphB2 and EphB4 signaling are required for the formation of capillary-like vascular structures and that EphB2 and EphB4 activation enhances SDF-1–induced signaling and chemotaxis in endothelial cells. These results provide evidence of signaling and functional cooperation between the receptors EphB2 and EphB4 and the chemokine SDF-1 in endothelial cells.
Materials and methods
Cells and cell cultures
Human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HDMECs; Emory University, Atlanta, GA) from neonatal foreskin were propagated through passage 6, as described.33,37 Human aortic endothelial cells (HAECs; Cambrex Bio Science, Walkersville, MD) were propagated in “one endothelial cell medium” (Cambrex Bio Science). Cell lines SK-N-MC, SK-NEP-1, A-204, and NIH3T3 fibroblasts (ATCC, Manassas, VA) were cultured, as described.38 Brain tissue was obtained from the Laboratory of Pathology of the National Cancer Institute (Bethesda, MD).
Chemokines, antibodies, and peptides
Recombinant (Escherichia coli–derived) human SDF-1α (SDF-1α), mouse ephrin B1-Fc chimera, mouse ephrin B2-Fc chimera, human leptin receptor-Fc chimera, affinity-purified goat IgG antibodies to mouse EphB2, mouse EphB3, mouse EphB4, mouse ephrin B1, mouse ephrin B2, human ephrin B3, and rat monoclonal antibody to mouse EphB6 were from R&D Systems (Minneapolis, MN). Affinity-purified goat anti–actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit IgG antibody to EphB1 C-terminal peptide was from Stratagene (La Jolla, CA). Texas Red–conjugated affinity-purified donkey anti–goat IgG was from Jackson ImmunoResearch (West Grove, PA). Anti–phosphotyrosine mouse monoclonal antibody (clone 4G10), anti–phospho-Akt (Ser473) rabbit monoclonal antibody (clone 193H12), anti–phospho-Akt rabbit polyclonal antibody (Ser473), and anti–Akt rabbit polyclonal antibody were from Cell Signaling Technology (Beverly, MA). FITC-conjugated goat anti–rabbit IgG (heavy and light chains) was from Molecular Probes (Eugene, OR). Horseradish peroxidase (HRP)–conjugated rabbit anti–goat IgG-Fc was from Calbiochem (La Jolla, CA), and HRP-conjugated donkey anti–mouse IgG and donkey anti–rabbit IgG antibodies were from Amersham Pharmacia Biotech (Piscataway, NJ). The SNEW peptide (SNEWIQPRLPQH) and scrambled peptide SCR-EPQ (EPQNHSWPIRQL), TNYL-RAW peptide (YNYLFSPNGPIARAW), and scrambled peptide SCR-WTL (WTLAIFARNYNGPSP)39 were synthesized and purified (95% purity) by Sigma Genosys (The Woodlands, TX). CTCE9908 peptide (KGVSLSYR-KCONH2-RYSLSVGK) was a gift of Chemokine Therapeutics (Vancouver, BC, Canada); AMD3100 was from the AIDS Research and Reference Reagent Program (NIH, Rockville, MD).40 Peptide concentrations were calculated based on OD280.
RNA isolation and reverse transcription–polymerase chain reaction
Total RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH). cDNA was synthesized from 1 μg total RNA (SuperScript preamplification system; Gibco-BRL, Carlsbad, CA). Amplifications (28-30 cycles) were performed in a 20-μL reaction mixture, as described.33 Primer sequences for EphB1, EphB2, EphB3, EphB4,17 EphB6,41 ephrin B2,17 ephrin B3,41 and GAPDH33 were described. Primers for ephrin B1 were as follows: sense, TCTGATGGCAAGCATGAGAC; antisense, CGTCATCTTCCTGCTCATCA. PCR products were loaded onto 1.8% agarose gels (NuSieve agarose; FMC, Rockland, ME) and were visualized with ethidium bromide.
Western blot analysis and immunoprecipitation
Cell lysates were prepared in tricine–SDS sample buffer (Novex, San Diego, CA). Brain tissue was treated with NP-40 buffer, sonicated, and solubilized in tricine–SDS sample buffer. Protein (50 μg) was boiled, separated through precast polyacrylamide gels (8% or 10%-20%; Novex), and blotted onto nitrocellulose membranes. After blocking, membranes were probed with primary antibodies (18 hours, 4°C), washed, and probed (1 hour, room temperature) with secondary antibodies (HRP-conjugated rabbit anti–goat, donkey anti–mouse, or donkey anti–rabbit). Membranes were stripped and restained after complete removal of signal. Immunoreactive bands were detected by enhanced chemiluminescence (ECL kit; Amersham Pharmacia Biotech, Uppsala, Sweden) and scanned.
Immunoprecipitation of EphB2 and EphB4 was carried out as previously described.39 Cells were lysed in modified RIPA buffer (150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40 [NP-40], 0.5% Na deoxycholate, 0.1% SDS, 20 mM Tris, pH 8.0) with protease inhibitors (Roche Complete; Roche Diagnostic, Indianapolis, IN) and 1 mM sodium orthovanadate. Goat anti–EphB2 or goat anti–EphB4 antibodies (3 μg; R&D Systems) were added (18 hours, 4°C) to precleared cell lysates (150 μg protein). EphB2- or EphB4-antibody complexes were precipitated with protein G–Sepharose beads (50 μL; Amersham Pharmacia Biotech), and the precipitates were eluted by boiling in 2 × SDS sample buffer (Novex), separated by SDS-PAGE electrophoresis (8%; Novex), and blotted onto nitrocellulose membranes. The membranes were immunoblotted with anti–phosphotyrosine mouse monoclonal antibody (4G10; Cell Signaling Technology) and peroxidase-conjugated donkey anti–mouse IgG antibody (Amersham Pharmacia Biotech). Membranes were reprobed with goat anti–EphB2 or anti–EphB4 goat antibodies (R&D Systems).
Immunofluorescence microscopy and flow cytometry
HUVECs (10-50 × 103 cells/mL) were incubated (0.75-18 hours, 37°C) onto gelatin (0.2%) or Matrigel (Collaborative; BD PharMingen, San Diego, CA)–coated glass chamber slides (Lab-Tech, Naperville, IL) in complete culture medium, washed, and fixed with 1% formaldehyde. Fixed cells were incubated (45 minutes at 4°C) with affinity-purified goat IgG antibodies to murine EphB2 or EphB4 (1 μg/mL; R&D Systems) or control goat IgG (1 μg/mL; Chemicon International, Temecula, CA) and DAPI (4,6 diamidino-2-phenylindole), followed by washing and incubation with Texas Red–conjugated affinity-purified donkey anti–goat IgG (1 μg/mL; Jackson Immuno Research). F-actin was detected with Alexa 488–conjugated antibody to phalloidin (Molecular Probes).33 Confocal images were acquired with a Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NJ) with a Zeiss acioverto 100M inverted microscope and 50-mW argon UV laser tuned to 364 nm and a 25-mW argon visible laser tuned to 488 nm. Either a 10×/0.3 NA Plan-Neofluar dry objective of a 25×/0.8 NA Plan-Neofluar oil immersion objective was used at various digital zoom settings. Emission signals after sequential excitation of FITC and DAPI by the 488-nm laser line and 364-nm laser line were collected with a BP505-550 filter and a BP385-470 filter, respectively, using individual photomultipliers. Images were imported into Adobe Photoshop (Adobe Systems, San Jose, CA).
For flow cytometric detection of phospho-Akt, HUVECs were detached (2 mM EDTA in PBS), washed in PBS, and suspended in 250 μL BD Cytofix/Cytoperm solution (Becton Dickinson, Franklin Lakes, NJ) for 20 minutes at 4°C. Cells were washed, blocked (10 minutes, room temperature) with 0.5% BSA in PBS, and stained (30 minutes, room temperature) with rabbit monoclonal antibody to phospho-Akt (Ser473193H12; 1:100 dilution; Cell Signaling Technology). Cells were washed and incubated (30 minutes, room temperature) with FITC-conjugated goat anti–rabbit IgG (Molecular Probes).
Matrigel cord formation and migration assays
Cord formation assay was carried out as described.37 Endothelial cells preincubated (37°C, 20 minutes) with or without peptides were plated (40 000-75 000 cells) onto 24-well tissue culture plates precoated with 200 to 300 μL solidified Matrigel (Collaborative; BD PharMingen) in complete culture medium. Cells were photographed with a digital camera (Retiga 1300; Qimaging, Burnaby, BC, Canada) under phase-contrast microscopy (1 × 51 with a 10 × 0.25 PhL lens; Olympus Optical, Melville, NY), and images were obtained with IPLab for Windows software (Scanalytics, Fairfax, VA) imported into Adobe Photoshop. Network formation was measured by counting the number of cord angles per field (each field was defined as the area visualized by a 4 × magnification lens).
For video microscopy, HUVECs (40 000-50 000 cells/chamber) were dispersed on Matrigel-coated LabTek chambers (Nalge Nunc, Naperville, IL) in complete culture medium. Time-lapse microscopy was performed with an inverted confocal scanning microscope (LSM510 META; Carl Zeiss, Thornwood, NJ) equipped with a heated stage (maintaining 37°C temperature and 5% CO2 with a CTI-Controller 3700 digital; Zeiss) and a 10 × (0.3 NA) plan apochromat objective. Images were recorded at 5-minute intervals over 13 to 14 hours using LSM510 software and were processed to Quick-time format using MetaMorph software (Universal Imaging, Glendale, WI). One second of the video corresponded to approximately 30 minutes of real time.
Migration assays were performed as described33 using 0.2% gelatin-coated polycarbonate filters (pore size, 8 μm) of 24-well transwells (Costar, Cambridge, MA). Chemotaxis medium (RPMI 1640 containing 0.5% BSA and 10 mM HEPES) alone or with SDF-1α (50-100 ng/mL) was added to the bottom chamber. HUVECs (0.5-1.0 × 106 cells/well) were placed in the upper chambers and were incubated (16-20 hours, 37°C). Viable cells from the lower chamber were counted.
Statistical analysis
Group differences were evaluated by Student t test; P values below .05 were considered significant.
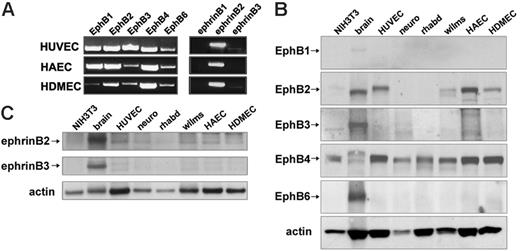
Expression of EphB receptors and ephrin B ligands in primary human endothelial cells from the umbilical vein, aorta, and dermal microvasculature. (A) EphB1, EphB2, EphB3, EphB4, and EphB6 receptor and ephrin B1, ephrin B2, and ephrin B3 ligand mRNA detected by semiquantitative RT-PCR with specific primer pairs in HUVECs, HAECs, and HDMECs. Representative results from 3 experiments. (B) EphB1, EphB2, EphB3, EphB4, and EphB6 were detected by Western blotting with specific antibodies in cell lysates from murine NIH3T3 cells, human brain tissue (brain), HUVEC, human SK-N-MC neuroblastoma cell line (neuro), human A-204 rhabdomyosarcoma cell line (rhabd), and human SK-NEP-1 Wilms tumor cell line (wilms), HAEC, and HDMEC. Results shown, which are representative of 4 independent immunoblotting experiments, reflect reprobing of a single membrane after stripping; sample loading was evaluated by reprobing for actin. (C) Immunoblotting analysis of ephrin B2 and ephrin B3 expression in HUVECs, HAECs, HDMECs, and other samples—murine NIH3T3 cells, human brain tissue (brain), human SK-N-MC neuroblastoma cell line (neuro), human A-204 rhabdomyosarcoma cell line (rhabd), and human SK-NEP-1 Wilms tumor cell line (wilms). A single membrane was used for immune detection; sample loading was evaluated by probing with antiactin antibodies. Results are representative of 3 immunoblotting experiments.
Expression of EphB receptors and ephrin B ligands in primary human endothelial cells from the umbilical vein, aorta, and dermal microvasculature. (A) EphB1, EphB2, EphB3, EphB4, and EphB6 receptor and ephrin B1, ephrin B2, and ephrin B3 ligand mRNA detected by semiquantitative RT-PCR with specific primer pairs in HUVECs, HAECs, and HDMECs. Representative results from 3 experiments. (B) EphB1, EphB2, EphB3, EphB4, and EphB6 were detected by Western blotting with specific antibodies in cell lysates from murine NIH3T3 cells, human brain tissue (brain), HUVEC, human SK-N-MC neuroblastoma cell line (neuro), human A-204 rhabdomyosarcoma cell line (rhabd), and human SK-NEP-1 Wilms tumor cell line (wilms), HAEC, and HDMEC. Results shown, which are representative of 4 independent immunoblotting experiments, reflect reprobing of a single membrane after stripping; sample loading was evaluated by reprobing for actin. (C) Immunoblotting analysis of ephrin B2 and ephrin B3 expression in HUVECs, HAECs, HDMECs, and other samples—murine NIH3T3 cells, human brain tissue (brain), human SK-N-MC neuroblastoma cell line (neuro), human A-204 rhabdomyosarcoma cell line (rhabd), and human SK-NEP-1 Wilms tumor cell line (wilms). A single membrane was used for immune detection; sample loading was evaluated by probing with antiactin antibodies. Results are representative of 3 immunoblotting experiments.
Results
EphB receptor and ephrin B ligand expression in human endothelial cells
Five known EphB receptors (EphB1-EphB4, EphB6) and 3 ephrin ligands (ephrin B1, ephrin B2, ephrin B3) are known to exist in mammals; some are expressed in primary human endothelial cells derived from different tissues.10-12,17,42,43 Reverse transcription–polymerase chain reaction (RT-PCR) analysis showed expression of all 5 EphB receptors in human umbilical vein endothelial cells (HUVECs), human aortic endothelial cells (HAECs), and human dermal microvascular endothelial cells (HDMECs) (Figure 1A). EphB1, EphB3, and EphB6 expression levels varied among HUVECs, HDMEDs, and HAECs, as judged by relative mRNA band intensities. Ephrin B2 was expressed in HUVECs, HAECs, and HDMECs, but expression of ephrin B1 and ephrin B3 was minimal. We used specific antibodies to examine the expression of EphB and ephrin B proteins. Specific bands attributable to EphB2 and EphB4 were detected in HUVECs, HAECs, and HDMECs but not bands clearly attributable to EphB1, EphB3, and EphB6 (Figure 1B). All EphB proteins were detected in human brain tissue extracts (brain)44 ; EphB1 was detected only at low levels. EphB2- and EphB4-related bands appeared to be of different sizes in brain tissue and endothelial cells, presumably because of different glycosylation patterns (Figure 1B). EphB4 protein was detected in murine NIH3T3 fibroblasts, SK-N-MC neuroblastoma (neuro),45 A-204 rhabdomyosarcoma (rhabd), and SK-NEP-1 Wilms tumor (Wilms) cell lines. EphB2 protein was detected in the Wilms tumor–derived cell line (Figure 1B).
Consistent with RT-PCR results, we detected ephrin B2 protein in HUVECs, HAECs, and HDMECs but not ephrin B3 (Figure 1C) or ephrin B1 (data not shown). We conclude that primary human endothelial cells from the umbilical vein, the aorta, and the dermal microvascular endothelium express the EphB2 and EphB4 receptors and the ephrin B2 ligand.
EphB2 and EphB4 receptor activity in HUVECs
When cultured in low serum medium on gelatin-coated dishes, HUVECs did not express a constitutively active EphB2 receptor detected by anti–phosphotyrosine antibodies after EphB2 immunoprecipitation (Figure 2A). By contrast, EphB2 was phosphorylated in HUVECs after 10- to 30-minute stimulation with ephrin B1-Fc (1 μg/mL), a dimeric form of the ephrin B1 ligand (Figure 2A). Ephrin B1-Fc preclustered by incubation with a monoclonal antibody to human IgG1-Fc11 also induced EphB2 phosphorylation similar in kinetics and magnitude to that induced by non–preclustered ephrin B1/Fc (data not shown).
We tested whether the SNEW peptide, which was shown to inhibit EphB2 receptor activity in COS cells,39 also inhibited EphB2 receptor activity in HUVECs. The SNEW peptide (100 μM), but not the control peptide (SCR-EPQ control, 100 μM), antagonized ephrin B1-Fc–induced phosphorylation of EphB2 in HUVECs (Figure 2B).
Ephrin B receptors and ligands are promiscuous, but their binding affinities vary.3 For example, EphB4 receptors are preferentially activated by ephrin B2 ligands.23,46 As shown (Figure 2C), ephrin B2-Fc induced EphB4 phosphorylation in HUVECs after 30 minutes, which was abrogated by the specific EphB4 peptide inhibitor TNYL-RAW (TNYL, 100 μM) but not by the SNEW peptide (100 μM) or a control peptide (SCR-WTL, 100 μM; Figure 2C and data not shown). In addition to promoting EphB4 phosphorylation, ephrin B2-Fc promoted EphB2 phosphorylation in HUVECs (Figure 2D). These results provide evidence that EphB2 and EphB4 receptors are functional in HUVECs and that EphB2 and EphB4 activation is inhibited by the specific SNEW and TNYL-RAW peptides.
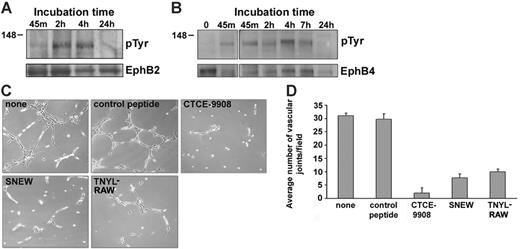
Detection of EphB2 and EphB4 phosphorylation in primary human endothelial cells. (A) Kinetics of EphB2 phosphorylation in HUVECs stimulated with ephrin B1-Fc. HUVECs were incubated in medium only or were treated with ephrin B1-Fc (1 μg/mL) for 10 minutes, 30 minutes, 2 hours, and 4 hours. Cell lysates (150 μg) were immunoprecipitated with goat IgG antibodies to EphB2, and the precipitates were immunoblotted with anti–phosphotyrosine mouse monoclonal antibodies (4G10). The membrane was reblotted with goat IgG antibodies to EphB2 (results are representative of 3 experiments). (B) Effect of the SNEW peptide (100 μM) on EphB2 phosphorylation in HUVECs stimulated for 10 minutes with ephrin B1-Fc (1 μg/mL) or with a control Fc-fusion protein (Fc, leptin receptor-Fc chimera, 1 μg/mL). As a control peptide (control), we used a scrambled form of SNEW (SCR-EPQ, 100 μM). EphB2 was immunoprecipitated, immunoblotted with anti–phosphotyrosine mouse monoclonal antibodies, and reblotted with goat IgG antibodies to EphB2 antibodies. (C) EphB4 phosphorylation was induced in HUVECs stimulated with ephrin B2-Fc (1 μg/mL). HUVECs were incubated for 30 minutes with a control Fc-fusion protein (Fc, leptin receptor-Fc chimera, 1 μg/mL) or with ephrin B2-Fc (1 μg/mL) in medium alone or in medium supplemented with the peptide TNYL-RAW (100 μM) or the peptide SNEW (100 μM). EphB4 was immunoprecipitated, immunoblotted with anti–phosphotyrosine mouse monoclonal antibodies, and reblotted with goat IgG antibodies to EphB4 antibodies. (D) EphB2 phosphorylation was induced in HUVECs after stimulation for 30 minutes with ephrin B2-Fc (1 μg/mL) but not with a control Fc-fusion protein (Fc, leptin receptor-Fc chimera, 1 μg/mL). Phosphorylated EphB2 was visualized after immunoprecipitation and immunoblotting with anti–phosphotyrosine mouse monoclonal antibodies; total EphB2 was visualized after reprobing with antibodies to EphB2.
Detection of EphB2 and EphB4 phosphorylation in primary human endothelial cells. (A) Kinetics of EphB2 phosphorylation in HUVECs stimulated with ephrin B1-Fc. HUVECs were incubated in medium only or were treated with ephrin B1-Fc (1 μg/mL) for 10 minutes, 30 minutes, 2 hours, and 4 hours. Cell lysates (150 μg) were immunoprecipitated with goat IgG antibodies to EphB2, and the precipitates were immunoblotted with anti–phosphotyrosine mouse monoclonal antibodies (4G10). The membrane was reblotted with goat IgG antibodies to EphB2 (results are representative of 3 experiments). (B) Effect of the SNEW peptide (100 μM) on EphB2 phosphorylation in HUVECs stimulated for 10 minutes with ephrin B1-Fc (1 μg/mL) or with a control Fc-fusion protein (Fc, leptin receptor-Fc chimera, 1 μg/mL). As a control peptide (control), we used a scrambled form of SNEW (SCR-EPQ, 100 μM). EphB2 was immunoprecipitated, immunoblotted with anti–phosphotyrosine mouse monoclonal antibodies, and reblotted with goat IgG antibodies to EphB2 antibodies. (C) EphB4 phosphorylation was induced in HUVECs stimulated with ephrin B2-Fc (1 μg/mL). HUVECs were incubated for 30 minutes with a control Fc-fusion protein (Fc, leptin receptor-Fc chimera, 1 μg/mL) or with ephrin B2-Fc (1 μg/mL) in medium alone or in medium supplemented with the peptide TNYL-RAW (100 μM) or the peptide SNEW (100 μM). EphB4 was immunoprecipitated, immunoblotted with anti–phosphotyrosine mouse monoclonal antibodies, and reblotted with goat IgG antibodies to EphB4 antibodies. (D) EphB2 phosphorylation was induced in HUVECs after stimulation for 30 minutes with ephrin B2-Fc (1 μg/mL) but not with a control Fc-fusion protein (Fc, leptin receptor-Fc chimera, 1 μg/mL). Phosphorylated EphB2 was visualized after immunoprecipitation and immunoblotting with anti–phosphotyrosine mouse monoclonal antibodies; total EphB2 was visualized after reprobing with antibodies to EphB2.
Contribution of EphB2 and EphB4 receptor activity to endothelial cell cord formation
When dispersed onto wells coated with Matrigel, a crude mixture of extracellular matrix proteins, HUVECs form cordlike structures.33,47,48 This morphogenic process, which occurs spontaneously during incubation in culture medium, recapitulates several steps in new vessel formation, including cell attachment to the extracellular matrix, cell movement, cell–cell adhesion, extension of lamellipodia and filopodia, and formation of cordlike structures.33,48 By 13- to 14-hour video microscopy, we monitored HUVEC formation of cordlike structures (Video S1, available on the Blood website; see the Supplemental Videos link at the top of the online article). Shortly after addition to Matrigel-coated chambers, HUVECs spread and begin to attach, as evidenced by their flattened appearance, apparently larger size, and presence of lamellipodia. Cells also start to move and, by 1 to 1.5 hours, join with neighboring cells to form many dispersed clusters composed of 2 to 5 cells. Between 1.5 and 4 hours, HUVECs within the clusters change from round to elongated, actively form new lamellipodia and filopodia, and move often vigorously while maintaining contact with each other. By approximately 4 hours, fragmented lines of various orientation and bends are formed, each composed of 2 to 5 elongated cells. Through constant movement of the cells and accelerated formation of filopodia, these fragmented lines become bridged to each other. As a result, by 7 to 8 hours, a network of endothelial cells forms in which individual cells appear attached to the extracellular matrix and anchored to each other.
We looked for potential changes in EphB2 (Figure 3A) and EphB4 (Figure 3B) phosphorylation during cord formation onto Matrigel. After 45-minute dispersion onto Matrigel, when HUVECs were attached to the matrix and began to come in contact with other cells, low-level EphB2 (Figure 3A) and EphB4 (Figure 3B) phosphorylation was detected. At 2 and 4 hours, when HUVECs clustered, changed shape from round to elongated, and generated fragmented, cordlike structures, EphB2 (Figure 3A) and EphB4 (Figure 3B) phosphorylation were detected. At 18 hours, when the network was established, EphB2 (Figure 3A) and EphB4 (Figure 3B) phosphorylation was low or undetectable. These results provide evidence for a temporal correlation between the occurrence of cell–cell contact and EphB2 and EphB4 activation during cord formation.
To test for a contribution of EphB2 or EphB4 signaling to HUVEC cord formation, we used the SNEW and TNYL-RAW peptides, which selectively block EphB2 (SNEW) or EphB4 (TNYL-RAW) receptor activation. The SNEW peptide (100 μM) and the TNYL-RAW peptide (100 μM) individually interfered with the ability of HUVECs to form the characteristic network of cordlike structures on Matrigel, but a control peptide (SCR-EPQ, 100 μM) did not (Figure 3C, representative images). Similarly, the addition of SNEW or TNYL-RAW peptides, but not of the control SCR-WTL (all at 100 μM) reduced Matrigel-dependent cord formation in HAECs and HDMECs (data not shown). Late addition of the SNEW or TNYL-RAW peptides (100 μM), as early as 45 minutes after the cells were dispersed onto the Matrigel, was ineffective at altering HUVEC cord formation (data not shown). Functional inactivation of CXCR4 by the peptide inhibitor CTCE-9908 (50 μg/mL; Figure 3C) and the synthetic compound AMD3100 (5 μg/mL; data not shown) prevented endothelial cell cord formation on Matrigel, as did the addition of soluble SDF-1α (1 μg/mL; data not shown). Additionally, in the presence of AMD3100 (5 μg/mL), EphB2 phosphorylation was minimal after 4-hour cell incubation on Matrigel (data not shown). Ephrin B1-Fc (1 μg/mL) added at the start has minimal effect on HUVEC cord formation (data not shown). Inhibition of endothelial cell network formation on Matrigel by the peptides SNEW, TNYL-RAW, and CTCE-9908 was reproducible and significant at 18 hours (P < .05; Figure 3D).
Analysis of EphB2 and EphB4 phosphorylation and function during endothelial cell cord formation on extracellular matrix. (A-B) HUVECs were incubated on Matrigel-coated wells for the indicated times. After incubation, cells were washed in PBS at 4°C and removed by trypsin digestion at 4°C, and cell lysates were immunoprecipitated with goat IgG antibodies to EphB2 (A) or EphB4 (B). Immunoprecipitates were immunoblotted with anti–phosphotyrosine mouse monoclonal antibody 4G10, and the membranes were reblotted with the immunoprecipitating antibodies to EphB2 or EphB4. Results derived from independent experiments, each representative of 3 experiments. (C) Effects of the peptides CTCE-9908, SNEW, and TNYL-RAW on Matrigel-dependent cord formation. HUVECs were incubated for 18 hours onto Matrigel-coated wells in medium alone (none), with control peptide SCR-WTL (100 μM), CTCE-9908 peptide (50 μg/mL), SNEW peptide (100 μM), or TNYL-RAW peptide (100 μM). Representative images from phase-contrast microscopy reflecting various degrees of cord formation after 18 hours of incubation (original magnification, ×5). Representative results from 5 experiments. (D) Quantitative analysis of HUVEC cord formation on Matrigel-coated wells under the same conditions listed in panel C and measured as a function of the number of vascular joints per visual field (magnification, ×10). Results reflect the mean ± SD of 3 independent experiments; in each experiment, 4 nonoverlapping fields were counted.
Analysis of EphB2 and EphB4 phosphorylation and function during endothelial cell cord formation on extracellular matrix. (A-B) HUVECs were incubated on Matrigel-coated wells for the indicated times. After incubation, cells were washed in PBS at 4°C and removed by trypsin digestion at 4°C, and cell lysates were immunoprecipitated with goat IgG antibodies to EphB2 (A) or EphB4 (B). Immunoprecipitates were immunoblotted with anti–phosphotyrosine mouse monoclonal antibody 4G10, and the membranes were reblotted with the immunoprecipitating antibodies to EphB2 or EphB4. Results derived from independent experiments, each representative of 3 experiments. (C) Effects of the peptides CTCE-9908, SNEW, and TNYL-RAW on Matrigel-dependent cord formation. HUVECs were incubated for 18 hours onto Matrigel-coated wells in medium alone (none), with control peptide SCR-WTL (100 μM), CTCE-9908 peptide (50 μg/mL), SNEW peptide (100 μM), or TNYL-RAW peptide (100 μM). Representative images from phase-contrast microscopy reflecting various degrees of cord formation after 18 hours of incubation (original magnification, ×5). Representative results from 5 experiments. (D) Quantitative analysis of HUVEC cord formation on Matrigel-coated wells under the same conditions listed in panel C and measured as a function of the number of vascular joints per visual field (magnification, ×10). Results reflect the mean ± SD of 3 independent experiments; in each experiment, 4 nonoverlapping fields were counted.
To confirm the results obtained in steady state experiments and to dissect potential differences in the inhibition of cord formation by the peptide inhibitors of CXCR4, EphB2, and EphB4, we used real-time videomicroscopy. If CXCR4 signaling accounted for cell clustering in response to cell-derived SDF-1 gradients, CTCE-9988 would be expected to impair HUVEC migration. If EphB2 and EphB4 became activated by cell–cell contact, the SNEW and TNYL-RAW peptides would be expected to inhibit events after the occurrence of cell clustering. If, however, EphB2 and EphB4 contributed to cell adhesion to the extracellular matrix, their inhibitors would impair early events during cord formation. In the presence of the CXCR4 inhibitor CTCE-9908 (50 μg/mL), the cells attached to Matrigel, but their movement toward each other to form cell clusters was markedly reduced in magnitude and was temporally delayed compared with control cells, such that few cell clusters were noted after approximately 7.5 hours (Video S2); such clusters were visible by 1.5 hours in control cells (Video S1). In spite of subsequent cell elongation and formation of cords from the few cell clusters, the overall network was incomplete. In the presence of the SNEW peptide (100 μM) or in the presence of TNYL-RAW peptide (100 μM), cell attachment and initial cell clustering appeared similar to those observed in control cells incubated in medium only (Videos S3, S4). Once clusters formed, however, cell elongation, movement, alignment into segments, and later connection of these segments onto a reticular pattern were substantially delayed and reduced in magnitude. These results confirm that CXCR4 plays an important role in promoting the initial endothelial cell movement and clustering on Matrigel and provide evidence for a critical contribution of EphB2 and EphB4 activation in promoting cord formation, particularly once the cells have clustered.
Modulation of EphB2 and EphB4 expression during endothelial cell cord formation
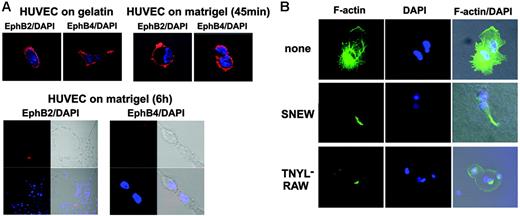
Given that endocytosis and cleavage of Eph and ephrin molecules have been described to follow their activation,1 we looked for potential changes of surface EphB2 and EphB4 expression during tube formation. We detected diffuse surface EphB2 and EphB4 on a proportion of HUVECs cultured onto gelatin-coated plates or incubated on Matrigel for 45 minutes (Figure 3A, top panel). After 6-hour incubation on Matrigel, when HUVECs made contact with each other and formed some cords, surface EphB2 and EphB4 were consistently undetectable (Figure 3A, bottom panel; representative images on the left depict the typical HUVEC network and on the right depict a higher magnification of cells within a cord). Surface EphB2 and EphB4 continued to be undetectable on HUVECs even after 18 hours on Matrigel, when the network structure was fully established (data not shown). Thus, EphB2 and EphB4 were down-regulated from the cell surface during endothelial cell cord formation.
Actin microfilaments organize into the so-called stress fibers during endothelial cell formation of cordlike structures.33 We examined whether EphB2 or EphB4 inactivation affects these characteristic changes. Confocal microscopy revealed the characteristic filamentous (F)–actin network spanning adjacent HUVECs incubated for 6 hours onto Matrigel (Figure 4B, top row; representative images reflect 2 adjacent cells). By contrast, F-actin staining was minimal or focally confined in HUVECs incubated for 6 hours onto Matrigel in the presence of the EphB2 peptide inhibitor SNEW (100 μM) or the EphB4 peptide inhibitor TNYL-RAW (100 μM), indicative of defective actin polymerization (Figure 4B; representative images reflect 2 adjacent cells each).
Surface EphB2 and EphB4 expression and actin polymerization during cord formation. (A) HUVECs were plated onto gelatin-coated or Matrigel-coated glass chamber slides and incubated at 37°C. After 45 minutes and 6 hours of incubation, cells were fixed with 1% formaldehyde, stained for EphB2 or EphB4 with specific goat IgG antibodies followed by Texas Red donkey anti–goat IgG and DAPI, and examined with a confocal system. Images were from phase-contrast and epifluorescence microscopy showing representative HUVECs cultured on gelatin (18 hours) or Matrigel (45 minutes and 6 hours) (original magnification, ×10 ×25). (B) HUVECs were plated onto Matrigel-coated glass chamber slides and were incubated at 37°C in medium alone, medium with the SNEW peptide (100 μM), or medium with the TNYL-RAW peptide (100 μM) for 6 hours and then stained with phalloidin-FITC and DAPI. Images from confocal epifluorescence microscopy show representative F-actin detection in HUVECs (original magnification, ×25).
Surface EphB2 and EphB4 expression and actin polymerization during cord formation. (A) HUVECs were plated onto gelatin-coated or Matrigel-coated glass chamber slides and incubated at 37°C. After 45 minutes and 6 hours of incubation, cells were fixed with 1% formaldehyde, stained for EphB2 or EphB4 with specific goat IgG antibodies followed by Texas Red donkey anti–goat IgG and DAPI, and examined with a confocal system. Images were from phase-contrast and epifluorescence microscopy showing representative HUVECs cultured on gelatin (18 hours) or Matrigel (45 minutes and 6 hours) (original magnification, ×10 ×25). (B) HUVECs were plated onto Matrigel-coated glass chamber slides and were incubated at 37°C in medium alone, medium with the SNEW peptide (100 μM), or medium with the TNYL-RAW peptide (100 μM) for 6 hours and then stained with phalloidin-FITC and DAPI. Images from confocal epifluorescence microscopy show representative F-actin detection in HUVECs (original magnification, ×25).
AKT phosphorylation by SDF-1 is enhanced by ephrin B1-Fc or ephrin B2-Fc
Given that CXCR4, EphB2, and EphB4 contributed to endothelial cell cord formation and that considerable interplay exists between Eph signaling and other signaling pathways,1,49,50 we examined whether interplay exists between SDF-1–induced and EphB2 or EphB4 signaling. SDF-1 stimulation leads to AKT phosphorylation in many cell types, including endothelial cells.51-53 Through flow cytometry, we detected phospho-AKT in approximately 14% of HUVECs stimulated for 15 minutes with SDF-1α (500 ng/mL), approximately 5% of HUVECs stimulated with ephrin B1-Fc (1 μg/mL) alone, and approximately 8% of cells stimulated with ephrin B2-Fc; less than 1% of unstimulated cells were positive (Figure 5A). Importantly, we detected phospho-Akt in approximately 44% and 67% of HUVECs costimulated for 15 minutes with SDF-1α (500 ng/mL) plus ephrin B1-Fc (1 μg/mL) or with SDF-1α (500 ng/mL) plus ephrin B2-Fc (1 μg/mL), respectively (Figure 5A). This synergy between SDF-1α and ephrin B1-Fc and SDF-1α and ephrin B2-Fc was also observed at SDF-1 concentrations of 50 and 100 ng/mL (data not shown) and was sustained for 30 minutes after stimulation, when SDF-1α and ephrin B1-Fc, individually, were only minimally stimulatory (data not shown).
Akt phosphorylation in HUVECs stimulated with SDF-1, ephrin B1-Fc, and ephrin B2-Fc. (A) Flow cytometric analysis of intracellular Akt in HUVECs incubated for 15 minutes in medium alone, SDF-1α (500 ng/mL), ephrin B1-Fc (1 μg/mL), ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL), or ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL). After permeabilization, Akt was detected by staining with a rabbit monoclonal antibody to phospho-Akt (Ser 473) followed by FITC-labeled goat anti–rabbit IgG antibodies. Background staining was set with FITC-labeled goat anti–rabbit IgG. FSC indicates forward scatter. Results are representative of 5 experiments. (B) Flow cytometric analysis of intracellular Akt. HUVECs were incubated for 15 minutes with ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) alone, with the SNEW peptide (100 μM) or with a control peptide (SCR-EPQ, a scrambled form of SNEW [100 μM]), with ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) alone, with the TNYL-RAW peptide (100 μM), or with a control peptide (SCR-WTL, a scrambled form of TNYL-RAW, 100 μM). (C, top left) After 3-hour starvation, HUVECs were treated with SDF-1α (500 ng/mL), ephrin B1-Fc (1 μg/mL), or ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) for 15 minutes. (C, top right) After 3-hour starvation, HUVECs were treated with SDF-1 (500 ng/mL), ephrin B2-Fc (1 μg/mL), or ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) for 15 minutes. (C, bottom) After overnight starvation, HUVECs were treated for 15 minutes with SDF-1 (100 ng/mL), ephrin B1-Fc (1 μg/mL), ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus SDF-1α (100 ng/mL), ephrin B2-Fc (1 μg/mL) plus SDF-1α (100 ng/mL), or with SDF-1 (100 ng/mL) plus ephrin B1-Fc (1 μg/mL) plus ephrin B2-Fc (1 μg/mL). Cell lysates were immunoblotted with a rabbit monoclonal antibody to phospho-Akt (Ser473) and were reblotted with rabbit antibodies against total Akt.
Akt phosphorylation in HUVECs stimulated with SDF-1, ephrin B1-Fc, and ephrin B2-Fc. (A) Flow cytometric analysis of intracellular Akt in HUVECs incubated for 15 minutes in medium alone, SDF-1α (500 ng/mL), ephrin B1-Fc (1 μg/mL), ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL), or ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL). After permeabilization, Akt was detected by staining with a rabbit monoclonal antibody to phospho-Akt (Ser 473) followed by FITC-labeled goat anti–rabbit IgG antibodies. Background staining was set with FITC-labeled goat anti–rabbit IgG. FSC indicates forward scatter. Results are representative of 5 experiments. (B) Flow cytometric analysis of intracellular Akt. HUVECs were incubated for 15 minutes with ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) alone, with the SNEW peptide (100 μM) or with a control peptide (SCR-EPQ, a scrambled form of SNEW [100 μM]), with ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) alone, with the TNYL-RAW peptide (100 μM), or with a control peptide (SCR-WTL, a scrambled form of TNYL-RAW, 100 μM). (C, top left) After 3-hour starvation, HUVECs were treated with SDF-1α (500 ng/mL), ephrin B1-Fc (1 μg/mL), or ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) for 15 minutes. (C, top right) After 3-hour starvation, HUVECs were treated with SDF-1 (500 ng/mL), ephrin B2-Fc (1 μg/mL), or ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) for 15 minutes. (C, bottom) After overnight starvation, HUVECs were treated for 15 minutes with SDF-1 (100 ng/mL), ephrin B1-Fc (1 μg/mL), ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus SDF-1α (100 ng/mL), ephrin B2-Fc (1 μg/mL) plus SDF-1α (100 ng/mL), or with SDF-1 (100 ng/mL) plus ephrin B1-Fc (1 μg/mL) plus ephrin B2-Fc (1 μg/mL). Cell lysates were immunoblotted with a rabbit monoclonal antibody to phospho-Akt (Ser473) and were reblotted with rabbit antibodies against total Akt.
Addition of the SNEW peptide (100 μM) during 5-minute HUVEC costimulation with SDF-1 (500 ng/mL) and ephrin B1-Fc (1 μg/mL) reduced the proportion of phospho-Akt–positive cells, but there was no such change with the control SCR-EPQ peptide (Figure 5B). A similar reduction in the proportion of phospho-Akt–positive cells was derived from addition of the TNYL-RAW (100 μM), but not the control SCR-WTL, peptide (100 μM) to HUVECs costimulated for 15 minutes with SDF-1 (500 ng/mL) and ephrin B2-Fc (1 μg/mL) (Figure 5B).
We confirmed by immunoblotting that the combination of SDF-1α (500 ng/mL) plus ephrin B1-Fc (1 μg/mL) and the combinations of SDF-1α (500 ng/mL) plus ephrin B2-Fc (1 μg/mL) produced greater levels of Akt phosphorylation in HUVECs than did SDF-1α, ephrin B1-Fc, or ephrin B2-Fc alone (Figure 5C, top panels). A similar enhancement of Akt phosphorylation was induced by ephrin B1-Fc (1 μg/mL) or ephrin B2-Fc (1 μg/mL) added to SDF-1α at a lower concentration (100 ng/mL) (Figure 5C, bottom panel). No further enhancement of AKT phosphorylation was derived by the combination of ephrin B1-Fc and ephrin B2-Fc (1 μg/mL each) with SDF-1α (100 ng/mL) (Figure 5C, bottom panel). These results together provide evidence for interplay between SDF-1–induced signaling and EphB2 or EphB4 signaling in HUVECs.
EphB2 and EphB4 signaling enhance SDF-1–induced chemotaxis
While searching for functional correlates of increased Akt phosphorylation induced by the combination of SDF-1 and ephrin B1-Fc or ephrin B2-Fc, we examined endothelial cell chemotaxis. First we incubated HUVECs (10 minutes, 37°C) with ephrin B1-Fc (1 μg/mL), ephrin B2-Fc (1 μg/mL), or medium only, and then we exposed them to SDF-1 gradients in transwell assays. SDF-1α (50 ng/mL) promoted the expected chemotactic response in HUVECs (Figure 6). Ephrin B1-Fc– and ephrin B2-Fc–activated HUVECs did not spontaneously (no SDF-1 gradient) migrate to the lower chamber but did display significantly (P < .05) increased chemotactic response to SDF-1 compared with non–preactivated HUVECs (Figure 6A). The SNEW peptide (100 μM), but not a control peptide, added before (20 minutes, 37°C) and during stimulation with ephrin B1-Fc significantly (P < .05) reduced migration to SDF-1 in HUVECs prestimulated with ephrin B1-Fc but not in control cells incubated with medium (Figure 6B). Significant (P < .05) inhibition of HUVEC migration to SDF-1 was also observed with the TNYL-RAW peptide (100 μM) added during stimulation with ephrin B2-Fc. These results provide evidence that EphB2 and EphB4 signaling can enhance SDF-1–induced chemotaxis in HUVECs.
Discussion
In this study, we show that endogenous EphB2 and EphB4 signaling is required for endothelial cell formation of extracellular matrix–dependent cordlike structures, a complex morphogenic process that recapitulates critical steps during new vessel formation. Previously, we demonstrated that endogenous SDF-1 and CXCR4 are also required for the formation of cordlike structures.33 We show here that EphB2 and EphB4 activation enhances SDF-1–induced endothelial cell chemotaxis, a step required for the formation of cordlike structures. Thus, we provide direct evidence for signaling and functional cooperation between endogenous EphB2/EphB4 and SDF-1 in endothelial cells. Previous studies in vitro have documented that soluble, dimeric forms of ephrin ligands and EphB receptors can promote endothelial cell sprouting and formation of capillary-like cords, presumably by stimulating forward or reverse signaling,11,14,15,21 and that interplay exists between Eph signaling and various signaling molecules.12,13,16,21,54,55 However, it was not previously shown that signaling of endogenous EphB2 and EphB4 is required for the formation of capillary-like structures or that in endothelial cells signaling and functional cooperation take place between SDF-1and EphB2 and EphB4.
Stimulation with ephrin B1-Fc or ephrin B2-Fc enhances SDF-1-induced HUVEC chemotaxis. (A) HUVECs (0.5 × 106) preincubated (10 minutes, 37°C) in medium only, with ephrin B1-Fc (1 μg/mL), or with ephrin B2-Fc (1 μg/mL) were placed on the top chamber of the transwell. SDF-1α (50 ng/mL) or chemotaxis medium only was placed in the bottom chamber. (B) HUVECs (0.5 × 106) were first incubated (20 minutes) in medium only, with the peptide SNEW (100 μM), or with a control scrambled form of SNEW (SCR-EPQ, 100 μM). Cells were subsequently incubated (10 minutes, 37°C) with medium only or with ephrin B1-Fc (1 μg/mL). Cells and additives were placed on the top chamber of the transwell. SDF-1α (50 ng/mL), or chemotaxis medium only was placed in the bottom chamber, and the transwells were incubated for 18 hours at 37°C. (C) HUVECs (0.5 × 106) were first incubated (20 minutes, 37°C) in medium only, with the peptide TNYL-RAW (100 μM), or with the peptide SNEW (100 μM); cells were subsequently incubated (10 minutes, 37°C) with medium only or with ephrin B2-Fc (1 μg/mL). Cells and additives were placed on the top chamber of the transwell. SDF-1α (50 ng/mL) or chemotaxis medium only was placed in the bottom chamber. All transwell plates (A-C) were incubated 18 hours at 37°C. Cells accumulated into the lower chamber were counted. Results reflect the mean ± SD number of migrated HUVECs under the different conditions tested (3-5 separate experiments, each performed in triplicate).
Stimulation with ephrin B1-Fc or ephrin B2-Fc enhances SDF-1-induced HUVEC chemotaxis. (A) HUVECs (0.5 × 106) preincubated (10 minutes, 37°C) in medium only, with ephrin B1-Fc (1 μg/mL), or with ephrin B2-Fc (1 μg/mL) were placed on the top chamber of the transwell. SDF-1α (50 ng/mL) or chemotaxis medium only was placed in the bottom chamber. (B) HUVECs (0.5 × 106) were first incubated (20 minutes) in medium only, with the peptide SNEW (100 μM), or with a control scrambled form of SNEW (SCR-EPQ, 100 μM). Cells were subsequently incubated (10 minutes, 37°C) with medium only or with ephrin B1-Fc (1 μg/mL). Cells and additives were placed on the top chamber of the transwell. SDF-1α (50 ng/mL), or chemotaxis medium only was placed in the bottom chamber, and the transwells were incubated for 18 hours at 37°C. (C) HUVECs (0.5 × 106) were first incubated (20 minutes, 37°C) in medium only, with the peptide TNYL-RAW (100 μM), or with the peptide SNEW (100 μM); cells were subsequently incubated (10 minutes, 37°C) with medium only or with ephrin B2-Fc (1 μg/mL). Cells and additives were placed on the top chamber of the transwell. SDF-1α (50 ng/mL) or chemotaxis medium only was placed in the bottom chamber. All transwell plates (A-C) were incubated 18 hours at 37°C. Cells accumulated into the lower chamber were counted. Results reflect the mean ± SD number of migrated HUVECs under the different conditions tested (3-5 separate experiments, each performed in triplicate).
Analysis of mutant mice has demonstrated indispensable roles for certain ephrin B ligands and EphB receptors in normal vascular development. Particularly striking is the phenotype of mice null for either ephrin B2 or EphB4, which die during embryogenesis and display an apparent block at the primitive capillary plexus stage.6-8 However, evidence for a continued role of EphB/ephrin B function in postnatal angiogenesis is limited and derives from altered expression of these molecules in hereditary vascular malformations, inflammation, and cancer.10,56,57 In these cases, it is unclear whether altered Eph/ephrin expression is a cause or a consequence of disease. Functional assays in vitro showed that various ephrin B and EphB molecules can promote adult endothelial cell growth, sprouting, migration, or assembly of capillary-like structures, presumably by activating forward or reverse signaling.6,11-16 Other experiments showed that ephrin B2-Fc produces repulsive signals and inhibits adult endothelial cell sprouting while activating EphB4 forward signaling15 and reduced VEGF-induced endothelial cell migration and growth.17 Here, using the EphB2 inhibitor SNEW peptide and the EphB4 peptide inhibitor TNYL-RAW,39 we demonstrate that EphB2 and EphB4 function is necessary for mature endothelial cell assembly into vascular structures and thus provide evidence for a continued role of Eph/ephrin in postnatal morphogenic processes. Consistent with the current results, interruption of EphB/ephrin B signaling with soluble forms of EphB2 or eph-rin B1 extracellular domains disrupted neuronal migration to the subventricular zone,58 and a monoclonal antibody directed at EphB2 killed cancer cells expressing the receptor.59
The downstream signaling of EphB/ephrin B is not well understood, in part because of the large number of adaptor proteins that link Eph signaling to various signaling pathways.1,20,60 Earlier studies in neuronal cells provided evidence that ephrin B reverse signaling mediated by the adaptor protein PDZ-RGS3 can neutralize CXCR4 signaling induced by SDF-1 and proposed that this result could explain migration patterns of cerebellar granule cells.24 In another study, stimulation of EphB4 by ephrin B2-Fc led to Akt phosphorylation in human mesenteric endothelial cells.12 Here, we observed greater Akt phosphorylation and chemotaxis when HUVECs were costimulated with ephrin B1-Fc or ephrin B2-Fc plus SDF-1 than when stimulated with SDF-1 alone. Thus, we provide evidence for a link between EphB2 and EphB4 forward signaling and SDF-1–induced signaling and for cooperation between the 2 axes in promoting endothelial cell chemotaxis.
Evidence indicates that a variety of molecules contribute to the endothelial cell morphogenic process, including the neuropilin-1/VEGFRs and Delta4/Notch systems.61-63 Our current results add EphB2 and EphB4 to the list and suggest a model in which endothelial cells dispersed on Matrigel monolayers migrate toward neighboring endothelial cells in response to SDF-1 gradients generated by some of the endothelial cells.33 This leads to the formation of endothelial cell clusters, which provides an opportunity for EphB2 and EphB4 activation by Ephrin B2 on neighboring cells. Consistently, we found that endogenous EphB2 and EphB4 were not phosphorylated when the cells were incubated with a CXCR4 inhibitor, even after 4 hours, onto Matrigel. We suggest that active EphB2 and EphB4 contribute to the morphogenic process by promoting cell adhesion, elongation, and active protrusion of filopodia. Importantly, by amplifying SDF-1 chemotaxis, active EphB2 and EphB4 would promote the movement of clustered endothelial cells and, in so doing, would contribute to the formation of the network. It is interesting to consider why a system of activation based on cell–cell contact such as EphB/ephrin B would participate in endothelial cell cord formation. One possibility is that by amplifying SDF-1 chemotaxis, EphB/ephrin B would ensure the movement of groups of cells already clustered rather than single cells.
Evidence for an exquisite interplay among molecules that orchestrate cell proliferation, differentiation, adherence, and movement can be seen in the nervous and the vascular systems, which have complex branching structures.61,64 It appears that SDF-1/ CXCR4 and the Eph/ephrin system cooperate in regulating endothelial cell movement.
Prepublished online as Blood First Edition Paper, July 13, 2006; DOI 10.1182/blood-2006-05-023341.
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and in part by a grant from the Ministero dell'Istruzione, dell'Università e della Ricerca, University of Brescia, Italy.
G.T. and O.S. designed the research, performed the experiments, and wrote the paper; M.L.S., J.A.M., and P.J.M. performed some of the experiments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Anna C. Berardi, Masashi Narazaki, Joshua Farber, and Cassin Kimmel-Williams for their help in various aspects of this work.


![Figure 5. Akt phosphorylation in HUVECs stimulated with SDF-1, ephrin B1-Fc, and ephrin B2-Fc. (A) Flow cytometric analysis of intracellular Akt in HUVECs incubated for 15 minutes in medium alone, SDF-1α (500 ng/mL), ephrin B1-Fc (1 μg/mL), ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL), or ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL). After permeabilization, Akt was detected by staining with a rabbit monoclonal antibody to phospho-Akt (Ser 473) followed by FITC-labeled goat anti–rabbit IgG antibodies. Background staining was set with FITC-labeled goat anti–rabbit IgG. FSC indicates forward scatter. Results are representative of 5 experiments. (B) Flow cytometric analysis of intracellular Akt. HUVECs were incubated for 15 minutes with ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) alone, with the SNEW peptide (100 μM) or with a control peptide (SCR-EPQ, a scrambled form of SNEW [100 μM]), with ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) alone, with the TNYL-RAW peptide (100 μM), or with a control peptide (SCR-WTL, a scrambled form of TNYL-RAW, 100 μM). (C, top left) After 3-hour starvation, HUVECs were treated with SDF-1α (500 ng/mL), ephrin B1-Fc (1 μg/mL), or ephrin B1-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) for 15 minutes. (C, top right) After 3-hour starvation, HUVECs were treated with SDF-1 (500 ng/mL), ephrin B2-Fc (1 μg/mL), or ephrin B2-Fc (1 μg/mL) plus SDF-1α (500 ng/mL) for 15 minutes. (C, bottom) After overnight starvation, HUVECs were treated for 15 minutes with SDF-1 (100 ng/mL), ephrin B1-Fc (1 μg/mL), ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus ephrin B2-Fc (1 μg/mL), ephrin B1-Fc (1 μg/mL) plus SDF-1α (100 ng/mL), ephrin B2-Fc (1 μg/mL) plus SDF-1α (100 ng/mL), or with SDF-1 (100 ng/mL) plus ephrin B1-Fc (1 μg/mL) plus ephrin B2-Fc (1 μg/mL). Cell lysates were immunoblotted with a rabbit monoclonal antibody to phospho-Akt (Ser473) and were reblotted with rabbit antibodies against total Akt.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/9/10.1182_blood-2006-05-023341/4/m_zh80210603040005.jpeg?Expires=1763462724&Signature=JxNPP27F53xnRvWd7fX2oSE34dRf6LBI~AiZrO1o8djDHbKKImqF9Dstm6C19zAmBetUHHaJPJIghUgF5DrNY4VjGxKSsJ9thisf1xlmtzjtghwrJz3gK8bkvFuPaKMcJ4cTYRG1cl19plEfOk47pidN6NU4ctv-hsZBgnVS12OYtNlhDfTTujTWm1RZeS3y-axjNU4zf5ogZb-SV9v8nr-HzPk-VkB~s90ctzZujdXgWyaZlFWB-Ror14jUE4hhGeyYtcVh0L3Zvs9KaFS5h7T~PBpd1pt341pjtNYqPaGrWM2oLmono9RTgwj9FpqvW7Yal6eqzpsTTLwJ7tiM9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal