Abstract
Haptoglobin-related protein (Hpr) is a primate-specific plasma protein associated with apolipoprotein L-I (apoL-I)-containing high-density lipoprotein (HDL) particles shown to be a part of the innate immune defense. Despite the assumption hitherto that Hpr does not bind to hemoglobin, the present study revealed that recombinant Hpr binds hemoglobin as efficiently as haptoglobin (Hp). However, in contrast to Hp, Hpr did not promote any high-affinity binding to the scavenger receptor CD163. Binding of hemoglobin to circulating native Hpr incorporated into the HDL fraction was indicated by hemoglobin-affinity precipitation of plasma Hpr together with apoL-I. In conclusion, plasma has 2 high-affinity hemoglobin-binding haptoglobins instead of one, but only Hp-hemoglobin complexes are efficiently recognized by CD163. Circulating Hpr-bound hemoglobin should therefore be taken into consideration when measuring “free” plasma hemoglobin. Furthermore, Hpr-bound hemoglobin might contribute to the biologic activity of the circulating apoL-I/Hpr-containing HDL particles.
Introduction
The primate-specific haptoglobin-related protein (Hpr) is a plasma protein with 91% sequence identity to the 1-1 phenotype of haptoglobin (Hp), the established hemoglobin-binding protein in plasma. Hpr is a core component of a subclass of HDL referred to as trypanosome lytic factor-1 (TLF1) based on its ability to induce lysis of the African parasite Trypanosoma brucei brucei, which primarily infects nonprimate mammals.1,2 Aside from Hpr, the main TLF1 protein components are apolipoprotein A-I (apoA-I)3,4 and the primate-specific apoL-I.5 Both Hpr and apoL-I have been reported to possess lytic activity.2,3,5-7
Like Hp, Hpr is synthesized as an approximate 45-kDa protein that is proteolytically cleaved to form an α- and a β-chain which remain connected via a disulfide bond. However, in contrast to Hp, Hpr lacks a glycosylation site and a cysteine involved in inter-α-chain bonding.8 Human Hp has either 1 (Hp1 genotype) or 2 (Hp2 genotype) of such cysteines, leading to the formation of the covalent (αβ)2 structure (Hp1-1 phenotype) or larger (αβ)-oligomers (Hp2-2 and Hp2-1 phenotypes).9 Furthermore, the α-chain of Hpr differs from that of Hp by containing the hydrophobic signal peptide,2,4 which may explain the association of Hpr to lipoprotein particles.
Hp avidly binds hemoglobin released into plasma during physiologic and pathologic hemolysis, thereby preventing heme-mediated oxidative side effects in the kidney and other organs.10,11 Formation of the Hp-hemoglobin complex promotes high-affinity binding to the monocyte/macrophage-specific scavenger receptor CD163 which mediates internalization of the complex.12 In contrast, immunoprecipitation experiments with TLF1 have suggested that Hpr is inactive in terms of hemoglobin binding.13,14 Because of the high degree of sequence similarity between Hp and Hpr, we have investigated the hemoglobin- and receptor-binding potential of Hpr using a direct molecular approach.
Study design
Expression and purification of recombinant Hp1-1 and Hpr
Hp1 cDNA was amplified from a human fetal liver cDNA library (Clontech, Palo Alto, CA) and ligated into the pcDNA5/FRT vector (Invitrogen, Taastrup, Denmark). A construct encoding Hpr was generated by multiple rounds of site-directed mutagenesis (Quick change; Invitrogen). Flp-In 293 cells (Invitrogen) were transfected using FuGENE 6 (Roche Diagnostics, Hvidovre, Denmark), and stable transfectants were selected using 150 μg/mL hygromycin B (Invitrogen). Serum-free 293 SFM II medium (Invitrogen) was collected from expression cultures and subjected to hemoglobin A0 (Sigma, Brøndby, Denmark) affinity chromatography essentially as described.15 For amino-terminal sequencing, the purified proteins were heated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for 3 minutes at 80°C, separated by SDS-gel electrophoresis, and subsequently transferred to a polyvinylidene diflouride membrane as described.16 Automated Edman degradation was performed by using an Applied Biosystems Model 477A sequencer (Applied Biosystems, Foster City, CA) with on-line phenylthiohydantoin analysis by high-performance liquid chromatography (HPLC; Model 120A; Applied Biosystems). In agreement with recent data demonstrating a very low degree of cleavage of recombinant Hp to α- and β-chains in 293 cells,17 amino-terminal sequencing of recombinant Hp yielded only the sequence of the mature α-chain amino-terminus (VDSGNDVTDIAD). Two amino-terminal sequences in equimolar amounts were obtained for recombinant Hpr (LYSGNDVTDISD and ILGGHLDAKGSF).
Surface plasmon resonance (SPR) analysis
SPR analysis was performed on a Biacore 3000 instrument (Biacore, Uppsala, Sweden) as described.18 Hemoglobin A0 (Sigma) was immobilized at a concentration of 10 μg/mL in 10 mM sodium acetate, pH 6.0. The resulting densities were 50 to 60 fmol/mm2 (Figures 1B and 1C, respectively) or 70 to 80 fmol/mm2 (experiments in Figure 1C). Sample and running buffer was 10 mM HEPES, 150 mM NaCl, 3.0 mM CaCl2, 1.0 mM EGTA, and 0.005% Tween-20, pH 7.4.
Hemoglobin-precipitation of human plasma proteins
Random plasma and serum samples with normal Hp levels (> 0.6 g/L) from the routine laboratory of the Department of Clinical Biochemistry, Aarhus Hospital, Denmark, or from steady-state patients with sickle cell anemia (Hp concentration, < 0.1 g/L) were incubated with hemoglobin-coupled Sepharose, bovine serum albumin (BSA)-coupled Sepharose, or underivatized Sepharose. Bound proteins were analyzed by immunoblotting (using rabbit polyclonal anti-human Hp antibody; DAKOCytomation, Glostrup, Denmark) and amino-terminal sequencing/mass spectrometry essentially as described.19 The concentration of Hp was determined by enzyme-linked immunosorbent assay using biotinylated anti-human Hp (DAKOCytomation).
Quantification of Hpr levels
Immunostained Hpr was visualized by using the enhanced chemiluminescence Western blot detection system (Amersham Biosciences, Hillerød, Denmark) and a FUJIFILM LAS-1000 luminescence image analyzer (Fujifilm, Tokyo, Japan). Quantification relatively to standards of recombinant Hpr was performed by using MultiGauge 3.0 software (Fujifilm). The concentration of recombinant Hpr was determined by protein hydrolysis and amino acid analysis. The present study was approved by the Research and Research Education Committee of the Faculty of Health Sciences, University of Aarhus, Denmark.
Results and discussion
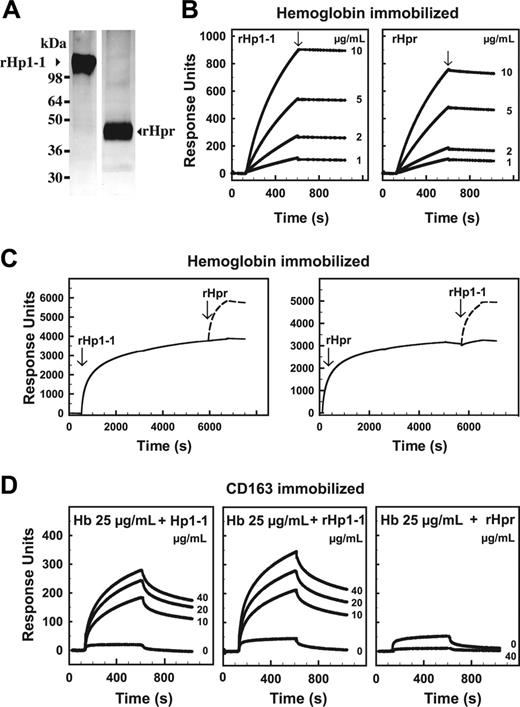
Hpr and Hp (1-1 phenotype) were expressed as recombinant proteins in a mammalian 293 cell expression system. Binding of hemoglobin to Hpr was indicated by the surprising observation that hemoglobin-affinity chromatography was applicable for purification of both expression products. In agreement with the findings on the authentic proteins,13 nonreducing denaturing gel electrophoresis of the recombinant proteins showed migration of Hpr and Hp corresponding to approximately 45-kDa and approximately 110-kDa proteins, respectively (Figure 1A). However, nondenaturing S-200 gel filtration18 showed identical elution of Hpr and Hp1-1 (data not shown), indicating that Hpr noncovalently also associates into a dimeric (αβ)2 structure.
SPR analysis showed an almost irreversible and virtually indistinguishable high-affinity binding of hemoglobin A0 to both recombinant proteins (Figure 1B). Similar SPR data were obtained for hemoglobin A0, hemoglobin A2, and hemoglobin S (data not shown). Hpr efficiently competed for binding of Hp to hemoglobin and vice versa (Figure 1C). The signal peptide of the recombinant Hpr α-chain was found to be removed in contrast to that of plasma-derived Hpr.2,4 However, because hemoglobin is known to bind to the β-chain of Hp,20-22 it seems unlikely that this difference affects hemoglobin binding to Hpr.
Identification of recombinant Hpr as a hemoglobin-binding protein by hemoglobin-affinity chromatography and SPR analyses. (A) Silver-stained SDS-polyacrylamide gels of hemoglobin affinity-purified recombinant human Hp (phenotype 1-1, rHp1-1) and Hpr (rHpr). (B) SPR analysis of the binding of a range of concentrations of rHp1-1 and rHpr to immobilized hemoglobin A0 (Kd calculated to 2.3 nM and 13 nM, respectively). The slow dissociation (arrows, Kd < 2 × 10-4 s-1) indicates an almost irreversible binding. The concentration of rHp1-1 and rHpr is shown to the right of each curve. (C) SPR analysis of binding of rHpr or rHp1-1 to hemoglobin A0 in the presence of rHp1-1 and rHpr, respectively. The curves (solid lines) show binding of rHp1-1 (left panel) or rHpr (right panel) to the hemoglobin chip and subsequent binding of rHpr (arrow, left panel) and rHp1-1 (arrow, right panel). The dashed lines are transposed curves (from an identical chip), showing binding to the chip without prebound rHp1-1/rHpr, that is, the expected binding in the absence of competitive inhibitor. (D) SPR analysis of the binding of hemoglobin A0 (Hb), complexed with plasma-derived Hp1-1, recombinant rHp1-1, or rHpr, to immobilized CD163. The concentration of Hp1-1, rHp1-1, and rHpr is shown to the right of each curve.
Identification of recombinant Hpr as a hemoglobin-binding protein by hemoglobin-affinity chromatography and SPR analyses. (A) Silver-stained SDS-polyacrylamide gels of hemoglobin affinity-purified recombinant human Hp (phenotype 1-1, rHp1-1) and Hpr (rHpr). (B) SPR analysis of the binding of a range of concentrations of rHp1-1 and rHpr to immobilized hemoglobin A0 (Kd calculated to 2.3 nM and 13 nM, respectively). The slow dissociation (arrows, Kd < 2 × 10-4 s-1) indicates an almost irreversible binding. The concentration of rHp1-1 and rHpr is shown to the right of each curve. (C) SPR analysis of binding of rHpr or rHp1-1 to hemoglobin A0 in the presence of rHp1-1 and rHpr, respectively. The curves (solid lines) show binding of rHp1-1 (left panel) or rHpr (right panel) to the hemoglobin chip and subsequent binding of rHpr (arrow, left panel) and rHp1-1 (arrow, right panel). The dashed lines are transposed curves (from an identical chip), showing binding to the chip without prebound rHp1-1/rHpr, that is, the expected binding in the absence of competitive inhibitor. (D) SPR analysis of the binding of hemoglobin A0 (Hb), complexed with plasma-derived Hp1-1, recombinant rHp1-1, or rHpr, to immobilized CD163. The concentration of Hp1-1, rHp1-1, and rHpr is shown to the right of each curve.
In contrast to the similar hemoglobin-binding properties, Hpr and Hp in complex with hemoglobin showed a significant difference in binding to CD163 (Figure 1D). Whereas the Hp-hemoglobin complex formation induced a considerable increase in binding to CD163 relative to hemoglobin alone,12,23 such effect was not apparent for the Hpr-hemoglobin complex (Figure 1D). Accordingly, SPR analysis showed no interference of Hpr with Hp-hemoglobin binding to CD163 (data not shown).
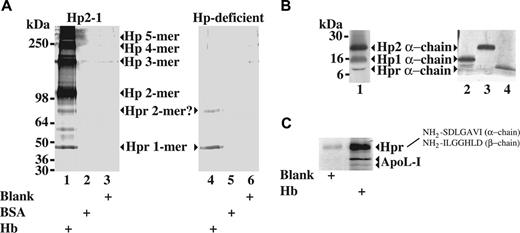
To show hemoglobin-binding activity of native Hpr in plasma, we identified human plasma proteins precipitated by hemoglobin-Sepharose. Immunoblotting and mass spectrometry/amino-terminal sequencing (Figure 2) identified Hp in multiple sizes and Hpr (containing the signal peptide) in a 45-kDa monomeric and a 90-kDa form. The 90-kDa form is in agreement with previous data reporting an SDS-resistant dimeric form of Hpr.13 Reducing SDS-PAGE and subsequent immunoblotting of hemoglobin affinity-purified proteins from the Hp2-1 serum displayed in Figure 2A showed the characteristic difference in migration of the α-subunits specific for Hp1, Hp2, and Hpr13 (Figure 2B). The staining intensities of these bands comply with a plasma concentration of Hpr amounting to approximately 1/10 to 1/20 of the Hp concentration. ApoL-I (Figure 2C) and apoA-I (not shown) were also identified as hemoglobin-precipitated proteins, suggesting that Hpr bound to hemoglobin-Sepharose was associated with TLF1 particles.
Identification of plasma Hpr as a hemoglobin-interacting protein by hemoglobin-affinity precipitation. (A) Immunoblot with an anti-Hp antibody (recognizing both Hp and Hpr) of proteins eluted from hemoglobin-coupled Sepharose (Hb), BSA-coupled Sepharose (BSA), or underivatized Sepharose (blank) after incubation with serum from a person (Hp phenotype 2-1) with a normal Hp level (0.9 g/L) and from a patient with Hp-deficient sickle cell disease. (B) Immunoblotting (reducing 12%-15% SDS-PAGE) of the hemoglobin-binding serum proteins from a person with the Hp phenotype 2-1 (same material as shown in Figure 2A) to identify the different α-chains of the Hp1, Hp2, and Hpr proteins. Lanes 2, 3, and 4 show silver-stained reducing (12%-15% gradient) SDS-polyacrylamide gel of Hp1-1 purified from human plasma, Hp2-2 purified from human plasma, and purified recombinant Hpr, respectively, demonstrating the distinct migration patterns of the Hp1, Hp2, and Hpr α-chains. The identity of the bands from the purified proteins was verified by mass spectrometry (data not shown). (C) Silver-stained (8%-16% gradient) SDS-polyacrylamide gel of proteins from normal human serum bound to hemoglobin-coupled Sepharose or underivatized Sepharose. The identity of the protein bands representing apoL-I and Hpr was determined by mass spectrometry and amino-terminal sequencing (as indicated).
Identification of plasma Hpr as a hemoglobin-interacting protein by hemoglobin-affinity precipitation. (A) Immunoblot with an anti-Hp antibody (recognizing both Hp and Hpr) of proteins eluted from hemoglobin-coupled Sepharose (Hb), BSA-coupled Sepharose (BSA), or underivatized Sepharose (blank) after incubation with serum from a person (Hp phenotype 2-1) with a normal Hp level (0.9 g/L) and from a patient with Hp-deficient sickle cell disease. (B) Immunoblotting (reducing 12%-15% SDS-PAGE) of the hemoglobin-binding serum proteins from a person with the Hp phenotype 2-1 (same material as shown in Figure 2A) to identify the different α-chains of the Hp1, Hp2, and Hpr proteins. Lanes 2, 3, and 4 show silver-stained reducing (12%-15% gradient) SDS-polyacrylamide gel of Hp1-1 purified from human plasma, Hp2-2 purified from human plasma, and purified recombinant Hpr, respectively, demonstrating the distinct migration patterns of the Hp1, Hp2, and Hpr α-chains. The identity of the bands from the purified proteins was verified by mass spectrometry (data not shown). (C) Silver-stained (8%-16% gradient) SDS-polyacrylamide gel of proteins from normal human serum bound to hemoglobin-coupled Sepharose or underivatized Sepharose. The identity of the protein bands representing apoL-I and Hpr was determined by mass spectrometry and amino-terminal sequencing (as indicated).
By quantitative Western blotting using recombinant Hpr as standard, we measured the Hpr monomer levels in 18 persons with normal Hp levels (> 0.6 g/L) to 40.7 ± 14.3 mg/L and in 13 patients with low Hp levels resulting from sickle cell anemia and extensive intravascular hemolysis (Hp concentration, < 0.1 g/L) to 49.9 ± 22.5 mg/L. Thus, in contrast to Hp the plasma concentration of Hpr appears to be unaffected by hemolysis. The depletion of Hp but not Hpr upon intravascular hemolysis was further confirmed by Western blot analysis (data not shown) of a large set (n = 15) of anonymous routine samples with low Hp levels and biochemical parameters indicating intravascular hemolysis.
The present data explain the previous observation13 that 2 patients with severe hemolysis, because of paroxysmal nocturnal hemoglobinuria, had depletion of approximately 98% of the Hp pool in plasma but virtually normal levels of Hpr. However, this observation is likely not due to lack of binding of Hpr to hemoglobin as originally suggested13 but may be a consequence of the pronounced difference in CD163 binding of Hpr-hemoglobin and Hp-hemoglobin. Furthermore, our data suggest that the hemoglobin previously reported to be present in TLF1 particles2,13 is not a contaminant but represents Hpr-bound hemoglobin.
The absent induction of high-affinity binding to CD163 when hemoglobin binds to Hpr indicates that some of the differences in the primary structure of Hp versus Hpr are essential for receptor binding. Furthermore, the very low affinity for CD163 and the association with high molecular weight particles resisting renal clearance suggest an extended circulation time of Hpr-bound hemoglobin relative to free and Hp-associated hemoglobin. Although a previous study13 of hemoglobin-exposed TLF1 particles did not reveal increased trypanosoma-lytic activity, it is intriguing to speculate that the oxidative potential of Hpr-bound hemoglobin may be a part of the innate immune defense as originally proposed.2 Moreover, it could be speculated that association of Hpr-bound hemoglobin with the antioxidant apoA-I24,25 in plasma may protect the circulatory system from the oxidative properties of hemoglobin which may be uncovered subsequent to internalization by T brucei brucei.
Nonimmune biologic activity of circulating hemoglobin bound to HDL particles should also be considered. Hemoglobin released into plasma or added to plasma as a blood substitute scavenges NO, and this is associated with smooth muscle dystonias, endothelial dysfunction, and thrombosis.26,27 It will be highly important to investigate whether this toxicity of hemoglobin is influenced by circulating plasma Hpr/HDL.
Finally, the presence of 2 hemoglobin-binding haptoglobins in plasma instead of one has to be taken into account when measuring haptoglobin and “free” hemoglobin in plasma, particularly in patients with low Hp levels where Hpr becomes a prominent hemoglobin-binding protein.
Prepublished online as Blood First Edition Paper, June 15, 2006; DOI 10.1182/blood-2006-05-022327.
Supported by the Lundbeck Foundation and the Danish Medical Research Council.
M.J.N. performed the research and wrote the paper; S.V.P. performed the amino-terminal sequencing and the MALDI mass spectrometry analysis; C.J. performed the SPR analysis; C.O. performed the amino acid analysis; D.R. studied the patients with sickle cell anemia; H.J.M. performed the gel filtration analysis and the serum haptoglobin measurements; and S.K.M. designed the study and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Gitte Ratz, Anne Marie Bundsgaard, Ida B. Thøgersen, and Kirsten Bank Petersen for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal