Abstract
HLA-DR is under investigation as a target for monoclonal antibody (mAb) therapy of malignancies. Here we describe a humanized IgG4 form of the anti-HLA-DR mAb L243, hL243γ4P (IMMU-114), generated to provide an agent with selectivity toward neoplastic cells that can kill without complement-dependent cytotoxicity (CDC) or antibody-dependent cellular-cytotoxicity (ADCC), so as to reduce reliance on intact immunologic systems in the patient and effector mechanism-related toxicity. In vitro studies show that replacing the Fc region of hL243γ1, a humanized IgG1 anti-HLA-DR mAb, with the IgG4 isotype abrogates the effector cell functions of the antibody (ADCC and CDC) while retaining its antigen-binding properties, antiproliferative capacity (in vitro and in vivo), and the ability to induce apoptosis concurrent with activation of the AKT survival pathway. Growth inhibition was evaluated compared with and in combination with the anti-CD20 mAb rituximab, with the combination being more effective than rituximab alone in inhibiting proliferation. Thus, hL243γ4P is indistinguishable from hL243γ1 and the parental murine mAb in assays dependent on antigen recognition. The abrogation of ADCC and CDC, which are believed to play a major role in side effects of mAb therapy, may make this antibody an attractive clinical agent. In addition, combination of hL243γ4P with rituximab offers the prospect for improved patient outcome.
Introduction
The human leukocyte antigen-DR (HLA-DR) is 1 of 3 polymorphic isotypes of the class II major histocompatibility complex (MHC) antigen. Because HLA-DR is expressed at high levels on a range of hematologic malignancies, there has been considerable interest in its development as a target for antibody-based lymphoma therapy. However, safety concerns have been raised regarding the clinical use of HLA-DR-directed antibodies, because the antigen is expressed on normal as well as tumor cells.1 HLA-DR is constitutively expressed on normal B cells, monocytes/macrophages, dendritic cells, and thymic epithelial cells. In addition, interferon-γ may induce HLA class II expression on other cell types, including activated T and endothelial cells.1 The most widely recognized function of HLA molecules is the presentation of antigen in the form of short peptides to the antigen receptor of T lymphocytes. In addition, signals delivered via HLA-DR molecules contribute to the functioning of the immune system by up-regulating the activity of adhesion molecules, inducing T-cell antigen counterreceptors, and initiating the synthesis of cytokines.2,3
Stimulation by HLA ligation by antibodies has been shown to affect growth, differentiation, and immunoglobulin secretion by B lymphocytes as well as production of cytokines, modulation of expression of growth factor receptors, cell adhesion, and costimulatory molecules by B cells and monocytes. HLA molecules have also been shown to serve as receptors that activate various cell death pathways, including caspase-dependent and a caspase-independent alternative pathway of apoptosis.2,4-6 HLA-DR ligation can lead to proliferation in activated T and B lymphocytes and apoptosis in resting B lymphocytes. It has been suggested that the induction of apoptosis by ligation of class II molecules in resting B lymphocytes prevents premature class II-mediated activation of B cells that have not been specifically primed by antigen. In addition, HLA class II-mediated death is a means of rapidly removing either T or B lymphocytes that have already served their role in the immune response, thereby avoiding the inflammatory responses associated with necrosis and concentrating the ligands for new T-cell receptor and/or CD4 interactions.5
Almost 20 years ago, Bridges et al7 demonstrated the ability of a monoclonal antibody (mAb) specific for the Ia antigen (murine MHC class II) to cure a B-cell lymphoma in a mouse model and suggested class II antigens as attractive molecules that could potentially be targeted by therapeutic agents. Although it was known that HLA-DR is not tumor specific, the substantial effect on lymphoma growth, the lack of modulation following mAb binding, and the observation that mechanisms other than complement-dependent cytotoxicity (CDC) play a major role in antibody-induced toxicity initiated interest in HLA-DR as a target for mAb therapy. Elasser et al8 demonstrated that murine anti-HLA class II mAbs, including L243, Lym-1, 1D10, and others, could induce antibody-dependent cellular-cytotoxicity (ADCC) by peripheral blood mononuclear cells. Lym-1 and humanized 1D10 (Hu1D10) have been studied clinically as antilymphoma therapeutics. Both mAbs recognize polymorphic variants on the HLA-DR β chain and have been detected in more than 80% and 60% of lymphoma patients, respectively.9,10 Radiolabeled Lym-1, a murine IgG2a, has shown promising results in targeted radioimmunodiagnosis and therapy of B-lymphocytic malignancies and has been studied in various clinical trials.11,12 Hu1D10 was developed as an unconjugated antibody and has been evaluated in clinical trials in patients with relapsed or refractory indolent non-Hodgkin lymphoma (NHL).13,14 In vitro functional studies have shown that in addition to mediating ADCC and CDC, both Lym-1 and Hu1D10 are capable of inducing apoptosis of malignant cells.13,15 Unlike Lym-1 and Hu1D10, L243 is a pan-HLA-DR mAb recognizing a conformational epitope in the α chain of HLA-DR.16 The effects of murine L243 on malignant cells have been studied extensively. In addition to B-cell malignancies, L243 has been used against melanoma17 and in models for autoimmune disease, specifically rheumatoid arthritis.18 Functions reported to be affected by incubation of cells with L243 have included signal transduction, growth inhibition, Fas-mediated apoptosis, interactions with actin microfilaments, TNF-α and TNF-β gene expression, cell adhesion, ADCC, and others.8,17,19-25
mAbs against cell-surface differentiation antigens, such as CD20 and CD52, exert their in vivo effect largely through immunologic effector mechanisms, including CDC and ADCC, although direct apoptosis also occurs to some degree.26,27 Thus, their efficacy is dependent, for the most part, on intact immunologic mechanisms in the treated patient. Moreover, it is likely that these effector mechanisms mediate side effects observed upon mAb administration, such as B-cell depletion28 and infusion-related toxicity. In contrast, the activity of anti-HLA-DR mAbs is largely a consequence of direct cytotoxic effects.
In this report, we describe a humanized IgG4 form of the murine anti-HLA-DR mAb L243 (mL243), hL243γ4P, generated by complementarity-determining region (CDR) grafting. The IgG4 antibody was generated to fill the need for an agent with selectivity toward neoplastic cells that is able to kill tumor cells without CDC or ADCC in order to reduce reliance on intact immunologic systems in the patient and effector mechanism-related toxicity.
Materials and methods
Antibodies
The hybridoma cell clone producing the anti-HLA-DR mAb, L243, was obtained from the American Type Culture Collection ([ATCC] Manassas, VA). Cells were cultured in HSFM medium (Life Technologies, Gaithersburg, MD) with 10% FBS (Hyclone, Logan, UT). The genes encoding the Vκ and VH regions of L243 were cloned by reverse transcriptase-polymerase chain reaction (RT-PCR). The humanized L243 (IgG1/κ isotype), hL243γ1, was generated as described previously.29-32 The IgG4/κ isotype of hL243γ4P (IMMU-114) was constructed by replacing the heavy chain constant region coding sequence for the human γ1 chain with that of γ4 chain. A point mutation, Ser241Pro (based on Kabat numbering), was introduced into the hinge region of the γ4 sequence to avoid formation of half molecules when the antibody is expressed and produced in mammalian cell cultures.33
Other mAbs used in the studies were rituximab, purchased from IDEC Pharmaceuticals (San Diego, CA), and hA20 (IMMU-106, humanized anti-CD20 IgG1) and hMN-14 (humanized anticarcinoembryonic antigen [anti-CEA] IgG1, used here as a negative control), provided by Immunomedics (Morris Plains, NJ). The construction and characterization of hA20 and hMN-14 have been described previously.34,35
Cells
The Burkitt lymphoma lines, Daudi, Raji, and Ramos, were purchased from ATCC. Non-Burkitt lymphoma cell lines were obtained as follows: RL and SU-DHL-6, which contain the chromosomal translocation t(14;18), were obtained from Dr John Gribben (Dana-Farber Cancer Institute, Boston, MA) and Dr Alan Epstein (University of Southern California, Los Angeles), respectively. SU-DHL-4, SU-DHL-10, and Karpas422 were provided by Dr Myron Czuczman (Roswell Park Cancer Institute, Buffalo, NY), and FSCCL (also called WSU-FSCCL) was obtained from Dr Mitchell Smith (Fox Chase Cancer Center, Philadelphia, PA). The cells were grown as suspension cultures in DMEM (Life Technologies) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM) (complete media).
Antigen-binding specificity of humanized L243 mAbs
Antigen-binding activity and specificity of hL243γ1 were shown by cell surface-binding assays. Raji cells were incubated in PBS/BSA (1%) containing a saturating concentration of purified hL243γ1 (20 μg/mL) for 1 hour at 4°C. After washing, cell surface-bound hL243γ1 was detected by incubating the Raji cells in the buffer containing a PE-conjugated second antibody (goat anti-human IgG, Fc fragment specific) and counting in a Guava PCA system (Guava Technologies, Hayword, CA).
A competition cell-binding assay was carried out to assess the reactivity of hL243γ4P relative to mL243. A constant amount of 125I-labeled mL243 or hL243γ4P (100 000 cpm, about 10 μCi/μg [3.7 × 108 Bq]) was incubated with human lymphoma cells (Raji, Daudi, or Ramos) in the presence of varying concentrations (0.2 to 700 nM) of purified hL243γ4P or mL243 at 4°C for 1 to 2 hours. Unbound mAbs were removed by washing the cells in PBS. The radioactivity associated with cells was determined after washing.
The antigen-binding affinity constant of hL243γ4P was determined by a direct cell surface-binding assay of the radiolabeled antibodies and Scatchard plot analysis. To measure specific cell-surface antigen binding, 2 sets of cells were prepared and used to estimate the nonspecific and total binding of radioactivity, respectively. The cells for nonspecific binding were preincubated with excess amount of unlabeled mAb to block all surface antigen sites prior to adding the radiolabeled antibody, while those for total binding were preincubated in PBS. After preincubation, varying amounts of either 125I-hL243γ4P or 125I-mL243 were added and incubated with 2 × 105 human lymphoma cells (Raji, Daudi, or Ramos) for 2 hours at 4°C. Unbound antibodies were removed by washing. The cell-associated radioactivity was counted, and specific cell-surface binding of the radiolabeled antibody at a given concentration of radiolabeled antibody was calculated.
Immunophenotyping
Determination of antigen expression levels on NHL cells was performed by indirect immunofluorescence assays using FITC-goat anti-human IgG (Tago, Burlingame, CA), as described previously.36 All flow cytometry experiments were performed and analyzed using a FACSCalibur (Becton Dickinson, San Jose, CA).
Cytotoxicity assays
CDC was determined by 2 methods, standard 51chromium (51Cr) release37 and viability assessment using the fluorescent dye resazurin (Molecular Probes, Eugene, OR). For the fluorescents assay, Daudi cells were plated at 50 000 cells per well in black 96-well plates and incubated with 2-fold serially diluted mAbs in the presence of human complement (final dilution 1:20; Quidel, San Diego, CA) at 37°C, 5% CO2, for 2 hours. C12 resazurin substrate was added to all wells at 5 μM and incubated an additional 5 hours. Plates were read using a Perkin Elmer Wallac (Wellesley, MA) Envision 2100 multilabel reader (excitation 563 nm, emission 587 nm). Cells treated with 0.25% Triton X-100 were included as 100% lysis control and cells treated with complement alone as 0% lysis. For the 51Cr release assay, a 1:20 final dilution of normal human serum complement was used, followed by a 3-hour incubation. All assays were performed in triplicate.
ADCC was measured using a calcein-acetoxymethyl (calcein-AM) (Molecular Probes) release assay essentially as described by Neri et al.38 Effector-target cell ratios of approximately 50:1 were used, and incubations were for 4 hours. All blood donors gave voluntary, written informed consent.
In vitro cell proliferation and viability assays
Colorimetric assays were performed for quantitation of viable cells using MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium dye) (Promega, Madison, WI) as described by the manufacturer. Briefly, cells were plated into sterile transparent flat-bottom 96-well plates at 2 × 104 per well and then incubated with 2-fold serial dilutions of the mAbs at 37°C, 5% CO2, for 48 or 72 hours. MTS substrate was then added to all wells, and incubation was continued for 3 hours. Absorbance readings were obtained at 490 nm using the Perkin Elmer Wallac Envision 2100 multilabel reader. In some assays, F(ab′)2 fragment goat anti-human IgG, Fcγ specific, was added immediately after the addition of the respective test or control IgG, at a final concentration of 20 μg/mL, constant across the plate.
mAb effects on proliferation were determined by measuring 3H-thymidine incorporation in the NHL cell lines with and without the presence of a cross-linking second antibody, essentially as described by Shan et al.39 All tests were performed in triplicate.
Assessment of cell death/apoptosis
DNA fragmentation. Flow cytometric analysis of cellular DNA was performed following propidium iodide (PI) staining.39,40 NHL cells were placed in 24-well plates (5 × 105 cells per well) and treated with mAbs (5 μg/mL) in the presence or absence of a second antibody (20 μg/mL). Following a 48-hour incubation (37°C, 5% CO2), cells were transferred to test tubes, washed with PBS, and then resuspended in hypotonic PI solution (50 mg/mL PI in 0.1% sodium citrate, 0.1% Triton X-100). Percent apoptotic cells (hypodiploid cells) was determined by flow cytometry using a FACSCalibur. This method has been used previously for determining HLA class II-induced apoptosis and is a method of choice for this application, because it circumvents problems resulting from HLA class II-induced cellular aggregation.41
Apoptosis assays. Apoptotic cells were quantitated using the Guava Nexin kit (Guava Technologies). Following incubation with the mAbs under evaluation, cells were stained with annexin V-PE and the cell impermeant dye 7-aminoactinomycin D (7-AAD). Staining was performed according to the manufacturer's directions, and analysis done using the Guava PCA system. Three populations of cells can be distinguished in this assay: nonapoptotic cells, annexin V negative and 7-AAD negative; early apoptotic cells, annexin V positive and 7-AAD negative; and late stage apoptotic and dead cells, annexin V positive and 7-AAD positive.
Changes in the intracellular levels of cleaved caspase-3 and phosphor-AKT (Ser473) were measured using Alexa Fluor 488-conjugated antibodies (Cell Signaling Technology, Beverly, MA) as per the manufacturer's directions. Changes in mitochondrial membrane potential were measured using the BD MitoScreen Kit (BD Biosciences PharMingen, San Diego, CA), which uses the dye JC-1, (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraeethyl-benzimidazolcarbocyanine). Analyses for these assays were performed on the FACSCalibur.
In vivo survival experiment
Seven-week-old female severe combined immunodeficiency (SCID) CB-17 mice (Taconic Farms, Germantown, NY) were given injections of 2.5 × 106 Raji cells intravenously. After 1 day, animals were given intraperitoneal injections of various doses of hL243γ4P or mL243 twice weekly for 4 weeks. Control groups were given injections of saline or the nonspecific control antibody, hMN14. Body weights were measured weekly. Mice were monitored daily and killed when they developed hind-leg paralysis or lost 20% of body weight. All animal studies were approved by the Center for Molecular Medicine and Immunology's Institutional Animal Care and Use Committee (Belleville, NJ) and performed in accordance with the Association of Assessment and Accreditation of Laboratory Animal Care International (AAALAC) regulations.
Results
Construction and characterization of humanized L243
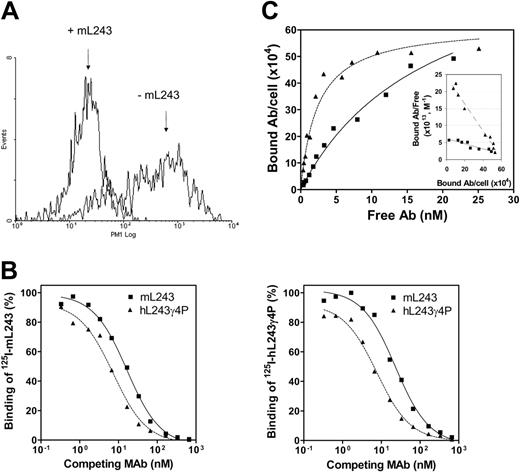
Two humanized anti-DR mAbs were generated, hL243γ1, designed to have human IgG1/κ constant regions, and hL243γ4P, constructed by replacing the heavy chain constant region coding sequence of the human γ1 chain with that of the human γ4 chain. A point mutation, Ser241Pro, was introduced into the hinge region of the γ4 sequence to avoid formation of half molecules when the antibody is expressed and produced in mammalian cell cultures.33 The ability of the 2 humanized L243 antibodies, γ1 and γ4P, to bind to Raji cells is shown in Figure 1. As seen in Figure 1A, hL243γ1 binding to Raji cells is specifically blocked by preincubation of the cells with the parental mL243, indicating that the antigen-binding specificity of mL243 is preserved in the humanized version.
To assess the reactivity of hL243γ4P relative to the parent mL243, a competition cell-binding assay was performed. A constant amount of 125I-labeled mL243 or hL243γ4P was incubated with human lymphoma cells (Raji, Daudi, or Ramos) in the presence of varying concentrations of purified hL243γ4P or mL243. As shown in Figure 1B, mL243 and hL243γ4P mAbs compete with each other for binding to the cell-surface antigen, indicating they recognize the same antigenic determinant. hL243γ4P showed an apparent approximately 2-fold higher binding avidity than mL243 (EC50 of about 7 versus about 16.5 nM). The maximum number of hL243γ4P and mL243 binding sites per cell and the apparent dissociation constants of the equilibrium binding were determined by Scatchard plot analysis. As shown in Figure 1C, the maximum binding of hL243γ4P and mL243 to Daudi cells was virtually the same, approximately 6 × 105 molecules per cell, consistent with binding to the same antigen. The apparent dissociation constant values for hL243γ4P and mL243 were calculated to be 2.6 nM and 14 nM, respectively. Similar results were obtained with Raji and Ramos cells (data not shown).
Binding characteristics of hL243γ4P and hL243γ1 relative to the parental murine L243. (A) Binding of hL243γ1 to Raji cells was measured using PE-conjugated second antibody (goat anti-human IgG, Fc fragment specific) and counting in a Guava PCA system. (B) Competitive binding assay. A cell-surface competitive binding assay was performed to compare the binding activity of hL243γ4P with the parental murine L243. Varying concentrations of mL243 (▪) or hL243γ4P (▴) were mixed with a constant amount of 125I-hL243γ4P and incubated with Raji (left) or Daudi cells (right). The cells were washed to remove unbound mAbs and counted for the bound residual radioactivity. (C) Direct cell-surface saturation binding and Scatchard plot analysis on Daudi cells. mL243, ▪; hL243γ4P, ▴.
Binding characteristics of hL243γ4P and hL243γ1 relative to the parental murine L243. (A) Binding of hL243γ1 to Raji cells was measured using PE-conjugated second antibody (goat anti-human IgG, Fc fragment specific) and counting in a Guava PCA system. (B) Competitive binding assay. A cell-surface competitive binding assay was performed to compare the binding activity of hL243γ4P with the parental murine L243. Varying concentrations of mL243 (▪) or hL243γ4P (▴) were mixed with a constant amount of 125I-hL243γ4P and incubated with Raji (left) or Daudi cells (right). The cells were washed to remove unbound mAbs and counted for the bound residual radioactivity. (C) Direct cell-surface saturation binding and Scatchard plot analysis on Daudi cells. mL243, ▪; hL243γ4P, ▴.
Antigen expression of cultured lymphoma cells
Flow cytometry analysis was performed using indirect immunofluorescent staining to show that hL243γ4P binds to a panel of cultured human B-cell lymphomas. A comparison with other surface antigens is shown Table 1. The strongest expression is observed on Daudi and Raji, but the level of fluorescence staining is strong on all the cell lines. Binding was compared with that of humanized mAbs against other B-cell antigens (CD74, CD22, and CD20); the murine-human chimeric anti-CD20 mAb, rituximab; and a humanized anti-CEA mAb (hMN-14, negative control). The staining with hL243γ4P is markedly greater than that of CD22 and CD74 on all 7 cell lines. In contrast, CD20 staining is considerably more variable, as shown by reactivity with the humanized (hA20) and chimeric (rituximab) mAbs. The Burkitt lines, Daudi, Raji, and Ramos, express intermediate levels of CD20, whereas the follicular and diffuse large B-cell lymphoma lines assessed varied. Specifically, compared with HLA-DR expression measured by hL243γ4P binding, SU-DHL-6 has higher CD20 expression, Namalwa and FSCCL lower CD20 expression, and RL approximately equal expression of both antigens.
Binding of humanized or chimeric mAbs on B-cell lines
. | Geometric mean fluorescence . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | None . | Anti-CEA: hMN14 . | Anti-CD74: hLL1 . | Anti-CD22: hLL2 . | Anti-CD20: hA20 . | Anti-CD20: rituximab . | Anti-HLA-DR: hL243γ4P . | ||||||
| Daudi | 3.2 | 3.2 | 48.8 | 51.7 | 241.0 | 380.5 | 937.4 | ||||||
| Namalwa | 2.6 | 2.4 | 7.8 | 6.4 | 10.1 | 14.1 | 260.9 | ||||||
| Raji | 6.9 | 7.0 | 95.1 | 22.6 | 267.1 | 394.6 | 972.0 | ||||||
| Ramos | 3.1 | 3.1 | 23.3 | 14.6 | 203.7 | 375.0 | 277.6 | ||||||
| RL | 2.4 | 2.8 | 7.9 | 5.1 | 127.5 | 147.8 | 112.2 | ||||||
| SU-DHL-6 | 4.6 | 4.9 | 17.3 | 11.0 | 1199.3 | 1308.9 | 572.3 | ||||||
| FSCCL | 2.7 | 2.7 | 8.7 | 4.2 | 8.9 | 12.5 | 466.8 | ||||||
. | Geometric mean fluorescence . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | None . | Anti-CEA: hMN14 . | Anti-CD74: hLL1 . | Anti-CD22: hLL2 . | Anti-CD20: hA20 . | Anti-CD20: rituximab . | Anti-HLA-DR: hL243γ4P . | ||||||
| Daudi | 3.2 | 3.2 | 48.8 | 51.7 | 241.0 | 380.5 | 937.4 | ||||||
| Namalwa | 2.6 | 2.4 | 7.8 | 6.4 | 10.1 | 14.1 | 260.9 | ||||||
| Raji | 6.9 | 7.0 | 95.1 | 22.6 | 267.1 | 394.6 | 972.0 | ||||||
| Ramos | 3.1 | 3.1 | 23.3 | 14.6 | 203.7 | 375.0 | 277.6 | ||||||
| RL | 2.4 | 2.8 | 7.9 | 5.1 | 127.5 | 147.8 | 112.2 | ||||||
| SU-DHL-6 | 4.6 | 4.9 | 17.3 | 11.0 | 1199.3 | 1308.9 | 572.3 | ||||||
| FSCCL | 2.7 | 2.7 | 8.7 | 4.2 | 8.9 | 12.5 | 466.8 | ||||||
An indirect flow cytometry assay was performed using FITC-GAH Fc-specific second antibody staining
Effector function assays
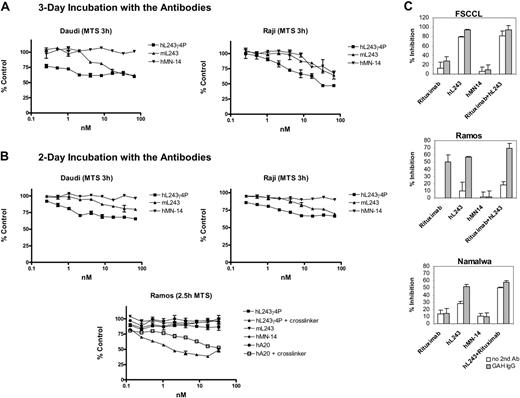
The goal of replacing the Fc region of hL43 with an IgG4 isotype Fc region was to abrogate effector cell functions through Fc receptor and complement binding. To assess CDC, Daudi cells were incubated with serial dilutions of the antibodies hL243γ1, hL243γ4P, hA20 (positive control), and hMN14 (negative control) in the presence of human complement, followed by the addition of the fluorescent dye resazurin to assess cell viability. The fluorescence level obtained is directly correlated with the number of viable cells. The results indicate that hL243γ4P does not produce any complement-mediated cytotoxic effect on cells compared with hL243γ1 (EC50 = 2.6 nM) and hA20 (EC50 = 0.66 nM) where CDC was observed (Figure 2A). The assay was repeated in Raji, Namalwa, and Ramos cell lines (using 51Cr to label target cells) and included rituximab and mL243 as positive controls. The observed activity of mL243 and the lack of activity of hL243γ4P were confirmed in these assays. Rituximab activity was higher than mL243 activity in Raji and Ramos and lower in Namalwa (Figure 2B), presumably due to its low CD20 expression (Table 1 and Goulet et al42 ).
Assessment of CDC and ADCC. (A) Daudi cells were treated with hL243γ4P or control mAbs, as indicated, at the concentrations shown in the presence of human complement. Cell viability was measured using resazurin and reported as percentage of viable population relative to cells treated with complement only (no mAb). (B) 51Cr-labeled NHL cell lines were incubated with anti-B-cell mAbs in the presence of human complement. Following a 3-hour incubation at 37°C, supernatants were collected and counted. Percentage of specific lysis of 3 cell lines is shown. (C) Calcein-AM cytotoxicity release assay for measurement of ADCC. Labeled NHL cell lines were incubated with anti-B-cell mAbs in the presence of human mononuclear cells. Following a 4-hour incubation at 37°C, supernatants were harvested and transferred to new plates. Samples were measured using a Spectromax Gemini dual-scanning microplate spectrofluorimeter (Molecular Devices, Sunnyvale, CA); excitation filter, 485 nm; bandpass filter, 530 nm. Percentage of specific lysis of 3 cell lines is shown. ▪, with PBMCs; □, without PBMCs. Error bars represent SD.
Assessment of CDC and ADCC. (A) Daudi cells were treated with hL243γ4P or control mAbs, as indicated, at the concentrations shown in the presence of human complement. Cell viability was measured using resazurin and reported as percentage of viable population relative to cells treated with complement only (no mAb). (B) 51Cr-labeled NHL cell lines were incubated with anti-B-cell mAbs in the presence of human complement. Following a 3-hour incubation at 37°C, supernatants were collected and counted. Percentage of specific lysis of 3 cell lines is shown. (C) Calcein-AM cytotoxicity release assay for measurement of ADCC. Labeled NHL cell lines were incubated with anti-B-cell mAbs in the presence of human mononuclear cells. Following a 4-hour incubation at 37°C, supernatants were harvested and transferred to new plates. Samples were measured using a Spectromax Gemini dual-scanning microplate spectrofluorimeter (Molecular Devices, Sunnyvale, CA); excitation filter, 485 nm; bandpass filter, 530 nm. Percentage of specific lysis of 3 cell lines is shown. ▪, with PBMCs; □, without PBMCs. Error bars represent SD.
Induction of ADCC was measured in 3 cell lines, Raji, Daudi, and SU-DHL-6, by calcein-am release. The activity of hL243γ4P was compared with that of mL243 and rituximab as positive controls. As expected, rituximab and mL243 but not hL243γ4P induced significantly more cell lysis than the negative controls, no mAb, and murine and humanized MN-14 (Figure 2C).
In vitro antiproliferative effects
Colorimetric assays using both MTS (for determination of the number of viable cells) and bromodeoxyuridine (BrdU) (for quantification of cell proliferation based on the measurement of BrdU incorporation during DNA synthesis) were performed. Daudi and Raji cells were incubated with serial dilutions of hL243γ4P for 2 and 3 days. Murine L243 and hMN14 were used as positive and negative controls, respectively. Results of the MTS assays performed following mAb incubations are shown in Figure 3A-B. BrdU assays gave similar results (not shown). These findings indicate that hL243γ4P inhibits proliferation of Raji and Daudi cell lines. However, in similar experiments in Ramos, which has a lower expression of HLA-DR compared with Raji or Daudi (Table 1), inhibition of proliferation was observed only in the presence of a cross-linking anti-Fc second antibody.
The effect of hL243γ4P on cellular proliferation was also assessed using the 3H-thymidine uptake assay on Ramos, FSCCL, and Namalwa. The effect of hL243γ4P was compared with that of rituximab and with rituximab combined with hL243γ4P in the presence or absence of a cross-linking anti-Fc antibody (Figure 3C; Table 2 [includes P values]). In FSCCL, which we previously showed to be relatively insensitive to rituximab,31 hL243γ4P yielded significantly greater inhibition of proliferation than rituximab. Because of the high level of activity of the hL243γ4P, the effect was not increased by using it in combination with rituximab. In Ramos, hL243γ4P and rituximab antiproliferative activities were similar, and the combination was more effective than either alone. Consistent with the results shown in Figure 3B, cross-linking with an anti-human Fc antibody is required for significant antiproliferative activity to be seen in Ramos. In Namalwa, as with FSCCL, hL243γ4P yielded significantly greater inhibition of proliferation than rituximab, and the combination of rituximab and hL243γ4P yielded significantly more inhibition of proliferation than either mAb alone.
Antiproliferative activity of mAbs with and without cross-linking
Antiproliferative activity of mAbs . | Rituximab + hL243γ4P . | Rituximab (P) . | hL243γ4P (P) . |
|---|---|---|---|
| Without cross-linking | |||
| Ramos | 18.2 ± 4.9 | –7.9 ± 3.6 (.001) | 10.1 ± 11.9 (.362) |
| FSCCL | 75.9 ± 10.2 | 13.4 ± 12.3 (.003) | 78.9 ± 1.7 (.661) |
| Namalwa | 50.1 ± 1.1 | 13.8 ± 5.6 (.006) | 27.8 ± 3.3 (.004) |
| In the presence of antihuman second antibody | |||
| Ramos | 69.0 ± 7.0 | 50.5 ± 9.4 (.052) | 56.8 ± 0.8 (.007) |
| FSCCL | 94.5 ± 0.9 | 28.1 ± 9.6 (.007) | 94.5 ± 0.8 (.998) |
| Namalwa | 58.1 ± 2.1 | 14.7 ± 7.0 (.005) | 51.5 ± 3.0 (.042) |
Antiproliferative activity of mAbs . | Rituximab + hL243γ4P . | Rituximab (P) . | hL243γ4P (P) . |
|---|---|---|---|
| Without cross-linking | |||
| Ramos | 18.2 ± 4.9 | –7.9 ± 3.6 (.001) | 10.1 ± 11.9 (.362) |
| FSCCL | 75.9 ± 10.2 | 13.4 ± 12.3 (.003) | 78.9 ± 1.7 (.661) |
| Namalwa | 50.1 ± 1.1 | 13.8 ± 5.6 (.006) | 27.8 ± 3.3 (.004) |
| In the presence of antihuman second antibody | |||
| Ramos | 69.0 ± 7.0 | 50.5 ± 9.4 (.052) | 56.8 ± 0.8 (.007) |
| FSCCL | 94.5 ± 0.9 | 28.1 ± 9.6 (.007) | 94.5 ± 0.8 (.998) |
| Namalwa | 58.1 ± 2.1 | 14.7 ± 7.0 (.005) | 51.5 ± 3.0 (.042) |
Numbers represent percent inhibition of 3H-thymidine uptake, and those in parentheses represent P values of the single mAbs compared with the combination of rituximab and hL243γ4P
Assessment of apoptosis induction
To evaluate the mechanism of hL243γ4P-induced cell death, assays measuring various markers of apoptosis were performed. These included induction of DNA fragmentation, detection of phosphatidylserine exposure on the external membrane, measurement of activated caspase-3, loss of mitochondrial membrane potential, and activation of the AKT survival pathway.
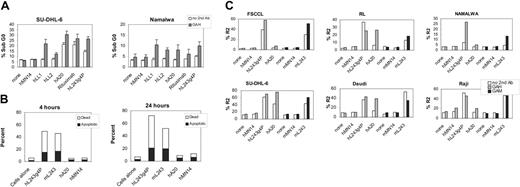
DNA fragmentation was evaluated by flow cytometry in SU-DHL-6 and Namalwa. Cells were cultured with the mAbs for 48 hours with or without a second mAb for cross-linking, followed by DNA staining with PI. Cells were analyzed by flow cytometry, and positive fluorescence below the G1 region represents DNA fragmentation and is a measure of apoptosis/cell death. Activity of hL243γ4P was compared with that of humanized mAbs against other B-cell antigens, including anti-CD74 (hLL1), anti-CD22 (hLL2, epratuzumab), anti-CD20 (hA20), and the murine-human chimeric mAb, rituximab. hL243γ4P induced the production of hypodiploid DNA in both cell lines at levels similar to or greater than the other anti-B-cell mAbs (Figure 4A).
Antiproliferative effects of hL243γ4P alone and in combination with rituximab. Effects of mAbs on proliferation of NHL cells lines were determined by MTS assays (A-B) and 3H-thymidine uptake assays. (C) Effect of combining hL243γ4P and rituximab on proliferation of cell lines. Cells were cultured with the mAbs with or without a second antibody for cross-linking. Error bars represent SD of triplicates. On the x-axis, hL243 refers to the γ4P form.
Antiproliferative effects of hL243γ4P alone and in combination with rituximab. Effects of mAbs on proliferation of NHL cells lines were determined by MTS assays (A-B) and 3H-thymidine uptake assays. (C) Effect of combining hL243γ4P and rituximab on proliferation of cell lines. Cells were cultured with the mAbs with or without a second antibody for cross-linking. Error bars represent SD of triplicates. On the x-axis, hL243 refers to the γ4P form.
The Guava Nexin kit was used to discriminate between apoptotic and nonapoptotic dead cells in Daudi lymhoma cells. This kit uses annexin V-PE to detect phosphatidylserine on the external membrane of apoptotic cells and a cell-impermeant dye, 7-AAD, as an indicator of membrane structural integrity. As shown in Figure 4B, the results of this study indicate that hL243γ4P induced levels of apoptosis similar to mL243 following both 4-hour and 24-hour treatment. In contrast, the anti-CD20 mAb did not induce measurable apoptosis in Daudi. This confirms previous reports demonstrating that hypercross-linking by a secondary agent, such as anti-human IgG or protein A, is necessary for induction of apoptosis by anti-CD20 mAbs in many cell lines, including Daudi.31,43
The effect of the humanized and murine L243 on mitochondrial potential was studied in SU-DHL-6, Daudi, Raji, FSCCL, RL, and Namalwa. Results summarized in Figure 4C demonstrate that apoptotic changes in the mitochondrial membrane potential are observed with both the murine and humanized L243 mAbs. Crosslinking with a second antibody is not needed but does increase the effect in 2 of 6 cell lines evaluated, FSCCL and Namalwa. The loss of mitochondrial membrane potential induced by hL243γ4P was markedly greater than that of the anti-CD20 mAb, hA20, without a cross-linking agent. With cross-linking, the hA20 levels are increased to those of hL243γ4P in 3 of the 6 cell lines (RL, SU-DHL-6, and Daudi).
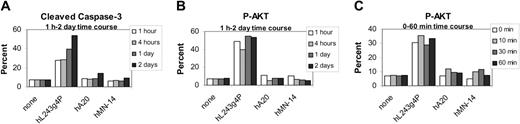
Induction of activated caspase-3 by humanized and murine L243 was assayed by flow cytometry in a panel of lymphoma cell lines. Results summarized in Table 3 show that both the murine and humanized L243 induce activation of caspase-3, at similar levels, in the absence of cross-linking with a second antibody. The induction of activated caspase-3 with the L243 mAbs is greater in all cell lines than that of hA20. With a second antibody, these levels are increased and the effect of hA20 is similar to that of the hL243γ4P, except in Namalwa and FSCCL, 2 cell lines that we routinely observe to be relatively insensitive to anti-CD20 mAbs. Cleaved caspase-3 also was assayed in Daudi over a 2-day time course (Figure 5A). We observed that the activity continues to increase for the 2 days of hL243γ4P incubation. Time points less than 1 hour were not determined.
Results of cleaved caspase-3 assay
. | Humanized mAbs, % relative to no mAb control . | . | . | Murine mAbs, % relative to no mAb control . | . | |||
|---|---|---|---|---|---|---|---|---|
. | hL243γ4P . | hA20 . | hMN-14 . | mL243 . | mMN-14 . | |||
| No cross-linking | ||||||||
| Ramos | 26.9 | 3.2 | 0.8 | 15.8 | 3.9 | |||
| Namalwa | 18.4 | –0.1 | 0.2 | 9.4 | 0.5 | |||
| FSCCL | 46.4 | 0.7 | 0.3 | 26.2 | –0.7 | |||
| Daudi | 48.1 | 7.9 | 0.9 | 45.8 | 1.0 | |||
| RL | 22.5 | 1.5 | –0.1 | 18.2 | –0.3 | |||
| SU-DHL-6 | 52.2 | 30.9 | 2.3 | 46.5 | 0.2 | |||
| Raji | 22.5 | 1.5 | –0.1 | 18.2 | –0.3 | |||
| With second antibody | ||||||||
| Ramos | 71.7 | 67.8 | 7.3 | 40.3 | 3.0 | |||
| Namalwa | 72.2 | 20.4 | 7.9 | 25.2 | –0.3 | |||
| FSCCL | 86.7 | 20.0 | 8.4 | 55.0 | 1.5 | |||
| Daudi | 68.9 | 72.0 | 2.9 | 51.2 | 0.0 | |||
| RL | 37.3 | 24.2 | 4.0 | 4.0 | 0.7 | |||
| SU-DHL-6 | 72.1 | 75.8 | 5.5 | 51.4 | –0.9 | |||
| Raji | 59.8 | 37.4 | 2.8 | 20.4 | –0.3 | |||
. | Humanized mAbs, % relative to no mAb control . | . | . | Murine mAbs, % relative to no mAb control . | . | |||
|---|---|---|---|---|---|---|---|---|
. | hL243γ4P . | hA20 . | hMN-14 . | mL243 . | mMN-14 . | |||
| No cross-linking | ||||||||
| Ramos | 26.9 | 3.2 | 0.8 | 15.8 | 3.9 | |||
| Namalwa | 18.4 | –0.1 | 0.2 | 9.4 | 0.5 | |||
| FSCCL | 46.4 | 0.7 | 0.3 | 26.2 | –0.7 | |||
| Daudi | 48.1 | 7.9 | 0.9 | 45.8 | 1.0 | |||
| RL | 22.5 | 1.5 | –0.1 | 18.2 | –0.3 | |||
| SU-DHL-6 | 52.2 | 30.9 | 2.3 | 46.5 | 0.2 | |||
| Raji | 22.5 | 1.5 | –0.1 | 18.2 | –0.3 | |||
| With second antibody | ||||||||
| Ramos | 71.7 | 67.8 | 7.3 | 40.3 | 3.0 | |||
| Namalwa | 72.2 | 20.4 | 7.9 | 25.2 | –0.3 | |||
| FSCCL | 86.7 | 20.0 | 8.4 | 55.0 | 1.5 | |||
| Daudi | 68.9 | 72.0 | 2.9 | 51.2 | 0.0 | |||
| RL | 37.3 | 24.2 | 4.0 | 4.0 | 0.7 | |||
| SU-DHL-6 | 72.1 | 75.8 | 5.5 | 51.4 | –0.9 | |||
| Raji | 59.8 | 37.4 | 2.8 | 20.4 | –0.3 | |||
It has been shown previously that HLA ligation on B cells promotes signal transduction via tyrosine phosphorylation of Syk and the downstream phosphatidylinositol-3 kinase (PI3 kinase)/AKT pathway.4,44 AKT is a protein that plays a critical role in controlling the balance between survival and apoptosis. The involvement of AKT in the mechanism of action of L243 was assayed in 6 cell lines by flow cytometry following treatment by both the murine and humanized IgG4 forms of the antibody. Cells were incubated with mAbs for 2 days and then assayed for activation of AKT as measured by phosphorylation of the amino acid residue Ser473. The results provided in Table 4 show that both forms of L243 activate AKT in all cell lines. The level of AKT phosphorylation following L243 treatment is variable among the cell lines and does not correlate with HLA-DR expression. Phospho-AKT levels in anti-CD20 (hA20)-treated cells as well as anti-CD74- and anti-CD22-treated cells (not shown) are similar to untreated cells on all cell lines. To determine the time course of P-AKT activation, Daudi cells were incubated with mAbs for various times; mAbs were removed (by centrifugation) at time points from 0 minutes up to 2 days (Figure 5B). These results show that the activation of AKT by L243 occurs faster than can be measured by this assay, because even at the 0 time point P-AKT levels are equal to the 2-day time point.
Results of P-AKT assay
. | Humanized mAbs, % relative to no mAb control . | . | . | Murine mAbs, % relative to no mAb control . | . | |||
|---|---|---|---|---|---|---|---|---|
. | hL243γ4P . | hA20 . | hMN-14 . | mL243 . | mMN-14 . | |||
| Namalwa | 8.4 | –2.8 | 1.3 | 3.5 | –4.4 | |||
| FSCCL | 25.1 | –1.4 | 3.9 | 16.3 | –1.7 | |||
| Daudi | 34.9 | 1.0 | –1.4 | 24.5 | –2.1 | |||
| RL | 5.9 | 1.8 | 0.0 | 1.3 | 1.3 | |||
| SU-DHL-6 | 29.8 | 0.2 | 1.2 | 26.1 | –0.5 | |||
| Raji | 5.1 | –0.9 | –1.6 | 17.2 | –4.2 | |||
. | Humanized mAbs, % relative to no mAb control . | . | . | Murine mAbs, % relative to no mAb control . | . | |||
|---|---|---|---|---|---|---|---|---|
. | hL243γ4P . | hA20 . | hMN-14 . | mL243 . | mMN-14 . | |||
| Namalwa | 8.4 | –2.8 | 1.3 | 3.5 | –4.4 | |||
| FSCCL | 25.1 | –1.4 | 3.9 | 16.3 | –1.7 | |||
| Daudi | 34.9 | 1.0 | –1.4 | 24.5 | –2.1 | |||
| RL | 5.9 | 1.8 | 0.0 | 1.3 | 1.3 | |||
| SU-DHL-6 | 29.8 | 0.2 | 1.2 | 26.1 | –0.5 | |||
| Raji | 5.1 | –0.9 | –1.6 | 17.2 | –4.2 | |||
Apoptotic effect of mAbs on NHL cell lines. (A) Induction of apoptosis was evaluated by flow cytometry determination of haploid DNA on the cell line panel with and without a second antibody for cross-linking, followed by staining with propidium iodide. Error bars represent SD. (B) Apoptosis was quantified in Daudi using annexin V/7-AAD staining. Percentage of apoptotic cells refers to the annexin V-positive, 7-AAD-negative cells; percentage of dead cells refers to the annexin V-positive, 7-AAD-positive population. Cells were 97% viable prior to treatment. (C) Changes in mitochondrial membrane potential were measured by flow cytometry using the JC-1 reagent following antibody incubation in the presence or absence of second antibody. GAM indicates F(ab′)2 goat anti-mouse IgG, Fcγ specific; GAH, F(ab′)2 goat anti-human IgG, Fcγ-specific antibody.
Apoptotic effect of mAbs on NHL cell lines. (A) Induction of apoptosis was evaluated by flow cytometry determination of haploid DNA on the cell line panel with and without a second antibody for cross-linking, followed by staining with propidium iodide. Error bars represent SD. (B) Apoptosis was quantified in Daudi using annexin V/7-AAD staining. Percentage of apoptotic cells refers to the annexin V-positive, 7-AAD-negative cells; percentage of dead cells refers to the annexin V-positive, 7-AAD-positive population. Cells were 97% viable prior to treatment. (C) Changes in mitochondrial membrane potential were measured by flow cytometry using the JC-1 reagent following antibody incubation in the presence or absence of second antibody. GAM indicates F(ab′)2 goat anti-mouse IgG, Fcγ specific; GAH, F(ab′)2 goat anti-human IgG, Fcγ-specific antibody.
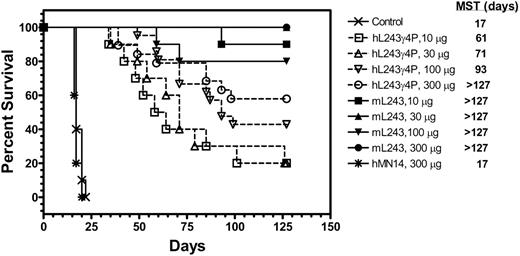
In vivo therapeutic efficacy of hL243γ4P in an NHL xenograft model (Raji)
A therapeutic study was performed to compare the in vivo efficacy of hL243γ4P and mL243 in a xenograft model of human NHL (Raji). Therapy was initiated 1 day after tumor cell administration; results are shown in Figure 6. Mice given injections of saline or nonspecific control antibody, hMN14, had a median survival time of 17 days. All the groups of mice treated with either humanized or mL243 had significantly improved life spans compared with mice given injections of saline or hMN14 (P < .001). Treatment with various doses of hL243γ4P resulted in a dose-response relationship, with mice receiving higher doses having better survival times. In the group of animals treated with various doses of mL243, the cure rate was in the range of 80% to 100%. Thus, despite the lack of effector functions (ADCC and CDC), hL243γ4P significantly prolonged the survival of animals compared with animals receiving saline. No evidence of toxicity was observed at any dose level, as assayed by body weight measurement.
Discussion
hL243γ4P is a humanized IgG4 form of the anti-HLA-DR mAb L243, generated to fill the need for a selective agent that can kill neoplastic B cells without CDC or ADCC. Characterization of hL243γ4P demonstrated that antigen-binding specificity, antiproliferative activity, and the ability to induce apoptosis and activation of the AKT survival pathway are preserved, while CDC and ADCC activity have been eliminated. Analogous to its murine counterpart, hL243γ4P bound to the 7 B-cell lymphoma cell lines examined, spanning Burkitt to diffuse large cell lymphoma. The cell lines vary in their levels of expression of HLA-DR and various other B-cell antigens, mAbs against which are either in clinical use or under evaluation as therapeutic agents for treatment of B-cell malignancies, including CD20, CD22, and CD74. Incubation of the B-lymphoma cell lines with hL243γ4P yielded substantial growth inhibition in Daudi, Raji, and FSCCL cell lines. In cell lines that express less HLA-DR, namely, Namalwa and Ramos, the effect was less pronounced but was increased by cross-linking with a second antibody.
Because it has been shown that CD20 and HLA-DR are physically and functionally coupled on B cells,45 the growth inhibition by hL243γ4P was evaluated in comparison with and in combination with the anti-CD20 mAb, rituximab. Importantly, the combination of the 2 mAbs was more effective than rituximab alone, implying that combination therapy may be more effective in patients. Moreover, hL243γ4P was also able to inhibit proliferation in a rituximab-resistant cell line, indicating that the anti-HLA-DR mAb can exert a therapeutic effect independently of rituximab. Enhanced therapeutic activity has also been reported by DeNardo et al for the combination of rituximab and Lym-1 in an in vitro evaluation in human lymphoma cell lines.11
In addition to the in vitro antiproliferative activity of hL243γ4P, a therapeutic effect was obtained in vivo using a SCID mouse model of disseminated NHL. hL243γ4P treatment yielded a significant survival benefit, suggesting that direct cytotoxic mechanisms rather than ADCC and CDC are responsible. At the highest hL243γ4P dose evaluated, we observed a 60% cure rate (defined as survival more than 127 days) compared with a 17-day median survival time in control mice. The mL243 yielded a greater survival rate in this study, with up to a 100% cure rate at the highest dose, suggesting that effector functions add to the antibody's efficacy. Human Fcγ receptors are known to have low affinity for the human IgG4 isotope.46 This was confirmed functionally by the absence of induction of ADCC by hL243γ4P in the in vitro assays. Specificity of murine Fcγ receptors II and III for human IgG4 was also reported to be lower than for human IgG1 and IgG3; however, a low level of Fc receptor binding may be present,46 and the possibility that in the mouse, ADCC may contribute somewhat to the therapeutic effect should be investigated further. No CDC activity could be detected using the SCID mouse serum (data not shown).
Time course studies in Daudi. (A) Cleaved caspase-3 and (B-C) P-AKT.
Therapeutic efficacy of hL243γ4P and murine L243 in Raji-bearing SCID mice. SCID mice (10 per group) were given injections of 2.5 × 106 Raji cells. Treatments were initiated 1 day after injection of cells and continued twice weekly for 4 weeks. Control animals were treated with saline. MST indicates median survival time.
Therapeutic efficacy of hL243γ4P and murine L243 in Raji-bearing SCID mice. SCID mice (10 per group) were given injections of 2.5 × 106 Raji cells. Treatments were initiated 1 day after injection of cells and continued twice weekly for 4 weeks. Control animals were treated with saline. MST indicates median survival time.
Nagy et al47 generated humanized anti-HLA-DR mAbs by screening the Human Combinatorial Antibody Library and engineered HLA-DR-specific antibodies of the IgG4 isotype. As noted in this report with hL243γ4P, the antibodies exhibited in vitro and in vivo cytotoxicity on lymphoma cell lines without the need for exogenous immunologic effector mechanisms. Interestingly, these mAbs were found to kill activated but not resting normal B cells in addition to tumor cells, suggesting a dual requirement for both MHC-II expression and cell activation for antibody-induced death. These data suggest that because most peripheral B cells are resting, the potential side effect due to killing of normal B cells may be minimal.
Several groups have reported that anti-HLA-DR antibodies targeting either the α or β chain induce apoptosis in B-cell malignancies. However, the mechanism of HLA-DR-mediated cell death remains controversial. There have been reports of anti-HLA-DR inducing apoptosis directly5 or through CD95 (Fas)-mediated mechanisms.41 Other studies describe caspase-dependent or caspase-independent pathways.4,48 The lack of agreement between these results may reflect the use of mAbs recognizing different HLA epitopes as well as the various cell models evaluated and different technical approaches used. Mone et al4 demonstrated that Hu1D10 induces caspase-independent apoptosis following secondary cross-linking in primary chronic lymphocytic leukemia cells; however, when used alone, in the absence of cross-linking, the Hu1D10 antibody did not induce apoptosis. They reported generation of reactive oxygen species, signaling through Syk and AKT, and loss of mitochondrial potential following incubation with Hu1D10 plus anti-human Fc antibody. This contrasts with our observations, in that hL243γ4P can induce apoptosis without hypercross-linking by anti-Fc antibodies and caspase-3 activation was detected. Consistent with the report of Mone et al,4 hL243γ4P induced loss of mitochondrial potential and AKT phosphorylation.
Thus, the anti-HLA-DR antibody, hL243γ4P, is a novel agent that possesses the intended functional attributes when tested in cell-based assays and in vivo in an animal model. Although it is indistinguishable from hL243γ1, the humanized IgG1, and the parental murine mAb, in assays dependent on antigen recognition, the abrogation of ADCC and CDC (which are believed to play a major role in mediating side effects) may make this antibody a more attractive agent for clinical applications.
Prepublished online as Blood First Edition Paper, June 15, 2006; DOI 10.1182/blood-2006-04-017921.
Supported in part by United States Public Health Service grant 1P01CA103985 from the National Cancer Institute, National Institutes of Health.
One of the authors (D.M.G.) has declared a financial interest in a company (Immunomedics, Inc) whose potential product was studied in the present work.
Several of the authors (D.S., Z.Q., D.M.G., H.J.H.) are employed by a company (Immunomedics, Inc) whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal