Abstract
Dendritic cells (DCs) are specialized antigen-presenting cells that play crucial roles in the initiation and regulation of immune responses. Maturation and activation of DCs are controlled by a balance of the inhibitory and activating signals transduced through distinct surface receptors. Many inhibitory receptors expressed by DCs have been identified, whereas the new members and their functions need further investigation. In this study, we functionally characterized DC-derived immunoglobulin receptor 2 (DIgR2) as a novel representative of a family of inhibitory receptors belonging to the immunoglobulin superfamily. We show that DIgR2 contains 2 immunoreceptor tyrosine-based inhibitory motifs (ITIMs) within its cytoplasmic region and that DIgR2 associates with Src homology-2 domain-containing protein tyrosine phosphatases-1 (SHP-1). Blockade of DIgR2 on DCs by pretreatment with DIgR2-Ig fusion protein or by silencing with specific small interfering RNA enhances DC-initiated T-cell proliferation and antigen-specific T-cell responses both in vitro and in vivo. Furthermore, immunization of mice with antigen-pulsed, DIgR2-silenced DCs elicits more potent antigen-specific CD4+ and CD8+ T-cell responses, thus protecting the vaccinated mice from tumor challenge more effectively. Our data suggest that DIgR2 is a functionally inhibitory receptor and can mediate negative signaling to regulate DC-initiated antigen-specific T-cell responses.
Introduction
Dendritic cells (DCs) play critical roles in the initiation of immune responses and induction of tolerance.1 Balanced signaling transmitted via different activating and inhibitory receptors in DCs can regulate the functional status of DCs, thus determining duration and magnitude of T-cell responses.2,3 Among the immune receptor families known to be expressed by DCs, the immunoglobulin superfamily (IgSF) seems to be unique in terms of structural features and roles in the regulation of DC development and function.4 IgSF receptors are mostly type I membrane proteins, characterized by 1 or more immunoglobulin-like domains in the extracellular regions, which are pivotal in cell-cell recognition and interaction. IgSF receptors are abundant in the immune system and are widely distributed in lymphoid and myeloid cells including T cells, B cells, monocytes, DCs, and natural killer (NK) cells. It is well recognized that a vast quantity of IgSF molecules are involved in shaping the synapse formed between T cells and antigen-presenting cells (APCs), regulating various processes ranging from antigen (Ag) recognition and cell-cell interaction to signal transduction.5
Many immune molecules belonging to the IgSF have recently been isolated and defined as key players in the innate and adaptive immune response, some of which act as inhibitory receptors with profound influence on immune responses. Inhibitory receptors are characterized by the presence of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic regions. Upon engagement with ligands, ITIMs contained in the inhibitory receptors serve as scaffolds for recruitment of protein-tyrosine phosphatases to mediate negative signaling,6 maintaining adequate thresholds for cell activation to avoid irrelevant cell activation and induction of autoimmunity.7
Considering the critical roles of DCs in the immune response, much attention has been focused on the isolation and functional analysis of receptors on DCs, especially on inhibitory receptors. Immunoglobulin-like transcript 3 (ILT3) and ILT4, 2 Ig superfamily receptors containing ITIMs, can negatively regulate antigen presentation, T-cell costimulation, and cytokine production by DCs. Up-regulation of ILT3/ILT4 by suppressor T cells is responsible for the tolerogenecity of monocytes and DCs.8,9 Similarly, paired immunoglobulin-like inhibitory receptor B (PIR-B), a murine homologue of ILT4, has also been defined as a key negative regulator of DC functions, and impaired maturation of DCs and imbalanced T helper 1 (Th1) and Th2 immune responses occurred in PIR-B-/- mice.10,11 Despite the fact that many inhibitory receptors belonging to Ig superfamily have been identified, unidentified members expressed by DCs remain to be isolated and investigated, and the identification of novel inhibitory immunoreceptors should further elucidate the sophisticated interactions between DCs and T cells.
Human CMRF35-A, previously isolated by recognition with the CMRF-35 monoclonal antibody (mAb), is a surface molecule with an IgV-like domain.12 Chromosome mapping and gene database searches have located CMRF35-A on human chromosome 17,13 where CMRF35-A and at least 6 other genes constitute an Ig family. Members of this family are involved in multiple facets of the immune regulation, such as modulation of mast cell activation and regulation of NK cell and eosinophil activity.14-16 Moreover, single nucleotide polymorphisms in this cluster are linked with psoriasis, atopic dermatitis, and psoriatic arthritis, underscoring the importance of CMRF35-A-like molecules in the regulation of immune response and immunologic pathogenesis.17,18
By searching a murine expressed sequence tags (ESTs) database19 with the human CMRF-35A sequence, we previously identified several novel receptors, one of which is preferentially expressed on APCs including DCs and designated as DC-derived immunoglobulin receptor 1 (DIgR1).20 Here we report the functional identification of another IgSF receptor, designated as DIgR2. We convincingly demonstrate that DIgR2 acts functionally as an inhibitory receptor and mediates negative regulation of DC-initiated T-cell responses, thus outlining a new pathway for immune regulation and providing a new target for immunotherapy.
Materials and methods
Animals and cells
Male C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from Shanghai Joint Venture SIPPR BK Experimental Animal (Shanghai, China) and maintained in a pathogen-free environment. Ovalbumin (OVA) (323-339)-specific T-cell receptor (TCR)-transgenic mice (DO11.10) were obtained from the Jackson Laboratory (Bar Harbor, ME). CD3+ T cells, CD4+ T cells, CD8+ T cells, B cells, and NK cells were freshly isolated from mouse splenocytes with CD3, CD4, CD8a, CD19, and NK1.1 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), respectively. Peritoneal macrophages were aspirated from peritoneal exudates. Murine bone marrow-derived DCs (BM-DCs) were prepared as described previously.21 The EG7-OVA cell line is derived from the murine lymphoma EL-4 cells transfected with OVA cDNA (kindly provided by Prof E Gilboa, Duke University, Durham, NC). Other cell lines were obtained from the American Type Culture Collection (Manassas, VA).
Identification of DIgR2
By Basic Local Alignment Search Tool (BLAST) analysis of National Center for Biotechnology Information (NCBI) mouse EST databases22 to identify homologues to human CMRF-35, 3 mouse ESTs (GenBank accession nos. AA839051, AI391276, and W07987) were found to be highly homologous to human CMRF-35, from which mouse DIgR2 full-length cDNA was obtained by contig. Full-length DIgR2 cDNA was obtained from mouse DCs using primers 5′-GGA ATT CTG TTC TCG CTG GCA GGC TC-3′ (sense) and 5′-GCT CTA GAA GCC GCC TTC AGA GAC CAA GAT T-3′ (antisense) and is available in the GenBank database with accession no. AY214460. The multiple alignments and the phylogenetic analysis were performed with clustalx1.81 software.56
Analysis of DIgR2 expression pattern
Northern blotting and reverse transcription-polymerase chain reaction (RT-PCR) analysis of DIgR2 expression were performed as described previously.20 Real-time PCR was conducted with the SYBR green I master mix kit (Applied Biosystems, Foster City, CA) as instructed. DIgR2 expression was quantified as a threshold cycle value relative to that of β-actin. Primers used for DIgR2 were 5′-GCA TGT AGC CTG TTC TGC CTG-3′ (sense) and 5′-ATC ACC GAC GCC ATC TTC AC-3′ (antisense).
Preparation of recombinant GST-DIgR2 fusion protein and generation of anti-DIgR2 polyclonal antibody
The code region of DIgR2 extracellular region without signal peptide was inserted into pGEX-2T (Pharmacia Biotech, Piscataway, NJ). Purification of the fusion protein and the preparation of polyclonal antibody (Ab) were performed as described previously.20
Eukaryotic expression vector construction and transfection
DIgR2 cDNA was inserted directly, or in frame with Flag tag, into pcDNA3.1/myc-His(-)B vector (Invitrogen, Carlsbad, CA). NIH3T3 cells or L929 cells were transfected with the vectors using PoLyFect transfection reagent (Qiagen, Valencia, CA) as instructed.
Subcellular localization of DIgR2
NIH3T3 cells were transfected with DIgR2 or CD54 (intercellular adhesion molecule [ICAM], positive control) expression vector. Forty-eight hours later, cells were fixed in 4% paraformaldehyde, perforated with 0.1% Triton X-100, incubated with anti-DIgR2 polyclonal Ab or anti-CD54 mAb for 1 hour, and then labeled with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG or anti-mouse IgG. Stained cells were viewed under fluorescence confocal microscopy (Leica, Wetzlar, Germany).
Immunoprecipitation and Western blot assay
L929 cells were transfected with DIgR2-Flag expression vector and total cell lysates were prepared as described.23 For sodium pervanadate stimulation, 1 × 108 cells were pretreated with 0.03% H2O2 and 100 μM Na3VO4 (Sigma, St Louis, MO) for 10 minutes. After preabsorption with protein A agarose (Pierce, Rockford, IL), cell lysates were incubated with anti-Flag M2 agarose beads (Sigma) for 8 hours. Then immunoprecipitated pellets were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and probed with anti-p-Tyr, -SHP-1, or -SHP-2 Ab (Santa Cruz Biotechnology, Santa Cruz, CA).
Preparation of DIgR2-Ig fusion protein
The DIgR2-Ig construct was made by fusing the extracellular domain of DIgR2 (residues 1-561), or the extracellular region without the IgV domain (residues 328-561), in-frame to the human IgG1 Fc in the pIg-Tail Plus vector (R&D Systems, Minneapolis, MN). COS-7 cells were transfected by diethylaminoethanol (DEAE)-dextran, and the DIgR2-Ig fusion protein was purified by chromatography through a protein A column.23 SDS-PAGE and silver staining demonstrated a purity of greater than 90%.
Binding assays
Freshly isolated immune cells were either directly stained with 40 μg/mL DIgR2-Ig fusion protein, hIg, DIgR2-Ig fusion protein without the IgV domain, or GST-DIgR2 (400 μg/mL) mixed with DIgR2-Ig, for 30 minutes on ice. After 3 washes, cells were incubated with FITC-conjugated goat anti-human IgG (Sigma). Stained cells were analyzed on a FACSCalibar (Becton Dickinson, Mountain View, CA).24
siRNA preparation and transfection
Day 6 BM-DCs were transfected with 21-bp small interfering RNA (siRNA) oligonucleotides specific for DIgR2 (DIgR2-siRNA: 5′-GAUGGCGUCGGUGAUGGGUTT-3′) or with the mutated control (DIgR2-mutsiRNA: 5′-GAUGGCGUCUUUGAUGGGUTT-3′), using Geneporter (Genlantis, San Diego, CA). Silencing efficiency was confirmed by both RT-PCR and Western blot.25
Adenovirus-mediated RNA interference
Recombinant DIgR2 siRNA or DIgR2-mut-siRNA adenoviruses (AV-DIgR2-siRNA and AV-DIgR2-mut-siRNA) were generated using the pSilencer adeno 1.0-CMV System (Ambion, Austin, TX). For infection, day 6 BM-DCs were plated in 24-well plates (2 × 105 cells/well) in 400 μL serum-free RPMI 1640 and exposed to adenovirus at different multiplicities of infection (MOIs) for 6 hours, then were washed with PBS and further incubated in fresh DC medium for 2 to 3 days.26
Allogeneic MLR
T cells purified from BALB/c splenocytes using nylon wool columns were used as responders (2 × 105/well) and were cocultured with mitomycinpretreated DCs (2 × 103 to 20 × 103/well) in the presence of DIgR2-Ig or human Ig (50 μg/mL). Alternatively, siRNA-treated DCs were used as stimulators. A 72-hour mixed lymphocyte reaction (MLR) was performed and the cells were pulsed with 1 μCi (0.037 MBq) [3H] thymidine for the last 18 hours. Radioactivity was counted using a Wallac 1450 Microbeta liquid scintillation counter (Shelton, CT).
In vitro assays of antigen-specific T-cell responses
CD4+ T cells from DO11.10 × C57BL/6 F1 hybrid mice were used as antigen-specific responders and cocultured for 3 days with siRNA-transfected and OVA (323-339) peptide-pulsed DCs (T/DC ratio = 10:1).21 Proliferation of T cells was analyzed via a [3H]-TdR incorporation assay. Supernatants were collected 48 hours after initiation of the cocultures for detection of IL-2, IFN-γ, and IL-12p70 levels by enzyme-linked immunosorbent assay (ELISA; R&D Systems). For intracellular staining, brefeldin A (Sigma) was added 6 hours before the end of culture. After staining for CD4 or CD11c, cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences, San Jose, CA), followed by intracellular staining of IFN-γ or IL-12p70.21,25
In vivo assays of antigen-specific T-cell proliferation
OVA (323-339)-specific TCR-transgenic splenic CD4 T cells (5 × 106) from DO11.10 × C57BL/6 F1 mice were injected intraperitoneally into F1 (BALB/c × C57BL/6) mice. Twenty-four hours later, 5 × 106 DIgR2-siRNA, DIgR2-mut-siRNA transfected, or control DCs, loaded with OVA peptide (323-339) and matured with LPS (100 ng/mL) ex vivo, were transferred intraperitoneally into the same recipients. After 5 days, splenocytes were pooled and double-stained with anti-CD4-FITC and KJ1-26 (BD PharMingen, San Diego, CA).21
DC immunization and assays of antigen-specific CD8+ T-cell responses
Day 5 BM-DCs were transduced with AV-DIgR2-siRNA or AV-DIgR2-mutsiRNA at an MOI of 100 and 1 day later pulsed with OVA and stimulated with LPS (100 ng/mL; Sigma) for 24 hours. Then the prepared DCs (1 × 106/mouse) were injected subcutaneously into mice. Fourteen days later, splenocytes were pooled and antigen-specific T-cell responses were analyzed.
To detect OVA-specific CD8+ T cells, H2-Kb/OVA tetramer assays were performed as described previously.25,27 Splenocytes from the immunized mice were double-stained with anti-CD8a-FITC and H2-Kb/ovalbumin-PE (Proimmune, Oxford, United Kingdom). For intracellular staining of IFN-γ-producing CD8+ cells, splenocytes from the immunized mice were restimulated in vitro with OVA peptide (SIINFEKL) for 2 to 3 days, and analysis was performed as described.25,27
CD8+ cytotoxic T-lymphocyte (CTL) responses were assessed with a standard 4-hour chromium release assay27,28 following restimulation in vitro with OVA peptide for 6 days. Percentage of lysis was calculated as (cpm experimental release - cpm spontaneous release)/(cpm maximal release - cpm spontaneous release) × 100, where cpm indicates counts per minute.
Establishment of the tumor model
C57BL/6 mice were given subcutaneous injections of 1 × 106 Ag-loaded, wild-type, or siRNA-treated DCs twice (at weekly intervals). Seven days after the final immunization, mice were given subcutaneous injections of 2 × 106 EG7 cells.28,29 Tumor size was monitored daily and measured twice a week. The largest perpendicular diameters of the tumors were measured and the size was recorded as tumor area (mm2).
Statistical analysis
Data are shown as mean ± SD, and statistical significance was determined by the Student t test, with P values less than .05 as statistically significant.
Results
DIgR2 is a member of the Ig superfamily containing ITIMs
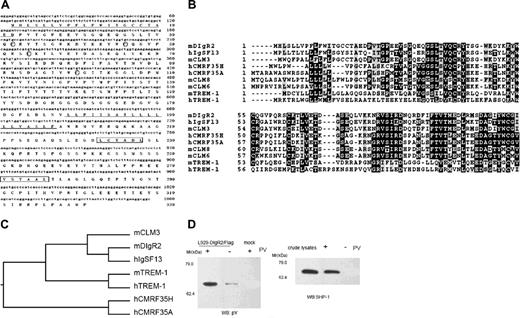
By comparing the mouse EST database22 with human CMRF35-A, we identified a mouse homologue of CMRF35-A. A full-length cDNA was isolated from mouse BM-DCs, which contained a single open reading frame encoding a transmembrane protein of 330 amino acids with a predicted molecular mass of 37 kDa (Figure 1A). The amino acid sequence begins with a hydrophobic signal peptide of 21 amino acids followed by an extracellular region composed of one IgV domain. The putative cytoplasmic domain is characterized by the presence of 2 tandem ITIMs spaced by one Tyr-x-x-Met motif, suggesting that an inhibitory signal might be transduced via these motifs. Therefore, this molecule could belong to the expanding Ig-like inhibitory receptor family and was designated as dendritic cell-derived Ig receptor 2 (DIgR2) following previous DIgR1 identification.20 DIgR2 is 96% identical to CLM-130 ; however, the extracellular region of DIgR2 is 7 residues longer than that of CLM-1. Genomic organization analysis revealed that DIgR2 and CLM-1 are derived from alternatively splicing in 2 different exon/intron junctions, so they may be 2 alternatively splicing variants. DIgR2 shows 48% sequence identity with IgSF13, a novel human inhibitory receptor of the Ig superfamily previously identified by our lab (Sui et al31 ). It is also closely related to other IgSF members, such as CLM-3 (81% identity) and murine triggering receptors expressed by myeloid cells-1 (TREM-1; 25% identity; Figure 1B-C).
Since DIgR2 contains 2 ITIMs in its cytoplasmic region, we investigated whether DIgR2 could associate with the tyrosine phosphatases SHP-1 and SHP-2. Such association represents a general way for inhibitory receptors to transmit their signals. We treated DIgR2-transfected L929 cells with sodium pervanadate and found that DIgR2 can be phosphorylated on the Tyr residue and could associate with SHP-1 (Figure 1D), indicating that DIgR2 is a structurally inhibitory IgSF receptor with the potential ability to transduce inhibitory signals.
DIgR2 is a membrane receptor preferentially expressed by APCs
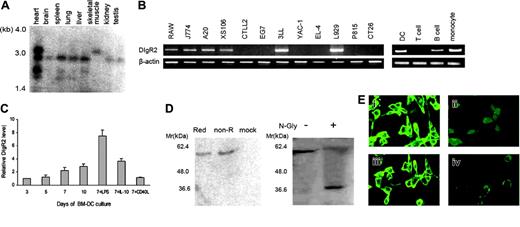
Northern blot analysis revealed that DIgR2 mRNA was widely distributed as approximately 1.4-kb and/or 3.0-kb transcripts in mouse tissues, including heart, brain, spleen, lung, liver, skeletal muscle, kidney, and testis, with a larger transcript (∼4.0 kb) highly expressed in heart tissues (Figure 2A). RT-PCR demonstrated that DIgR2 mRNA was highly expressed in monocytes (Raw264.7, J774), DCs (XS106), and B cells (A20) but not in T cells (EL-4, CTLL-2, and EG7). This expression pattern was further confirmed by analysis of freshly isolated primary cells (Figure 2B). Moreover, DIgR2 was also abundantly expressed by BM-DCs, and the level increased during culture of DCs. We found that DIgR2 is up-regulated by LPS (8-fold increase) and IL-10 (moderate increase) but is minimally up-regulated following CD40L stimulation (Figure 2C), indicating that DIgR2 might be involved in DC differentiation and activation.
DIgR2 is a member of the Ig superfamily containing ITIMs. (A) Sequences of nucleotides and deduced amino acids of DIgR2. Lines indicate signal and transmembrane sequence. The ITIM is boxed, and circled cysteines in the stalk region may allow homodimerization/heterodimerization through disulfide bridge. (B) Alignment of the amino acid sequence of DIgR2 with the most homologous human or mouse sequences. Identical amino acids are outlined in black and conservative substitutions are shaded. (C) Phylogenetic tree of the IgV of murine DIgR2 and related human and mouse sequences. (D) DIgR2 associates with SHP-1 in pervanadate-treated cells. L929 cells transiently transfected with DIgR2/Flag expression vector were pretreated with or without pervanadate (PV), then digitonin lysates of the cells were incubated with anti-Flag M2-agarose beads, and precipitates were subjected to Western blot (WB) analysis with antiphosphotyrosine (anti-pY; left) or anti-SHP-1 (right). Data represent 1 of 3 independent experiments.
DIgR2 is a member of the Ig superfamily containing ITIMs. (A) Sequences of nucleotides and deduced amino acids of DIgR2. Lines indicate signal and transmembrane sequence. The ITIM is boxed, and circled cysteines in the stalk region may allow homodimerization/heterodimerization through disulfide bridge. (B) Alignment of the amino acid sequence of DIgR2 with the most homologous human or mouse sequences. Identical amino acids are outlined in black and conservative substitutions are shaded. (C) Phylogenetic tree of the IgV of murine DIgR2 and related human and mouse sequences. (D) DIgR2 associates with SHP-1 in pervanadate-treated cells. L929 cells transiently transfected with DIgR2/Flag expression vector were pretreated with or without pervanadate (PV), then digitonin lysates of the cells were incubated with anti-Flag M2-agarose beads, and precipitates were subjected to Western blot (WB) analysis with antiphosphotyrosine (anti-pY; left) or anti-SHP-1 (right). Data represent 1 of 3 independent experiments.
To define the biochemical characteristics of DIgR2, we carried out immunoprecipitations from DIgR2-transfected NIH3T3 cells and detected a prominent band of 60 kDa under both nonreducing and reducing conditions, indicating that the mature protein did not form a disulfide-linked dimer despite several cysteine residues within its extracellular region. The discrepancy between the apparent molecular mass (∼60 kDa) and deduced mass (∼37 kDa) could result from glycosylation of the 2 N-linked glycosylation sites in the extracellular region of DIgR2, because the molecular mass was reduced to approximately 37 kDa after PNGase treatment (Figure 2D). Furthermore, we stained DIgR2-transfected NIH3T3 cells with anti-DIgR2 Ab and found DIgR2 expressed as a surface molecule on the cells (Figure 2E), consistent with its predicted protein structure.
DIgR2 binds with its ligand on T cells and inhibits DC-induced T-cell proliferation in vitro
The preferential expression of DIgR2 on APCs, especially inducible expression on DCs, suggested the possible involvement of DIgR2 in APC/T-cell interactions and possibly in immune responses. We examined lymphocyte and myeloid cell subsets for the presence of putative ligand(s) of DIgR2 and demonstrated that the DIgR2-Ig fusion protein bound strongly to CD3+ T cells (Figure 3A), whereas no significant binding was detected to B cells, NK cells, macrophages, or DCs. Further fractionation of CD4+ and CD8+ T cells showed that DIgR2-Ig fusion protein bound strongly to CD4+ T cells and faintly to CD8+ T cells (about 8%; Figure 3B). The specificity of binding to CD3+ T cells was confirmed by the observation that human immunoglobulin (hIg) did not bind to CD3+ T cells and excessive GST-DIgR2 fusion protein could abolish the binding of DIgR2-Ig to T cells (Figure 3A). Additionally, DIgR2-Ig fusion protein lacking the IgV region showed no binding to T cells, indicating that the IgV domain of DIgR2 was necessary for the observed binding (data not shown). Therefore, it is reasonable to speculate that putative ligand(s) for DIgR2 exists on T cells and that DIgR2 might be involved in DC-T-cell interactions.
To further explore this possibility, we added excess DIgR2-Ig fusion protein into the allogenic MLR to interfere with and block the putative interaction between DCs and T cells via DIgR2. We found that proliferation of T cells was significantly up-regulated or increased by DCs when DIgR2 was blocked with soluble DIgR2-Ig, whereas no increase was seen in the presence of hIg (Figure 3C). To exclude the direct effects of DIgR2-Ig on T cells, a small interfering RNA (siRNA) was also used to specifically block DIgR2 expression on DCs. The levels of DIgR2 in DIgR2 siRNA-transfected DCs were decreased about 60% 48 hours after transfection compared with DIgR2-mut-siRNA-DCs, and an approximate 50% reduction could even be observed 3 days after transfection (Figure 3D). Additionally, we did not detect any significant change in the phenotype (CD40, CD80, CD86, and Iab expression) or cytokine production (IL-12 and IL-10) with or without LPS stimulation (data not shown) as a result of siRNA transfection. The proliferation of T cells stimulated with DIgR2-siRNADCs was also significantly increased, compared with DIgR2-mut-siRNADCs or control DCs (Figure 3E), strengthening the evidence that DIgR2 is a functional molecule involved in DC-T-cell interaction.
DIgR2 is a membrane receptor preferentially expressed by APCs. (A) Northern blots showing the expression of several DIgR2 transcripts in immune and nonimmune tissues. (B) RT-PCR analysis of various mouse cell lines (left) and freshly isolated cells (right) demonstrating restricted expression of DIgR2 in B cells and myeloid cells. (C) Quantitative RT-PCR (Q-RT-PCR) analysis of DIgR2 levels during BM-DC culture. The relative expression of DIgR2 mRNA in the cultured cells was determined daily by real-time quantitative PCR. On day 7 of the culture, the relative expression of DIgR2 mRNA in the cells cultured with LPS, IL-10, and CD40L was also determined in triplicate. DIgR2 levels were normalized to the cells of the third-day culture. Error bars indicate SE. (D) DIgR2 is a cell-surface monomeric glycoprotein. Lysates of DIgR2-transfected NIH/3T3 cells were treated in nonreducing or reducing conditions (left) or with N-glycosidase F in reducing conditions (right) and were then subjected to SDS-PAGE and blotted with anti-DIgR2 polyclonal antibody. (E) DIgR2 is expressed as a transmembrane protein. NIH/3T3 cells transfected with DIgR2 expression vector were stained with anti-DIgR2 polyclonal antibody (i) or purified rabbit serum (ii); NIH/3T3 cells transfected with CD54 were stained with anti-CD54 mAb (iii), and NIH/3T3 cells transfected with pcDNA3.1 were stained with anti-DIgR2 polyclonal antibody (iv). All of these transfectants were then counter stained with FITC-conjugated goat antirabbit or goat antimouse antibody and analyzed by fluorescence confocal microscopy. Data shown are representative of 2 independent experiments. Images were visualized using a Leica DMIRE2 microscope and a Leica 506140 40×/0.85 numeric aperture objective. Images were captured using a Leica DMIRE2 camera and Leica confocal software version 2.61.
DIgR2 is a membrane receptor preferentially expressed by APCs. (A) Northern blots showing the expression of several DIgR2 transcripts in immune and nonimmune tissues. (B) RT-PCR analysis of various mouse cell lines (left) and freshly isolated cells (right) demonstrating restricted expression of DIgR2 in B cells and myeloid cells. (C) Quantitative RT-PCR (Q-RT-PCR) analysis of DIgR2 levels during BM-DC culture. The relative expression of DIgR2 mRNA in the cultured cells was determined daily by real-time quantitative PCR. On day 7 of the culture, the relative expression of DIgR2 mRNA in the cells cultured with LPS, IL-10, and CD40L was also determined in triplicate. DIgR2 levels were normalized to the cells of the third-day culture. Error bars indicate SE. (D) DIgR2 is a cell-surface monomeric glycoprotein. Lysates of DIgR2-transfected NIH/3T3 cells were treated in nonreducing or reducing conditions (left) or with N-glycosidase F in reducing conditions (right) and were then subjected to SDS-PAGE and blotted with anti-DIgR2 polyclonal antibody. (E) DIgR2 is expressed as a transmembrane protein. NIH/3T3 cells transfected with DIgR2 expression vector were stained with anti-DIgR2 polyclonal antibody (i) or purified rabbit serum (ii); NIH/3T3 cells transfected with CD54 were stained with anti-CD54 mAb (iii), and NIH/3T3 cells transfected with pcDNA3.1 were stained with anti-DIgR2 polyclonal antibody (iv). All of these transfectants were then counter stained with FITC-conjugated goat antirabbit or goat antimouse antibody and analyzed by fluorescence confocal microscopy. Data shown are representative of 2 independent experiments. Images were visualized using a Leica DMIRE2 microscope and a Leica 506140 40×/0.85 numeric aperture objective. Images were captured using a Leica DMIRE2 camera and Leica confocal software version 2.61.
DIgR2 negatively regulates DC-initiated antigen-specific T-cell responses both in vitro and in vivo
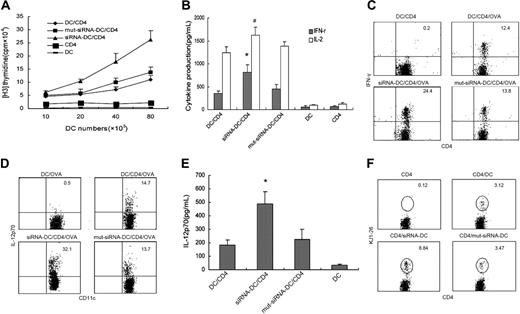
To further investigate the role of DIgR2 in the antigen-specific immune response, we used CD4+ T cells purified from DO11.10 × C57BL/6 F1 mice as responders and DIgR2-siRNA-DCs, DIgR2-mut-siRNA-DCs, or control DCs from C57 BL/6xBalb/c F1 mice as stimulators in an MLR assay. After 3 days, DIgR2-silenced DCs showed a more than 50% increase in their ability to promote Ag-specific responses, as determined by the increased proliferation of DC-stimulated DO11.10 T cells (Figure 4A). More importantly, DIgR2-siRNA-DCs stimulated DO11.10 T cells to produce significantly higher levels of IFN-γ (by ELISA assay and intracellular staining, P < .01; Figure 4B-C). IL-2 levels produced by DIgR2-siRNA-DC-stimulated DO11.10 T cells were also significantly increased (P < .05; Figure 4B).
The data described established a critical role of DIgR2 in the fine-tuning of immune responses. It was important to determine how DIgR2 executes its effects on the immune response and if the engagement of DIgR2 with putative ligand(s) on T cells delivers a signal to regulate DC function. When the phenotype and cytokine production of DIgR2-silenced DCs were compared with those of control DCs, we found that the percentage of CD11c+ DCs expressing intracellular IL-12p70 was increased from 14.7% (DCs) and 13.7% (DIgR2-mut-siRNA-DCs) to 32.1% (DIgR2-siRNADC; Figure 4D), whereas the percentage of IL-10-positive DCs was only slightly increased in similar conditions (data not shown). A significant increase in IL-12 production by DIgR2-siRNA-DCs was also confirmed by ELISA (Figure 4E). In view of the key role of IL-12 in enhancing DCs' ability to stimulate T cells to produce IFN-γ, the increased IL-12 production by DIgR2-silenced DCs may be responsible, at least in part, for subsequent increased T-cell proliferation and enhanced IFN-γ production.
We also tested the ability of DIgR2-silenced DCs to prime an Ag-specific response in vivo. DO11.10 T cells were preinjected into recipient mice, and 1 day later peptide-pulsed and DIgR2-silenced DCs were adoptively transferred into the same recipients. Increased proliferation of antigen-specific CD4+ T cells stimulated by DIgR2-siRNA-DCs was evidenced by the increased frequency of DO11.10 (KJ1-26+) T cells in the spleen of mice that had received DIgR2-siRNA-DCs (Figure 4F). This provided further in vivo evidence that DIgR2 signaling could negatively regulate the ability of DCs to prime Ag-specific T-cell responses.
Blockade of DIgR2 expression in DCs promotes DC-initiated antigen-specific Th1 and CTL responses in vivo
To better understand the contribution of DIgR2 in the negative regulation of DC-initiated T-cell responses in vivo, we also evaluated the immune response elicited by immunization with recombinant DIgR2-siRNA adenovirus-infected DCs (AV-DIgR2-siRNA). As assessed by real-time quantitative PCR, levels of DIgR2 decreased in an adenovirus particle-dependent manner in DCs transduced with AV-DIgR2-siRNA. We chose 100 MOIs in subsequent studies because levels of DIgR2 can be reduced by more than 60% 48 hours after infection, whereas the viability and morphology of DCs were minimally affected (data not shown).
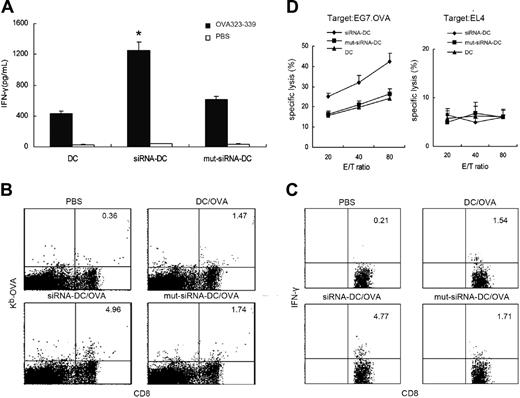
We then immunized C57BL/6 mice with DIgR2-silenced, OVA protein-pulsed, and LPS-matured DCs. Two weeks later, splenocytes from immunized mice were collected and used to assess the Ag-specific immunity. Results showed that more IFN-γ was produced when splenocytes from AV-DIgR2-siRNA mice were restimulated with OVA 17 peptide (323-339) compared with that produced in AV-DIgR2-mut-siRNA mice and control mice (Figure 5A). These data indicate that AV-DIgR2-siRNA-DC immunization induced more potent Th1 responses. Additionally, tetramer staining showed that 4.96% of total CD8+ T cells were positive for OVA tetramer in AV-DIgR2-siRNA-DCs mice, whereas only 1.74% and 1.47% CD8+ T cells were positive for OVA tetramer in AV-DIgR2-mut-siRNA mice or mock mice, respectively (Figure 5B). These data indicate that immunization with DIgR2-silenced DCs resulted in augmented specific CD8+ CTL responses. The functional status of CD8+ T cells was further evaluated by intracellular staining of IFN-γ production and cytotoxicity against OVA+ target cells. Both the percentage of IFN-γ+CD8+ cells (Figure 5C) and the cytotoxicity of splenocytes against OVA-specific target EG7.OVA cells (Figure 5D) were significantly increased in mice immunized with DIgR2-silenced DCs compared with those of the mice immunized with control DCs or AV-DIgR2-mut-siRNA-DCs.
DIgR2 binds with its unknown ligand expressed by T cells and inhibits DC-induced T-cell proliferation in vitro. The binding of DIgR2-Ig fusion protein to CD3+ T cells (A), CD4+ T cells, CD8+ T cells, B cells, NK cells, monocytes, and DCs (B) was analyzed by fluorescence-activated cell sorting (FACS). CD3+, CD4+, and CD8+ T cells, B cells, and NK cells were freshly isolated from mouse splenocytes with CD3, CD4, CD8a, CD19, and NK1.1 MicroBeads, respectively. Macrophages were isolated from peritoneal exudates, and bone marrow-derived DCs were prepared conventionally. Purity of each cell subset exceeds more than 90%. Cells were stained with 40 ng/mL DIgR2-Ig or hIg (CD3+ T cells were also stained with DIgR2-Ig mixed with 400 ng/mL GST-DIgR2) and then stained with FITC-conjugated sheep anti-human IgG (Sigma). Data shown are representative of 3 independent experiments. (C) Mixed lymphocyte reaction (MLR) of DCs from C57BL/6 mice incubated with splenocytes from Balb/c mice, with 50 μg/mL DIgR2-Ig or hIg added into the coculture. Data represent the mean (+SE) of [3H] thymidine uptake in 3 independent experiments. (D) Analysis of DIgR2 levels in siRNA-transfected DCs. Day 6 BM-DCs were transfected with synthetic DIgR2 siRNA, siRNA mutant duplexes, or mock. Expression of DIgR2 was determined in triplicate after stimulation with LPS (100 ng/mL) by Western blotting 48 hours after transfection (bottom panel), and DIgR2 mRNA was assessed by RT-PCR on different days after transfection (top panel). Data shown are representative of 3 independent experiments. (E) MLR was conducted as described. DCs were transfected with DIgR2-siRNA, DIgR2-mut-siRNA, or mock before coculturing with splenocytes. Data represent the mean (+SE) of [3H] thymidine uptake in 3 independent experiments.
DIgR2 binds with its unknown ligand expressed by T cells and inhibits DC-induced T-cell proliferation in vitro. The binding of DIgR2-Ig fusion protein to CD3+ T cells (A), CD4+ T cells, CD8+ T cells, B cells, NK cells, monocytes, and DCs (B) was analyzed by fluorescence-activated cell sorting (FACS). CD3+, CD4+, and CD8+ T cells, B cells, and NK cells were freshly isolated from mouse splenocytes with CD3, CD4, CD8a, CD19, and NK1.1 MicroBeads, respectively. Macrophages were isolated from peritoneal exudates, and bone marrow-derived DCs were prepared conventionally. Purity of each cell subset exceeds more than 90%. Cells were stained with 40 ng/mL DIgR2-Ig or hIg (CD3+ T cells were also stained with DIgR2-Ig mixed with 400 ng/mL GST-DIgR2) and then stained with FITC-conjugated sheep anti-human IgG (Sigma). Data shown are representative of 3 independent experiments. (C) Mixed lymphocyte reaction (MLR) of DCs from C57BL/6 mice incubated with splenocytes from Balb/c mice, with 50 μg/mL DIgR2-Ig or hIg added into the coculture. Data represent the mean (+SE) of [3H] thymidine uptake in 3 independent experiments. (D) Analysis of DIgR2 levels in siRNA-transfected DCs. Day 6 BM-DCs were transfected with synthetic DIgR2 siRNA, siRNA mutant duplexes, or mock. Expression of DIgR2 was determined in triplicate after stimulation with LPS (100 ng/mL) by Western blotting 48 hours after transfection (bottom panel), and DIgR2 mRNA was assessed by RT-PCR on different days after transfection (top panel). Data shown are representative of 3 independent experiments. (E) MLR was conducted as described. DCs were transfected with DIgR2-siRNA, DIgR2-mut-siRNA, or mock before coculturing with splenocytes. Data represent the mean (+SE) of [3H] thymidine uptake in 3 independent experiments.
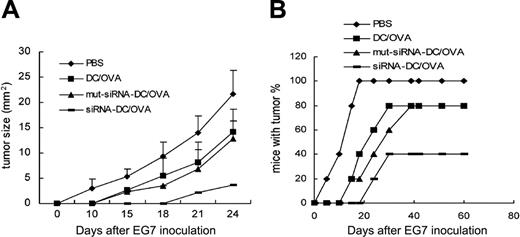
Blockade of DIgR2 expression in DCs promotes DC vaccines to induce antitumor effects in vivo
Immunization with antigen-pulsed DCs can effectively activate antigen-specific CTLs; however, their efficacy in inducing antitumor immunity in vivo has been seen to be far less satisfactory and needs further improvement.32 We investigated whether blockade of DIgR2-mediated inhibitory signals in DCs could promote Ag-specific immunity and protect mice from tumor challenge. C57BL/6 mice preimmunized twice with OVA-pulsed, DIgR2-silenced DC vaccines were inoculated with EG7.OVA lymphoma cells one week after the final immunization. Both the nontransduced DC vaccines and the DIgR2-mut-siRNA-DC vaccines could inhibit tumor growth, as demonstrated by smaller tumor size 24 days after tumor inoculation (14.1 ± 2.3 mm and 12.8 ± 2.8 mm, respectively) compared with mice treated with PBS (21.7 ± 4.1 mm); DIgR2-siRNA-DC vaccine was significantly more potent, resulting in superior suppression of tumor growth (3.6 ± 0.3 mm; Figure 6A). Moreover, 60% of the mice immunized with DIgR2-siRNA-DCs remained tumor free 60 days after tumor inoculation, whereas only 20% of the mice immunized with DIgR2-mut-siRNA-DCs or control DCs remained tumor free (Figure 6B).
Discussion
Positive or negative regulation of T-cell immune responses by DCs depends on specialized DC subsets and on their maturation or activation status.33,34 Although DC subsets or status may be preprogrammed to direct either tolerance or immunity, accumulating evidence demonstrates that integration of inhibitory and activating signals in DCs can instruct DC differentiation, maturation, and activation and result in complete flexibility of a basic program.35,36 The balance between divergent receptors establishes a threshold of DC activation and allows for homeostasis between induction of tolerance or immunity, whereas interference with unilateral signaling always results in an altered balance of signals on DCs, which in turn affects the maturation and function of the DCs themselves. It is worth noting that positive and negative receptor pairs frequently exist together in the closely linked clusters in the IgSF, such as activating versus inhibitory IgG Fc receptors: PIR-A versus PIR-B receptors and signal regulatory protein-α (SIRP-α) versus SIRP-β receptors.11,37-39 Here we have characterized DIgR2, a novel IgSF receptor containing functional ITIMs, that is preferentially expressed by DCs and can mediate negative regulation of DC-initiated T-cell responses both in vitro and in vivo. Given the similarity in extracellular structure, contrasting with completely different motifs in cytoplasm, between DIgR2, DIgR1, and other CLMs, it is interesting to speculate that DIgR2, together with its activating isoform, is required to enable immunologic equilibrium under physiologic conditions. Our identification of a novel ITIM-containing IgSF member and the observations that this molecule can negatively regulate DC function and then subsequent T-cell responses demonstrate a novel molecular pathway for the modulation of immune responses.
Interestingly, the Ig domain of DIgR2 is related to another recently identified Ig family, TREM, that includes several activating and inhibitory molecules encoded by a group of clustered genes.35 Members of TREM have been identified as key regulators in the immune system and beyond. For example, TREM-1 appears to be an amplifier of acute inflammation, as engagement of TREM-1 with agonist mAbs in granulocytes and monocytes can stimulate the production of proinflammatory chemokines and cytokines.40,41 TREM-2 may play important roles in development and function of DCs, as suggested by the observation that ligation of TREM-2 on immature DCs can induce partial maturation and that inefficient differentiation of myeloid precursors of DCs was found in TREM-2-deficient people.42 In view of the close relationship between DIgR2 and related Ig families (CMRF35, TREM, etc),43 it is reasonable to speculate that DIgR2, like other related Ig family receptors, can regulate DC-initiated T-cell responses, which is convincingly supported by data presented here.
DIgR2 negatively regulates the ability of DCs to prime Ag-specific T-cell responses. siRNA-transduced DCs were pulsed with OVA-II peptide (323-329), matured with LPS stimulation, and then cocultured with DO11.10 T cells. (A) Proliferation of T cells. (B) Cytokine production by T cells. (C) Percentages of IFN-γ+ T cells in the gated CD4+ T cells quantitated by double-staining of IFN-γ and CD4, with brefeldin A present in the coculture. (D) Percentages of IL-12p70+ DCs in the gated CD11c+ DCs quantitated by double-staining of IL-12 and CD11c. (E) IL-12p70 production quantitated by ELISA analysis. (F) Reduced ability of DIgR2-siRNA-DCs to prime Ag-specific T-cell responses in vivo. DO11.10 T cells were transferred, together with OVA-pulsed DCs one day later, into recipient mice; after 5 days, collected splenocytes were harvested and double-stained with CD4-FITC and KJ1-26-PE for flow cytometry. The numbers in CD4-gated plots indicate percentage of DO11.10 cells (KJ1-26+) among total CD4+ T cells. Data represent 1 of at least 3 experiments with similar results. *P < .01 and #P < .05 versus DIgR2-mut-siRNA-DCs. Error bars indicate SE.
DIgR2 negatively regulates the ability of DCs to prime Ag-specific T-cell responses. siRNA-transduced DCs were pulsed with OVA-II peptide (323-329), matured with LPS stimulation, and then cocultured with DO11.10 T cells. (A) Proliferation of T cells. (B) Cytokine production by T cells. (C) Percentages of IFN-γ+ T cells in the gated CD4+ T cells quantitated by double-staining of IFN-γ and CD4, with brefeldin A present in the coculture. (D) Percentages of IL-12p70+ DCs in the gated CD11c+ DCs quantitated by double-staining of IL-12 and CD11c. (E) IL-12p70 production quantitated by ELISA analysis. (F) Reduced ability of DIgR2-siRNA-DCs to prime Ag-specific T-cell responses in vivo. DO11.10 T cells were transferred, together with OVA-pulsed DCs one day later, into recipient mice; after 5 days, collected splenocytes were harvested and double-stained with CD4-FITC and KJ1-26-PE for flow cytometry. The numbers in CD4-gated plots indicate percentage of DO11.10 cells (KJ1-26+) among total CD4+ T cells. Data represent 1 of at least 3 experiments with similar results. *P < .01 and #P < .05 versus DIgR2-mut-siRNA-DCs. Error bars indicate SE.
Unavailability of the natural ligands often makes it difficult to directly explore functions of “orphan receptors.” However, emerging approaches of silencing genes provide promising alternatives for investigations into the functions of novel receptors. Soluble recombinant Fc-tagged fusion proteins or specific mAbs have been widely used as antagonists to block surface molecules to study function of CD200, TREM-1, PD-1, and so forth.44-46 siRNA technology is another powerful approach to investigate the function of the gene of interest in specific cells, including primary cells such as DCs.24,47-49 In our studies we used the DIgR2-Ig fusion protein and specific siRNA to block DIgR2 expression in DCs and evaluated the physiologic role of DIgR2. The finding that DIgR2 can selectively bind to CD4+ T cells indicates that DIgR2 may participate in the interaction between DCs and T cells and influence the immune response, which was strongly supported by the observation that DC-induced T-cell proliferation and cytokine production was increased either by the addition of recombinant DIgR2-Fc protein or by silencing DIgR2 on DCs both in vitro and in vivo. The consequences of blocking DIgR2 on DCs are akin to those recently described in studies on PD-L1- and PD-L2-blocked DCs, where DCs treated with anti-PD-L1 or anti-PD-L2 mAb were shown to be better inducers of T-cell responses (as characterized by enhanced T-cell proliferation and cytokine production), emphasizing the roles of inhibitory molecules in establishing and maintaining T-cell tolerance.46,50 Our promising studies demonstrate that blockade of DIgR2 expression in DCs can promote the induction of antigen-specific antitumor immunity, which further highlights the potential roles of DIgR2 in immune regulation and suggests the potential of DIgR2 as a target for immunotherapy of cancer.
Induction of more potent Th1 and CTL responses by immunization with DIgR2-siRNA-DCs. C57BL/6 mice were immunized once with DIgR2-siRNA adenovirus-infected DCs that were pulsed with ovalbumin proteins and ex vivo matured with LPS (100 ng/mL) for 24 hours before immunization (1 × 106 DCs per mouse). Two weeks later, splenocytes were pooled from 3 mice from each group. (A) IFN-γ production by CD4+ T cells after pooled splenocytes were restimulated with OVA-II peptide (1 mg/mL) for 48 hours. (B) Percentages of H2-Kb/OVA tetramer+ in the total gated CD8+ T-cell population. (C) Percentages of IFN-γ+ T cells in the total gated CD8+ T-cell population when pooled splenocytes were restimulated with OVA-I peptide (10 mg/mL) for 3 days. (D) Cytotoxicity against OVA+ EG7 (left) or OVA- EL4 (right) target cells by splenocytes restimulated in vitro with OVA-I peptide (10 mg/mL) for 6 days in the presence of 50 U/mL IL-2. E/T indicates effector-target ratio. Data shown are representative of 3 independent experiments. *P < .01 versus AV-DIgR2-mut-siRNADCs. Error bars indicate SE.
Induction of more potent Th1 and CTL responses by immunization with DIgR2-siRNA-DCs. C57BL/6 mice were immunized once with DIgR2-siRNA adenovirus-infected DCs that were pulsed with ovalbumin proteins and ex vivo matured with LPS (100 ng/mL) for 24 hours before immunization (1 × 106 DCs per mouse). Two weeks later, splenocytes were pooled from 3 mice from each group. (A) IFN-γ production by CD4+ T cells after pooled splenocytes were restimulated with OVA-II peptide (1 mg/mL) for 48 hours. (B) Percentages of H2-Kb/OVA tetramer+ in the total gated CD8+ T-cell population. (C) Percentages of IFN-γ+ T cells in the total gated CD8+ T-cell population when pooled splenocytes were restimulated with OVA-I peptide (10 mg/mL) for 3 days. (D) Cytotoxicity against OVA+ EG7 (left) or OVA- EL4 (right) target cells by splenocytes restimulated in vitro with OVA-I peptide (10 mg/mL) for 6 days in the presence of 50 U/mL IL-2. E/T indicates effector-target ratio. Data shown are representative of 3 independent experiments. *P < .01 versus AV-DIgR2-mut-siRNADCs. Error bars indicate SE.
Enhanced induction of antitumor effect by immunization with DIgR2-siRNA-DCs. C57BL/6 mice (n = 5 mice/group) mice were preimmunized twice at weekly intervals with 1 × 106 of OVA-pulsed, transduced, or mock DCs with ex vivo maturation. Seven days after the final immunization, OVA+ EG7 tumor cells (2 × 106) were inoculated subcutaneously. Tumor size (A) and percentage of mice with tumor (B) were monitored twice a week. The data represent 1 of 2 independent experiments. Error bars indicate SE.
Enhanced induction of antitumor effect by immunization with DIgR2-siRNA-DCs. C57BL/6 mice (n = 5 mice/group) mice were preimmunized twice at weekly intervals with 1 × 106 of OVA-pulsed, transduced, or mock DCs with ex vivo maturation. Seven days after the final immunization, OVA+ EG7 tumor cells (2 × 106) were inoculated subcutaneously. Tumor size (A) and percentage of mice with tumor (B) were monitored twice a week. The data represent 1 of 2 independent experiments. Error bars indicate SE.
Despite the data showing that DIgR2 contains functional ITIMs that can associate with the tyrosine phosphatase SHP-1 and that interference with DIgR2 on DCs has a negative influence on T-cell responses, we do not yet fully understand the mechanisms of inhibitory signaling transduced through DIgR2 on DCs. To clarify some of the underlying mechanisms of DIgR2 signaling in the DC-T-cell interaction, we explored if interference with DIgR2 on DCs can affect the maturation and function of DCs. The most pronounced change after blocking DIgR2 expression was a remarkable increase in IL-12 production. Considering that IL-12 produced by DCs plays a crucial role in triggering IFN-γ production and in Th1 polarization of T cells, it seems that restriction of IL-12 production by DIgR2 signaling in DCs might be an important mechanism for immune regulation. As uncontrolled IL-12 production and responsiveness can result in some organ-specific autoimmune diseases, it is urgent that potent negative regulatory feedback mechanisms for IL-12 production are investigated.51,52 Because IL-12 is significantly down-regulated by DIgR2 signaling in DCs and DIgR2 is itself rapidly induced upon LPS stimulation, it is conceivable that up-regulation of DIgR2 by LPS is a part of a feedback circuit of LPS signaling, thus providing an explanation to the puzzling observation that DCs can produce IL-12 upon LPS stimulation only transiently.53 Further studies are needed to define the cross-talk between DIgR2 signaling and other signaling, such as TLR signaling and CD40L signaling in DCs, in order to better understand the precise mechanisms of DIgR2 in immune regulation and immunologic pathogenesis.54,55
Prepublished online as Blood First Edition Paper, June 22, 2006; DOI 10.1182/blood-2006-04-015404.
Supported by grants from the National Key Basic Research Program of China (2003CB515503, 2001CB510002) and the National Natural Science Foundation of China (30471588, 30490240, 30121002). L.S., K.L., and D.X. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We sincerely thank Prof Frances Gotch of the Department of Immunology at Imperial College London for critical review of this manuscript and Drs Bin Liu and Zhenhong Guo for helpful discussion. We also acknowledge expert technical assistance of Y. Rui, Y. Han, Y. Zhang, Y. Li, and M. Jin.

![Figure 3. DIgR2 binds with its unknown ligand expressed by T cells and inhibits DC-induced T-cell proliferation in vitro. The binding of DIgR2-Ig fusion protein to CD3+ T cells (A), CD4+ T cells, CD8+ T cells, B cells, NK cells, monocytes, and DCs (B) was analyzed by fluorescence-activated cell sorting (FACS). CD3+, CD4+, and CD8+ T cells, B cells, and NK cells were freshly isolated from mouse splenocytes with CD3, CD4, CD8a, CD19, and NK1.1 MicroBeads, respectively. Macrophages were isolated from peritoneal exudates, and bone marrow-derived DCs were prepared conventionally. Purity of each cell subset exceeds more than 90%. Cells were stained with 40 ng/mL DIgR2-Ig or hIg (CD3+ T cells were also stained with DIgR2-Ig mixed with 400 ng/mL GST-DIgR2) and then stained with FITC-conjugated sheep anti-human IgG (Sigma). Data shown are representative of 3 independent experiments. (C) Mixed lymphocyte reaction (MLR) of DCs from C57BL/6 mice incubated with splenocytes from Balb/c mice, with 50 μg/mL DIgR2-Ig or hIg added into the coculture. Data represent the mean (+SE) of [3H] thymidine uptake in 3 independent experiments. (D) Analysis of DIgR2 levels in siRNA-transfected DCs. Day 6 BM-DCs were transfected with synthetic DIgR2 siRNA, siRNA mutant duplexes, or mock. Expression of DIgR2 was determined in triplicate after stimulation with LPS (100 ng/mL) by Western blotting 48 hours after transfection (bottom panel), and DIgR2 mRNA was assessed by RT-PCR on different days after transfection (top panel). Data shown are representative of 3 independent experiments. (E) MLR was conducted as described. DCs were transfected with DIgR2-siRNA, DIgR2-mut-siRNA, or mock before coculturing with splenocytes. Data represent the mean (+SE) of [3H] thymidine uptake in 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/8/10.1182_blood-2006-04-015404/4/m_zh80200602270003.jpeg?Expires=1765887643&Signature=Uq7L77e0e-Fn3VtHva1dcEoy0r1Nk4076CMbjdUwjDy~Tf6Jb5FKE2apyUD-o3~VDCfLoHOFoLLy45pAQHMDtUuIKrfxjvG4FZUnUy6N9jYnGIhNo1N9G6Txf05u0FqRn2z4tE9WpLMbycHQLoP9Z726bBMoLWnY3cJdyMOdYEpxMkOy2QRq9AtiMHdF8l46O45KF-apkabzQJ-dQtGc4VDP~3qjS8B4Cnii4k~wZjExlUS9hyWZHKTL~qsAiQaygQ4xC8w03xWbLEz09hLs7ZaupNsmr2yt85w5ELryM9zaABX1H-AK-PuKbUCuYyl3WifIEX5ScAapPCxFx7oEGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal