Abstract
Endothelial cells respond to vascular endothelial growth factor (VEGF) to produce new blood vessels. This process of angiogenesis makes a critical contribution during embryogenesis and also in the response to ischemia in adult tissues. We have studied the intracellular trafficking of the major VEGF receptor KDR (VEGFR2). Unlike other related growth factor receptors, we find that a significant proportion of KDR is held in an endosomal storage pool within endothelial cells. We find that KDR can be delivered to the plasma membrane from this intracellular pool and that VEGF stimulates this recycling to the cell surface. KDR recycling appears to be distinct from the previously characterized Rab4- and Rab11-dependent pathways, but, instead, KDR+ recycling vesicles contain Src tyrosine kinase and VEGF-stimulated recycling requires Src activation. Taken together, these data show that intracellular trafficking of KDR is markedly different from other receptor tyrosine kinases and suggest that the regulation of KDR trafficking by VEGF provides a novel mechanism for controlling the sensitivity of endothelial cells to proangiogenic signals.
Introduction
Angiogenesis is the fundamental physiologic process by which new blood vessels are generated from preexisting vasculature. It plays a crucial role in embryogenesis, where it is required for elaboration of the vasculature from the primary vascular plexus. In normal adult physiology, angiogenesis is significant in a relatively limited number of processes—primarily in the formation of endometrial vessels in the uterus and development of the corpus luteum during the ovulation cycle.1 Angiogenesis becomes more widely important to adult physiology through its critical involvement in a number of pathologic conditions. Active angiogenesis makes a positive contribution to the wound-healing process and also in the response to tissue ischemia—hypoxic tissues generate proangiogenic signals that stimulate the formation of new vessels and so improve perfusion.2 Angiogenesis makes an unwanted contribution to the growth of solid tumors, with cancer cells secreting proangiogenic factors to provide a blood supply for the growing mass.3 Deregulated angiogenesis is an underlying cause of proliferative diabetic retinopathy, a major vascular complication of both type I and type II diabetes,4 and also contributes to other disease states such as age-related macular degeneration, rheumatoid arthritis, and psoriasis.5,6 Understanding of the regulatory mechanisms of angiogenesis is hence seen not only as a fundamental problem in human biology but also as an important goal in medical research.
Angiogenesis is controlled by a wide range of positive and negative signals. Vascular endothelial growth factor (VEGF) is the most critical and potent of the proangiogenic regulators7-9 and is secreted by tissues in response to hypoxia or inflammation.9 VEGF binds to VEGF receptors (VEGFRs) on the surface of endothelial cells, triggering a cascading series of signaling pathways that stimulate endothelial cell sprouting, migration, tube formation, proliferation, and survival.10 There are 3 human members of the VEGFR family: VEGFR1/Flt-1, VEGFR2/Flk-1/KDR, and VEGFR3/Flt-4.11 These proteins are members of the larger family of receptor tyrosine kinases (RTKs), which includes the platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors.12 In common with these other RTKs, the VEGFRs undergo tyrosine phosphorylations on activation. These phosphorylations allow recruitment of multiple downstream signaling components, triggering a strikingly similar profile of signaling pathways to other RTKs, including the activation of p42/44 MAP kinase, phosphatidylinositol 3-kinase, and phospholipase C-γ.10
While we have learned much about VEGFR signaling, we still know comparatively little about VEGFR trafficking. The intracellular trafficking of other RTKs makes a critical contribution to their cellular function.13 Receptors such as the EGFR show a low constitutive rate of endocytosis in resting cells that is dramatically increased on binding of growth factor. The internalized active receptor is sorted through a number of intracellular endocytic compartments, eventually being targeted to the lysosome for degradation.14,15 This down-regulation desensitizes cells to further growth factor stimulation, and inhibition of these pathways gives rise to hyperproliferative signals.15 As well as this straightforward desensitization process, it is now known that the endocytic compartment plays a key role in developing and shaping growth factor receptor signals. Many signals from RTKs arise from receptors on endocytic vesicles within the cell, and some pathways are only triggered on internalization of the receptor from the plasma membrane.13,16 As a result of this, the routes and kinetics of receptor trafficking have the potential to determine the balance of signals produced on receptor activation.
It is clear that a full understanding of the signals elaborated by RTKs can only come from understanding the intracellular trafficking of these proteins. Here we examine the intracellular trafficking of VEGFR2/KDR/Flk-1, which is the major mediator of proangiogenic signaling7-9,17 and is absolutely required for embryonic angiogenesis.18,19 Unlike other RTKs, we find that a significant pool of KDR exists in an intracellular endocytic storage compartment in resting endothelial cells. Surface KDR undergoes constitutive internalization that is not further stimulated by VEGF ligation; however, VEGF stimulation increases recycling of the intracellular KDR pool to the cell surface. These data show that while the signaling pathways activated downstream of KDR are broadly similar to other RTKs, the intracellular trafficking of this critical proangiogenic receptor is very different. Importantly, the ability of VEGF to mobilize the intracellular store of KDR suggests ways in which the sensitivity of endothelial cells to proangiogenic signals can be governed by regulation of receptor trafficking.
Materials and methods
Constructs and antibodies
Recombinant human VEGF165 was from R&D Systems (Minneapolis, MN). Rabbit polyclonal and mouse monoclonal (clone KDR/EIC) antibodies to KDR were from Abcam (Cambridge, United Kingdom). A rabbit monoclonal antibody to KDR (55B11) was from Cell Signaling Technologies (Beverly, MA). The CD63 monoclonal antibody (RFAC4) and cathepsin D rabbit polyclonal antibody were from Biogenesis (Poole, United Kingdom). The EEA1 monoclonal antibody (clone 14) was from Becton Dickinson (Franklin Lakes, NJ). PECAM monoclonal antibody (9G11) was from R&D Systems. The tubulin monoclonal antibody (TUB2.1) was from Sigma (Poole, United Kingdom). Alexa-488-conjugated phalloidin was from Molecular Probes (Paisley, United Kingdom). Cy2- and Cy3-conjugated secondary antibodies were from Jackson Immunoresearch (Cambridgeshire, United Kingdom). GFP-tagged Rab4 and Rab11 cDNA constructs and GFP-tagged Src were generous gifts from Robert Lodge (Université du Quebec, QC, Canada) and Margaret Frame (Beatson Institute, United Kingdom), respectively. The highly specific Src tyrosine kinase inhibitor AP23464 was generously provided by ARIAD Pharmaceuticals (Cambridge, MA).
Cell culture and immunofluorescence microscopy
Primary human umbilical vein endothelial cells (HUVECs) were prepared by collagenase digestion of donor cords as previously described.20 Primary human dermal microvascular endothelial cells (HMVECs) were from PromoCell (Heidelberg, Germany). Both cell types were cultured in complete endothelial cell growth media (ECGM; PromoCell), supplemented with 100 μg/L streptomycin sulfate and 100 U/mL benzylpenicillin. Cells were maintained in a humidified incubator at 37°C with 5% carbon dioxide and were used between passage 4 and 6. For cell imaging, cells were cultured onto acid-washed, fibronectin-coated glass coverslips in complete ECGM and allowed to adhere overnight. Where indicated, cells were transfected using GeneFECTOR according to the manufacturer's protocol (Venn Nova, Pompano Beach, FL). For preparation for confocal immunofluorescence microscopy, cells were fixed for 15 minutes in 4% fresh paraformaldehyde in PBS, washed in PBS, and then permeabilized in 0.2% Triton X-100 in PBS for 5 minutes. The cells were then washed again in PBS and incubated with 0.1% sodium borohydride for 10 minutes. Where microtubule staining was required, the cells were alternatively fixed and permeabilized by immersion in methanol at -20°C for 2 minutes. After fixation, cells were washed 3 times in PBS then incubated with primary antibody in 1% BSA for 1 hour. The cells were washed 3 times in PBS then incubated for 45 minutes with secondary antibody in PBS. Where appropriate, cells were costained with 5 μM DAPI for 5 minutes. The cells were washed 3 times in PBS and mounted over MOWIOL 4-88 (Calbiochem, San Diego, CA) containing 0.6% 1,4-diazabicyclo-(2.2.2)octane (Sigma) as an antiphotobleaching agent. Confocal microscopy was performed using a Leica AOBS SP confocal laser-scanning microscope (Heidelberg, Germany) with an attached Leica DM IRE2 inverted epifluorescence microscope under a Plan Apo BL ×63/1.4 numeric aperture oil-immersion objective. Fluorophores were excited using the 405-nm line of a diode laser (DAPI), the 488-nm line of a Kr/Ar laser (Alexa 488, Cy2), and the 543-nm line of a HeNe laser (Cy3). A series of images was taken at 0.5-μm intervals through the Z-plane of the cell and were processed to form a projected image. Adobe Photoshop CS (Adobe Systems, San Jose, CA) was used to scale and crop images for publication. Where image contrast/brightness was enhanced, only linear operations were used (ie, gamma curves were not manipulated).
Biochemical quantification of KDR distribution
To measure the relative proportions of the surface and internal pools of KDR, cell surface proteins were covalently labeled using a membrane-impermeant biotinylation reagent (NHS-SS-biotin; Pierce, Rockford, IL). All steps were performed at 4°C. Cells were washed 3 times in PBS and then incubated with 0.15 mg/mL sulfo-NHS-SS-biotin (Pierce) in PBS for 10 minutes with rocking. The unreacted biotinylation reagent was quenched by washing once with TBA (25 mM Tris, pH 8; 137 mM NaCl; 5 mM KCl; 2.3 mM CaCl2; 0.5 mM MgCl2; and 1 mM Na2 HPO4), and the cells were then washed a further 3 times with PBS. Cells were then solubilized in lysis buffer (20 mM Tris, pH 7.5; 125 mM NaCl; 10% glycerol; 1% NP40; 1 μg/mL PMSF) containing protease inhibitor cocktail (Calbiochem), used according to the manufacturer's instructions. Cell lysates were centrifuged at 14 000g for 10 minutes at 4°C and a sample (10 μL) was taken from the supernatant, which represented the total cellular KDR. Streptavidin-agarose beads (Upstate Biotechnology, Lake Placid, NY) were added to the remaining supernatant (100 μL packed beads per 500 μL lysate) and left to tumble at 4°C for 2 hours. Beads were collected by centrifugation at 14 000g for 10 seconds at 4°C and supernatant was removed; this sample represents the internal KDR pool. The beads were then washed 3 times in lysis buffer at 4°C and protein was extracted from the beads by heating at 95°C with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer; this represents the surface KDR pool. Equivalent volumes of all 3 samples were resolved by SDS-PAGE and analyzed by Western blotting.
Biochemical quantification of KDR trafficking
Measurements of KDR trafficking were performed by a modification of our previous methodology for quantifying integrin trafficking.21 To measure the internalization of KDR, serum-starved HUVECs were transferred to ice, washed twice in cold PBS, and surface labeled at 4°C with 0.2 mg/mL NHS-SS-biotin in PBS for 30 minutes. Labeled cells were washed twice in ice-cold PBS and transferred immediately to prewarmed cell culture medium at 37°C, without or with 50 ng/mL VEGF to allow internalization. Where appropriate, incubations included Src tyrosine kinase inhibitor AP23464 at 1 μM or vehicle (DMSO). The cell culture medium also contained 0.6 mM primaquine to prevent recycling of the internalized receptor, which would otherwise confound the measurements of internalization rate. At the indicated times, the medium was aspirated and the dishes were rapidly transferred to ice and washed twice with ice-cold PBS. Biotin was removed from proteins remaining at the cell surface by reduction with the membrane-impermeant reducing agent Sodium 2-mercaptoethanesulphonate (MesNa; Pierce). Briefly, a solution of 20 mM MesNa in 50 mM Tris, 100 mM NaCl was adjusted to pH 8.6 with 10 M NaOH and immediately added to the monolayers. Reduction was allowed to proceed for 15 minutes on ice at 4°C with gentle rocking. MesNa was quenched by addition of 20 mM iodoacetamide (IAA) for 10 minutes. Cells were lysed in 100 μLof a buffer containing 200 mM NaCl, 75 mM Tris (pH 7.5); 15 mM NaF, 1.5 mM Na3VO4, 7.5 mM EDTA, and 7.5 mM EGTA, 1.5% Triton X-100, 0.75% Igepal CA-630, 50 μg/mL leupeptin, 50 μg/mL aprotinin, and 1 mM 4-(2-aminoethyl)benzynesulphonyl fluoride (AEBSF), and were scraped from the dish with a rubber policeman. Lysates were passed 3 times through a 27-gauge needle and clarified by centrifugation at 10 000g for 10 minutes. Supernatants were corrected to equivalent protein concentration using the BCA protein assay (Pierce), and levels of biotinylated KDR were determined by capture-enzyme-linked immunosorbent assay (ELISA) internalization assay. Data were expressed as a percentage of the value obtained from cells that had not been reduced; this was taken to be the “total.”
For determination of KDR recycling, serum-starved cells were surface labeled with 0.2 mg/mL NHS-SS-biotin for 30 minutes at 4°C as for measurements of receptor internalization. Cells were washed twice in ice-cold PBS and transferred to serum-free medium at 22°C for 20 minutes to allow internalization of KDR and its subsequent delivery to the Rab4+ compartment (data not shown). Cells were transferred to ice and washed twice with ice-cold PBS, and biotin was removed from proteins remaining at the cell surface by reduction with MesNa. The internalized fraction was then chased from the cells by returning them to 37°C in serum-free medium in the absence or presence of 50 ng/mL VEGF for 15 minutes. Cells were returned to ice and biotin was removed from recycled proteins by a second reduction with MesNa. Unreacted MesNa was quenched with 20 mM IAA for 10 minutes and cells were lysed as for measurements of receptor internalization. Levels of biotinylated KDR were determined by capture-ELISA (see “Biochemical quantification of KDR degradation”) and expressed as a proportion of the levels found in cells that had not been warmed to 37°C during the chase period; this represents the internal pool from which the receptor recycles.
To quantify KDR in the samples from the internalization and recycling assays, we used capture-ELISA. Maxisorb 96-well plates (Life Technologies, Gaithersburg, MD) were coated overnight with 5 mg/mL purified anti-mouse IgG1 (clone: A85-3; BD Pharmingen, Heidelberg, Germany) in 0.05 M Na2CO3 (pH 9.6) at 4 °C, followed by a 2-hour incubation with 5 μg/mL mouse monoclonal antibody against human KDR (A-3; Santa Cruz Biotechnology, Santa Cruz, CA) in PBS containing 0.1% Tween-20 (PBS-T). Nonspecific binding sites were blocked in with 5% BSA in PBS-T for 1 hour at room temperature. KDR was captured by overnight incubation of 50 μL of HUVEC lysate at 4°C. Unbound material was removed by extensive washing with PBS-T and wells were incubated with streptavidin-conjugated horseradish peroxidase in PBS-T containing 1% BSA for 1 hour at 4°C. Following further washing, biotinylated KDR was detected by chromogenic reaction with 0.56 mg/mL ortho-phenylenediamine in a buffer containing 25.4 mM Na2HPO4, 12.3 mM citric acid (pH 5.4) with 0.003% H2O2 at room temperature for 10 minutes. The reaction was stopped with 8 M H2SO4 and absorbance read at 490 nm.
Biochemical quantification of KDR degradation
Time courses of degradation of KDR were determined as previously described for EGF receptor.22 Briefly, cells (approximately 2 million per condition) were cultured in 6-well cell culture dishes. The cells were placed in supplement-free ECGM containing 0.1% (wt/vol) fatty acid-free BSA for 2 hours, prior to treatment with 100 ng/mL VEGF165 in the same media. At various time points, the cells were washed 3 times in ice-cold PBS and then extracted into 95°C SDS-PAGE sample buffer. The samples were heated at 95°C for 5 minutes and then subjected to SDS-PAGE. Proteins were transferred by electrophoresis to PVDF membrane (Millipore, Gloucestershire, United Kingdom) and receptor levels were detected and quantified by Western blotting.
Results
In unstimulated endothelial cells, a significant proportion of KDR is contained in an internal vesicular pool
We first examined the cellular distribution of endogenous KDR in unstimulated endothelial cells. With other RTKs, such as the EGFR and PDGFR, the majority of unliganded receptor is present at the cell surface in resting cells. Surprisingly, KDR showed a largely punctate staining pattern, with little obvious staining of receptor at the plasma membrane (Figure 1A). Similar results were seen with 2 other anti-KDR antibodies (data not shown). We saw essentially identical results with primary human dermal microvascular endothelial cells (HMVECs; Figure 1B). In the course of preparing this manuscript, Bhattacharya et al23 have reported a similar punctate pattern of KDR staining in endothelial cells. It is difficult to easily compare by cell imaging the relative intensities of receptors clustered in small endocytic vesicles with receptors distributed across the much larger area of the plasma membrane. We therefore used a biochemical assay to accurately quantify the relative distribution of endogenous receptor between surface and internal pools in unstimulated cells. In these assays we compare the fraction of total KDR that is accessible to a membrane-impermeant biotinylation reagent with the fraction that is inaccessible, and then reference both to the total cellular pool of KDR. Quantification in this way reveals that only 60% of cellular KDR is present at the cell surface and available for interaction with VEGF in unstimulated cells, with the remaining 40% of receptor present in an internal vesicular pool (Figure 1D).
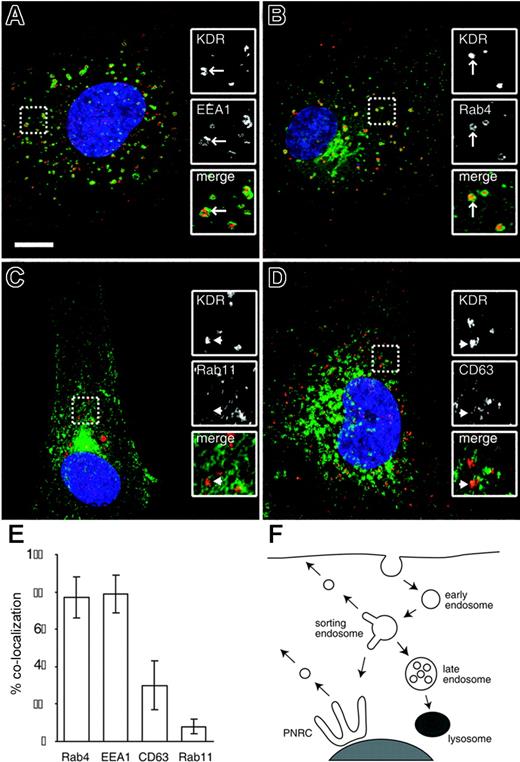
The internal KDR pool is comprised mainly of early endosomes and Rab4+ recycling endosomes
The endocytic pathway is composed of a set of subcompartments that direct the sorting of internalized receptors to a number of distinct fates24,25 (Figure 2F). Receptors entering the cell through clathrin-mediated endocytosis are contained in early endosomes. These then fuse with an early sorting endosomal compartment, marked by the EEA1 protein.26 Receptors can leave this compartment and return to the plasma membrane through 2 recycling pathways. The small GTPase Rab4 mediates so-called short-loop recycling to the plasma membrane, with a half-time of approximately 5 minutes. Receptors can also be sorted through a juxta-nuclear recycling compartment mediated by Rab11, the so-called long-loop, which has a half-time of approximately 15 to 30 minutes.24,27 RTKs such as the EGF and PDGF receptors have very low rates of recycling from sorting endosomes and instead are marked for degradation by the covalent addition of monoubiquitin.25 This leads to their retention in sorting endosomes, which mature into late endosomes.25,28 Late endosomes fuse with the lysosomal compartment, delivering RTKs for degradation.
We used a number of well-characterized markers of endosomal subcompartments to determine the nature of the intracellular KDR pool. The majority of KDR+ vesicles colocalized with the early/sorting endosomal marker EEA1 and with Rab4, a marker of the short-loop recycling pathway (Figure 2). Little or no colocalization of KDR was seen with Rab11, a marker of the long-loop recycling pathway (Figure 2). No colocalization was seen between KDR and the lysosomal marker cathepsin D (data not shown), although we saw a significant number of KDR+ vesicles that colocalized with CD63, a marker of late endosomes.29 In endothelial cells, a fraction of CD63 also marks specialized cigar-shaped organelles called Weibel-Palade bodies,30 which store and secrete von Willebrand factor, a key regulator of hemostasis. We saw no significant colocalization between KDR and von Willebrand factor (data not shown), consistent with the KDR+/CD63+ pool representing late endosomes only. We also observed a small fraction of KDR colocalizing with markers of the Golgi (data not shown), which we take to represent the biosynthetic pool of nascent receptor. The localization data are summarized in Figure 2E. From these measurements we conclude that the internal pool of KDR corresponds to at least 2 distinct compartments, the largest being EEA1+/Rab4+ sorting/recycling endosomes, with a smaller but significant pool of CD63+ late endosomes.
In unstimulated endothelial cells, KDR is contained in an internal vesicular pool. (A) Unstimulated HUVECs and (B) unstimulated HMVECs stained for endogenous KDR (red). The boundaries of the cells are visualized by staining with the endothelial cell-cell adhesion molecule VE-cadherin (green). Nuclei are stained with DAPI (blue). (C) Analysis of the surface and intracellular pools of KDR in unstimulated HUVECs. Surface KDR was labeled with the membrane-impermeant biotinylation reagent sulfo-NHS-SS-biotin, as described in “Biochemical quantification of KDR trafficking.” Biotinylated surface KDR was collected by binding to streptavidin-agarose. Aliquots of the total cell lysate, surface fraction, and internal fraction were then analyzed by Western blotting with an anti-KDR antibody. No KDR was retrieved in the surface fraction in the absence of biotinylation. (D) Densitometric quantification of the relative surface and internal pools of KDR (mean ± SD; n = 3).
In unstimulated endothelial cells, KDR is contained in an internal vesicular pool. (A) Unstimulated HUVECs and (B) unstimulated HMVECs stained for endogenous KDR (red). The boundaries of the cells are visualized by staining with the endothelial cell-cell adhesion molecule VE-cadherin (green). Nuclei are stained with DAPI (blue). (C) Analysis of the surface and intracellular pools of KDR in unstimulated HUVECs. Surface KDR was labeled with the membrane-impermeant biotinylation reagent sulfo-NHS-SS-biotin, as described in “Biochemical quantification of KDR trafficking.” Biotinylated surface KDR was collected by binding to streptavidin-agarose. Aliquots of the total cell lysate, surface fraction, and internal fraction were then analyzed by Western blotting with an anti-KDR antibody. No KDR was retrieved in the surface fraction in the absence of biotinylation. (D) Densitometric quantification of the relative surface and internal pools of KDR (mean ± SD; n = 3).
VEGF stimulation redirects KDR to the late endosomal compartment, but only a fraction of receptor undergoes subsequent lysosomal degradation
Stimulation of the EGF and PDGF receptors leads to an initial desensitization by removal of the receptor from the plasma membrane by endocytosis, followed by a more enduring down-regulation of growth factor signaling through lysosomal proteolysis of the receptor.15,25 Surprisingly, stimulation with VEGF for extended periods of up to 24 hours only partially depleted cellular KDR, with a significant intracellular pool of receptor present throughout (Figure 3). Quantification of total cellular KDR by Western blotting showed that approximately 40% of receptor was degraded within 30 minutes of stimulation but that no significant further degradation occurred beyond this point (Figure 3I). We saw essentially identical results in HMVECs (data not shown). Duval et al31 have previously reported an almost complete degradation of KDR in bovine aortic endothelial cells stimulated with VEGF for 30 minutes (ie, similar to other RTKs). The reasons for the apparent discrepancy between these 2 sets of data are unclear but may reflect differences in cultured bovine endothelial cells.
We were interested to see what happened to the cellular distribution of KDR on stimulation with VEGF and followed this by examining the colocalization of KDR with EEA1 and CD63 over a time course of VEGF treatment. Although stimulation with VEGF did not lead to loss of the intracellular KDR pool, it changed the relative distribution of the receptor between endosomal subcompartments. VEGF treatment led to an increase in CD63+/KDR+ endosomes at the expense of EEA1+/KDR+ vesicles (Figure 3). A shift of receptor into late endosomes is consistent with the degradation of the receptor observed; however, it is important to note that this increased pool of CD63+/KDR+ vesicles is maintained after up to 24 hours of stimulation (ie, long after further significant receptor degradation is occurring). This suggests that the CD63+ late endosomes can act as a storage compartment for KDR, without necessarily delivering the receptor to the lysosome for degradation.
VEGF stimulation provokes recycling of KDR to the cell surface
The second surprising observation on stimulation of endothelial cells with VEGF was the redistribution of a subset of KDR+ vesicles to the cell periphery (Figure 4). This redistribution was observed 30 to 60 minutes after VEGF stimulation, with small KDR+ vesicles being clustered just beneath the plasma membrane or, in some cases, associated with plasma membrane protrusions enriched in KDR (Figure 4A). The timing of the appearance of these vesicles suggests that they represent recycling endosomes rather than nascent endocytic vesicles; indeed, these structures do not stain with clathrin antibodies (data not shown). Further examination showed that these vesicles are associated with microtubules positioned just below the cell surface (Figure 4D). To examine the possible effects of VEGF on KDR recycling, we used a novel biochemical assay of KDR internalization and traffic, adapted from similar assays used previously for other RTKs.32,33 Surface KDR was biotinylated using a membrane-impermeant, cleavable biotinylation reagent. The cells were then stimulated with VEGF and, at various times, the surface accessibility of the labeled KDR was determined by using a membrane-impermeant reducing agent (MesNa) to cleave the biotin modification. In this way, the kinetics of internalization of a cohort of surface receptors can be measured as a loss of MesNa-accessible biotinylated KDR, and the recycling of this receptor pool can be measured as subsequent reappearance of the original cohort of biotinylated KDR at the cell surface. In unstimulated cells, KDR showed a relatively fast constitutive rate of internalization with a half-time of approximately 5 minutes. Unlike other RTKs, internalization was not increased by VEGF stimulation (Figure 4E). In unstimulated cells, approximately 40% of internalized KDR was recycled to the cell surface within 15 minutes (Figure 4F). Surprisingly, VEGF stimulation robustly increased this rate of recycling (Figure 4F). The net behavior of the KDR receptor on VEGF stimulation is then very different than other RTKs; rather than stimulating removal of KDR from the cell surface, VEGF actually provokes the return of internalized receptor through an endocytic recycling pathway.
The internal KDR pool is composed mainly of early endosomes and Rab4+ recycling endosomes. Unstimulated HUVECs were either stained for endogenous EEA1 (A), transfected with GFP-Rab4 (B) or GFP-Rab11 (C), or stained for endogenous CD63 (D; all in green). Cells were costained for endogenous KDR in each case (red). Nuclei are stained with DAPI (blue). Inset panels show magnified portions of each image, as indicated (dashed squares). Bar represents 10 μm. (E) The percentage of total KDR+ vesicles that colocalize with each of the 4 endosomal markers (mean ± SD; n ≥ 14). (F) A model of the canonical pathways of endocytic sorting. Receptors enter the cells through clathrin-mediated endocytosis and are trafficked in EEA1+ early endosomes to an EEA1+/Rab4+ sorting endosomal compartment. Receptors can be recycled from here either through the Rab4-dependent short-loop or through the long-loop via the perinuclear recycling compartment (PNRC), marked by Rab11. RTKs such as the EGF and PDGF receptors are not recycled but are instead sorted to CD63+ late endosomes and then to the lysosomal compartment for degradation.
The internal KDR pool is composed mainly of early endosomes and Rab4+ recycling endosomes. Unstimulated HUVECs were either stained for endogenous EEA1 (A), transfected with GFP-Rab4 (B) or GFP-Rab11 (C), or stained for endogenous CD63 (D; all in green). Cells were costained for endogenous KDR in each case (red). Nuclei are stained with DAPI (blue). Inset panels show magnified portions of each image, as indicated (dashed squares). Bar represents 10 μm. (E) The percentage of total KDR+ vesicles that colocalize with each of the 4 endosomal markers (mean ± SD; n ≥ 14). (F) A model of the canonical pathways of endocytic sorting. Receptors enter the cells through clathrin-mediated endocytosis and are trafficked in EEA1+ early endosomes to an EEA1+/Rab4+ sorting endosomal compartment. Receptors can be recycled from here either through the Rab4-dependent short-loop or through the long-loop via the perinuclear recycling compartment (PNRC), marked by Rab11. RTKs such as the EGF and PDGF receptors are not recycled but are instead sorted to CD63+ late endosomes and then to the lysosomal compartment for degradation.
VEGF stimulation redirects KDR to the late endosomal compartment, but only a fraction of receptor undergoes subsequent lysosomal degradation. HUVECs were stimulated with 100 ng/mL VEGF for 0 hours (A,D), 1 hour (B,E), or 24 hours (C-D) and then fixed and stained (green) for endogenous EEA1 (A-C) or CD63 (D-F). All cells were costained for endogenous KDR (red). Nuclei are stained with DAPI (blue). Inset panels show magnified portions of each image, as indicated (dashed squares). Bar represents 10 μm. (G) The percentage of KDR+ vesicles that colocalized with EEA1+ vesicles or CD63+ vesicles at the 3 time points was quantified (mean ± SD; n ≥ 16). Degradation of KDR over the same time course of VEGF stimulation was determined by Western blotting of HUVEC lysates (H). The lower portion of the Western blot was probed for tubulin to confirm equal loading between samples. (I) Densitometric quantification of the degradation time course experiments (mean ± SD; n = 4).
VEGF stimulation redirects KDR to the late endosomal compartment, but only a fraction of receptor undergoes subsequent lysosomal degradation. HUVECs were stimulated with 100 ng/mL VEGF for 0 hours (A,D), 1 hour (B,E), or 24 hours (C-D) and then fixed and stained (green) for endogenous EEA1 (A-C) or CD63 (D-F). All cells were costained for endogenous KDR (red). Nuclei are stained with DAPI (blue). Inset panels show magnified portions of each image, as indicated (dashed squares). Bar represents 10 μm. (G) The percentage of KDR+ vesicles that colocalized with EEA1+ vesicles or CD63+ vesicles at the 3 time points was quantified (mean ± SD; n ≥ 16). Degradation of KDR over the same time course of VEGF stimulation was determined by Western blotting of HUVEC lysates (H). The lower portion of the Western blot was probed for tubulin to confirm equal loading between samples. (I) Densitometric quantification of the degradation time course experiments (mean ± SD; n = 4).
Peripheral KDR+ vesicles carry Src tyrosine kinase
We again used markers of endosomal subcompartments to define the identity of the peripheral KDR+ vesicles observed on VEGF stimulation. As the internal pool of KDR shows extensive colocalization with Rab4, we examined whether these vesicles were Rab4+ recycling endosomes. While there were still a significant number of juxtanuclear KDR+/Rab4+ vesicles in VEGF-treated cells, the peripheral KDR+ vesicles did not contain Rab4 (Figure 5C). These peripheral vesicles also did not colocalize with the early endosomal marker EEA1 or the long-loop recycling endosomal marker Rab11 (data not shown). Taken together, these data suggest that KDR is not recycled through a conventional endocytic recycling pathway in VEGF-stimulated cells. Endothelial cells contain a specialized subplasmalemmal endosomal recycling compartment defined by the presence of the endothelial cell adhesion molecule PECAM-1/CD31.34 Rather than being vesicular in morphology, this compartment is a flattened reticulum located just beneath the plasma membrane at the site of cell-cell junctions, with regular connections to the cell surface. PECAM constitutively recycles from this compartment to junctions between cells34 as part of its function in regulating endothelial integrity and permeability.35 As previously reported, the PECAM recycling compartment resembled a broad ribbon of subplasmalemmal staining at cell-cell contact sites (Figure 5F). The peripheral KDR+ vesicles showed no significant colocalization with PECAM at these sites; however, we observed clusters of KDR+ vesicles beneath the subjunctional PECAM compartment (Figure 5F). From this we conclude that the recycling pathways for KDR and PECAM are distinct, although the KDR recycling pathway may return receptor to sites of cell-cell adhesion.
One of the unique features of VEGF as a proangiogenic growth factor is its ability to disrupt endothelial barrier formation and so increase vascular permeability.6 These effects of VEGF are mediated by Src family tyrosine kinases, which are activated downstream of KDR.36 Src kinases promote activation of αvβ5 integrin on VEGF stimulation37 and also disrupt the endothelial barrier by destabilizing VE-cadherin/β-catenin complexes.38 Both of these processes contribute to the vascular permeability response. Recent studies by Frame and coworkers (Sandilands et al39 ) have identified a novel endocytic recycling pathway for Src. This operates in actively spreading fibroblasts and takes Src from an intracellular endocytic storage compartment to the plasma membrane where it activates focal adhesion kinase (FAK),39 a key regulator of cell adhesion. The clustering of KDR+ vesicles near sites of cell protrusion (Figure 4A) and sites of cell-cell adhesion (Figure 5F) prompted us to examine whether this Src recycling pathway also operates in endothelial cells and indeed whether this is the pathway used by KDR. The intracellular KDR compartment showed extensive colocalization with Src in unstimulated endothelial cells (data not shown), but, unlike other markers of recycling compartments, Src also colocalized with the peripheral KDR+ vesicles in VEGF-stimulated cells (Figure 5G). To examine the potential involvement of Src in KDR recycling, we performed receptor recycling assays in the presence or absence of AP23464, a highly specific inhibitor of Src kinase activity.40 Inhibition of Src activation completely inhibited VEGF-stimulated KDR recycling, returning the recycling rate to basal levels (Figure 5J). We conclude that in VEGF-stimulated endothelial cells, Src and KDR are trafficked to the cell surface together through the same endocytic recycling pathway and that this pathway depends on Src activation.
VEGF stimulation provokes recycling of KDR to the cell surface. HUVECs were stimulated with 100 ng/mL VEGF for 30 minutes and then fixed and stained (green) for F-actin (C) and endogenous KDR (B; red). Nuclei are stained with DAPI (blue). Panel A shows the merged image. In panel B, peripheral clusters of KDR+ vesicles (arrow) and a KDR-enriched membrane protrusion (arrowhead) are indicated. (D) HUVECs were stimulated with 100 ng/mL VEGF for 30 minutes and then fixed and stained for tubulin (green) and endogenous KDR (red). Peripheral KDR+ vesicles can been seen aligned with microtubules directly beneath the cell surface. Bar represents 10 μm. (E) HUVECs were surface biotinylated and then incubated in the presence (•) or absence (○) of 50 ng/mL VEGF. At the time points indicated, biotin on surface KDR was cleaved by incubation with MesNa, and then the remaining biotinylated KDR (internalized) was quantified by ELISA, as described in “Biochemical quantification of KDR trafficking.” Data represent the mean ± SEM of 3 independent experiments. (F) A similar assay was used to measure the rates of KDR recycling over a 35-minute time course (20 min internalization, 15 min recycling), as detailed in “Biochemical quantification of KDR trafficking.” Cells were treated with (▪) or without (□) 50 ng/mL VEGF. VEGF stimulation caused a significant increase in the rate of KDR recycling (P < .001). Data represent the mean ± SEM of 5 independent experiments.
VEGF stimulation provokes recycling of KDR to the cell surface. HUVECs were stimulated with 100 ng/mL VEGF for 30 minutes and then fixed and stained (green) for F-actin (C) and endogenous KDR (B; red). Nuclei are stained with DAPI (blue). Panel A shows the merged image. In panel B, peripheral clusters of KDR+ vesicles (arrow) and a KDR-enriched membrane protrusion (arrowhead) are indicated. (D) HUVECs were stimulated with 100 ng/mL VEGF for 30 minutes and then fixed and stained for tubulin (green) and endogenous KDR (red). Peripheral KDR+ vesicles can been seen aligned with microtubules directly beneath the cell surface. Bar represents 10 μm. (E) HUVECs were surface biotinylated and then incubated in the presence (•) or absence (○) of 50 ng/mL VEGF. At the time points indicated, biotin on surface KDR was cleaved by incubation with MesNa, and then the remaining biotinylated KDR (internalized) was quantified by ELISA, as described in “Biochemical quantification of KDR trafficking.” Data represent the mean ± SEM of 3 independent experiments. (F) A similar assay was used to measure the rates of KDR recycling over a 35-minute time course (20 min internalization, 15 min recycling), as detailed in “Biochemical quantification of KDR trafficking.” Cells were treated with (▪) or without (□) 50 ng/mL VEGF. VEGF stimulation caused a significant increase in the rate of KDR recycling (P < .001). Data represent the mean ± SEM of 5 independent experiments.
Discussion
Taken together, our data present a picture of KDR trafficking that is strikingly different from what we know of other RTKs. These differences have implications for the role of receptor trafficking in proangiogenic signaling from KDR. Central to this is the observation that a significant pool of cellular KDR is contained within an intracellular endosomal storage pool, rather than being presented on the cell surface. This reservoir of KDR is inaccessible to growth factor signals at the surface, and resting cells would appear to be far below their full potential sensitivity to VEGF stimulation. VEGF can stimulate recycling of intracellular KDR to the cell surface and so potentially increase the sensitivity of endothelial cells to subsequent growth factor stimulation; however, we find that endothelial cells stimulated with VEGF for extended periods still have a significant intracellular pool of KDR. Clearly, VEGF is unable to fully mobilize this compartment, and it is intriguing to speculate about the possible existence of stimuli that could do this; such mobilization would lead to a dramatic increase in the surface expression of KDR and presumably to a consequent increase in the magnitude of response to VEGF stimulation.
The KDR store mobilized by VEGF may play a more sophisticated role in proangiogenic signaling than simply regulating the surface expression of the receptor. The peripheral KDR+ vesicles in VEGF-stimulated cells are frequently clustered at discrete sites beneath the plasma membrane—sites of membrane protrusion and sites of cell-cell contact. Targeted recycling of endocytic vesicles is known to play an important role in generating cell polarity. In fibroblasts migrating to EGF, the internalized growth factor receptor is recycled to the leading edge of the cell.41-43 This leads to an increased concentration of receptor in the forward protrusion, and it has been proposed that this sensitizes the cell to chemotactic signals, reinforcing the direction of forward movement.44 Similar findings have recently been reported for the guided embryonic migrations regulated by EGF and PDGF receptors in Drosophila.45 It is intriguing to speculate that the VEGF-stimulated recycling of KDR may function in the same way, to sensitize regions of the endothelial cell surface to further VEGF stimulation by concentrating receptor at these places. In support of this hypothesis, studies by Gerhardt et al46 have shown that KDR is concentrated at the tips of vascular sprouts during sprouting angiogenesis. Specifically, KDR is highly enriched in the protrusive filopodia at the sprout tip, which sense the gradient of VEGF and allow for guidance of sprout toward the angiogenic signal.46 It is also important to consider the relevance of the cotrafficking of Src tyrosine kinase with recycling KDR. Src plays a key role in increasing vascular permeability in response to VEGF but also is an important regulator of integrin signaling and cell adhesion. It will be important to investigate whether recycling KDR is delivered with other downstream signaling partners in addition to Src. Clustering of KDR with a selective package of signaling partners would potentially alter the blend of signals developed at the sites of delivery and so further impact on the angiogenic process.
Peripheral KDR+ vesicles carry Src tyrosine kinase. HUVECs were stimulated with 100 ng/mL VEGF for 30 minutes and then fixed and stained for endogenous KDR (red). (A-C) Cells were transfected with GFP-Rab4. (D-F) Cells were costained for PECAM (green), which stained a broad ribbon beneath the sites of cell-cell contact. Clusters of peripheral KDR+ vesicles could be observed at the base of some of these structures (F; arrows); however, these KDR+ vesicles did not contain PECAM (D). (G-I) Cells were transfected with GFP-Src. Panels H and I show a magnified section of panel G (dashed square). Arrowheads indicate the positions of peripheral KDR+ vesicles that colocalize with Src. Nuclei are stained with DAPI (blue). Bar represents 10 μm. (J) HUVECs were surface biotinylated and then incubated in the presence or absence of 50 ng/mL VEGF or with VEGF and 1 μM AP23464. KDR recycling over a 35-minute time course (20 min internalization, 15 min recycling) was measured as described in “Biochemical quantification of KDR trafficking.” Data represent the mean ± SEM of 3 independent experiments. As for measurements of receptor internalization, VEGF stimulation significantly stimulated KDR recycling (P = .006), whereas addition of Src inhibitor reduced the VEGF stimulation back to the basal rate (P = .002).
Peripheral KDR+ vesicles carry Src tyrosine kinase. HUVECs were stimulated with 100 ng/mL VEGF for 30 minutes and then fixed and stained for endogenous KDR (red). (A-C) Cells were transfected with GFP-Rab4. (D-F) Cells were costained for PECAM (green), which stained a broad ribbon beneath the sites of cell-cell contact. Clusters of peripheral KDR+ vesicles could be observed at the base of some of these structures (F; arrows); however, these KDR+ vesicles did not contain PECAM (D). (G-I) Cells were transfected with GFP-Src. Panels H and I show a magnified section of panel G (dashed square). Arrowheads indicate the positions of peripheral KDR+ vesicles that colocalize with Src. Nuclei are stained with DAPI (blue). Bar represents 10 μm. (J) HUVECs were surface biotinylated and then incubated in the presence or absence of 50 ng/mL VEGF or with VEGF and 1 μM AP23464. KDR recycling over a 35-minute time course (20 min internalization, 15 min recycling) was measured as described in “Biochemical quantification of KDR trafficking.” Data represent the mean ± SEM of 3 independent experiments. As for measurements of receptor internalization, VEGF stimulation significantly stimulated KDR recycling (P = .006), whereas addition of Src inhibitor reduced the VEGF stimulation back to the basal rate (P = .002).
In summary, the unique endocytic itinerary of KDR, together with the ability of VEGF to directly affect this, suggests ways in which proangiogenic signals can be shaped and refined through regulation of receptor traffic. In addition to regulating the sensitivity of endothelial cells to proangiogenic signals, these VEGF-controlled trafficking pathways have the potential to contribute to morphologic responses, such as the cellular remodeling and cell migration that underlie sprouting angiogenesis. Further studies directed at separating the contributions of receptor signaling and receptor trafficking will allow us to dissect out the role of endocytic sorting in the complex processes of angiogenesis.
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2005-12-007484.
Supported by a Wellcome Trust University Award (H.M.), a British Heart Foundation Project Grant (H.M.), and a Medical Research Council (MRC)
Infrastructure Award to the School of Medical Sciences Imaging Centre.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Jon Larty and Andres Lopez-Bernal for assistance with the preparation of HUVECs, Robert Lodge for the generous gift of GFP-tagged Rab4 and Rab11 constructs, and Margaret Frame for the GFP-Src construct.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal