Comment on Chng et al, page 2755
Waldenström macroglobulinemia (WM) shares traits with both chronic lymphocytic leukemia (CLL) and multiple myeloma (MM). Chng and colleagues now demonstrate that by gene expression WM appears more closely related to CLL than MM.
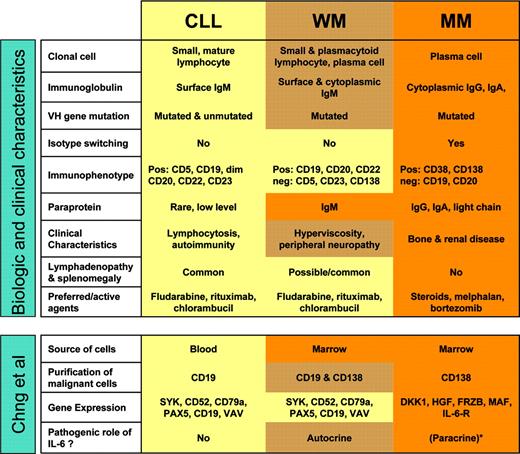
Waldenström macroglobulinemia (WM) is a rare hematologic malignancy characterized by an IgM monoclonal gammopathy and bone marrow infiltration by small lymphocytes that can undergo plasmacytoid and plasma cell differentiation.1 It has long been recognized that WM shows features suggestive of an intermediate process between chronic lymphocytic leukemia (CLL) and multiple myeloma (MM). WM cells express B-cell markers but not CD5 or CD23 in contradistinction to CLL. WM cells appear to derive from a postgerminal B cell that has undergone somatic hypermutation but not switch recombination. The clonal cells reside primarily in the bone marrow similar to MM but secrete an IgM paraprotein that causes many of the clinical sequelae. Effective therapeutic agents in WM are by and large the same as in CLL (see figure).
Gene expression profiling can establish molecular diagnoses and uncover or better define distinct diseases.2 This approach has been especially successful when the clinical material was relatively homogeneous, for instance lymph node biopsies or purified leukemic cells. Chng and colleagues used genomic-scale gene expression analysis to investigate the relationship between WM, CLL, and MM. Such a comparison is no easy undertaking due to distinct expression of cell-surface markers, differences in anatomic distribution, and the variable degree of blood or marrow involvement. The malignant cells studied here were obtained from peripheral blood (CLL) or bone marrow (WM, MM) and purified for CD19 (CLL), CD138 (MM), and combined CD19 and CD138 (WM) expression. The nature of the diseases and the approach chosen introduced confounding factors, notably differences in gene expression apparently contributed by contaminating cells, and made the use of statistical filters necessary. Nevertheless, the authors made several interesting observations (see figure).
Summary of characteristics of WM, CLL, and MM and of methods and results from the study by Chng et al. See the complete figure in the article beginning on page 2755.
Summary of characteristics of WM, CLL, and MM and of methods and results from the study by Chng et al. See the complete figure in the article beginning on page 2755.
Gene expression among the 23 WM cases appeared homogeneous, supporting the concept of a common biology despite the morphologic variability of the clonal cells. WM shared expression of typical B-cell markers with CLL and by hierarchic clustering analysis most WM samples aligned with the CLL samples. A small set of genes that interestingly included IL-6 and CD1c were overexpressed in WM. A role for IL-6 in WM has been previously suggested by the observation of IL-6-induced differentiation of the clonal B cells into plasma cells and the secretion of IL-6 by WM cells in vitro.3 The current study thus provides a valuable confirmation of IL-6's potential pathogenic role in WM. The CD1 family of MHC-like glycoproteins presents glycolipids to antigen-specific T cells and plays important roles in innate immunity and autoimmunity.4 Because WM cells often produce IgM paraproteins reactive with phospholipids, it is tempting to spe culate that phospholipid antigens could contribute to the pathogenesis of WM.
In summary, this study adds further evidence that WM constitutes more a B-cell than a plasma cell malignancy and that WM is more closely related to CLL than MM. The unique gene expression profile of WM can provide a framework for further studies and focus attention on the possible pathogenic role of IL-6 production by the malignant clone. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal