Abstract
The “holy grail” of allogeneic stem cell transplantation is to preserve the graft-versus-tumor (GVT) effect while eliminating graft-versus-host disease (GVHD). Endogenous donor-derived interleukin 15 (IL-15) has been implicated in the pathogenesis of acute GVHD, yet the mechanism by which it impacts this lethal process remains unclear. Using the well-described and clinically relevant C57BL/6 → B6D2F1 murine model of acute GVHD, we demonstrate that in trans presentation of IL-15 by donor bone marrow–derived cells is required for the rapid onset of acute GVHD. Recipients of IL-15–/– C57BL/6 bone marrow cells show diminished type 1 polarization of T cells, yet there is no decrease in donor T-cell reconstitution. A molecular basis for these findings is provided with the observation that expression of T-bet, the master control gene for type 1 T-cell functions, is necessary for IL-15–mediated acute GVHD lethality. Finally, we demonstrate that in the absence of donor-derived IL-15, the GVT effect is maintained. These findings thus establish a mechanism by which endogenous donor-derived IL-15 impacts the pathobiology of acute GVHD and GVT activity.
Introduction
Acute graft-versus-host disease (GVHD) is the most common severe side effect of allogeneic bone marrow transplantation (BMT), reaching grade III-IV status in 29% to 41% of transplant recipients.1 It is mediated by donor-derived T cells from the allograft and results in a shock-like “cytokine storm” within the first week after transplantation, followed by CD4+ and CD8+ T-cell–mediated tissue destruction in the liver, gut, and skin.2 Acute GVHD can largely be averted by using syngeneic or T-cell–depleted allogeneic grafts, although these methodologies are associated with higher rates of malignant relapse.3
A major goal of BMT research, therefore, is to discover factors that, when modulated through pharmacologic means or by prescreening donors, can lessen acute GVHD lethality while maintaining graft-versus-tumor (GVT) activity. Interleukin 15 (IL-15) is a cytokine critical for the survival and homeostasis of memory CD8+ T cells,4 which are known to actively promote acute GVHD.5,6 Exogenously administered IL-15 can increase both autoimmune disease7 and memory CD8+ T-cell function against autologous tumor targets.8,9 Recently, we and others have shown that deregulation of endogenous IL-15 expression or administration of exogenous IL-15 can increase acute GVHD lethality in the presence of donor-derived allogeneic T cells.10,11 Yet the mechanism by which endogenous IL-15 can promote acute GVHD, alter posttransplantation immune reconstitution, and influence the GVT effect remain unclear. We report that in the absence of donor-derived IL-15 expression, acute GVHD lethality is significantly decreased, yet donor T-cell reconstitution and GVT effects are maintained. Further, we demonstrate that donor-derived IL-15 is necessary for optimal type 1 T-cell polarization in acute GVHD and provide evidence for the cellular and molecular mechanisms by which this is accomplished. Taken together, these data support the notion that targeting IL-15 in allogeneic stem cell transplantation may move us closer to dissecting harmful acute GVHD from the beneficial GVT effect.
Materials and methods
Reagents, monoclonal antibodies, and flow cytometry
Fluorochrome-conjugated antimouse antibodies were all purchased from BD Pharmingen (San Jose, CA). Routine cell-surface staining was performed using standard techniques; data were acquired on a Becton Dickinson (Franklin Lakes, NJ) FACScalibur flow cytometer using CellQuest software. Recombinant human (rh) IL-15 was generously provided by Amgen (Thousand Oaks, CA); recombinant murine (rm) IL-15 was purchased from R&D Systems (Minneapolis, MN).
Mice
Female C57Bl/6 (B6, H-2b), B6D2F1 (H-2b/d), and T-bet–/– (B6 background, H-2b) mice (6 to 7 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). Female IL-15–/– B6 mice (6 to 7 weeks old) were purchased from Taconic Farms (Germantown, NY).12 IL-15 Rα–/– B6 mice were generously provided by Averil Ma (University of California San Francisco) and used to establish a breeding colony at The Ohio State University.13 IL-15 transgenic (tg) B6 mice were created as described and maintained at The Ohio State University.14 All mice were between 8 and 12 weeks old at the beginning of each experiment. Mice that underwent transplantation were maintained in sterilized microisolators and received irradiated rodent chow and acidified water plus oral antibiotic (Baytril, 0.2 mg/mL) for 21 days following transplantation. All animal research was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee (ILACUC) at The Ohio State University.
Bone marrow transplantation
The B6 → B6D2F1 model of experimental acute GVHD has been described in detail elsewhere.10,15 Briefly, T cells were depleted from bone marrow (BM) cells harvested from donor mice by labeling with PE-conjugated anti-CD3, anti-CD4, and anti-CD8 antibodies followed by anti-PE microbeads and passage through magnetic LD columns (Miltenyi Biotec, Auburn, CA). The efficiency of T-cell depletion was more than 98%. Splenic T cells were purified by negative selection from wild-type (wt) B6 mice by labeling splenocytes with PE-conjugated anti–Ter 119, anti-NK1.1, anti–Gr-1, anti-CD11b, anti-CD11c, anti–I-A/I-E, and anti-B220 (BD Pharmingen), followed by anti-PE microbeads and passage through LD columns (Miltenyi Biotec). Negatively selected T cells were more than 95% CD3+. Recipients were conditioned with 1300 cGy whole-body gamma irradiation (Gammacell 40; MDS Nordion, Ottawa, Ontario) split into 2 doses 24 hours prior to intravenous infusion of the combined bone marrow and T-cell graft. In most cases, recipients were given 1 × 107 T-cell–depleted BM cells and 5 × 106 splenic T cells. Because of the losses associated with T-cell purification and because of the limited number of animals available, experiments involving T-bet–/– B6 donors used whole splenocytes adjusted to contain 5 × 106 splenic T cells in all groups to induce GVHD. At the time they were killed, spleens of recipient mice were removed and splenocytes prepared by standard techniques. Intraepithelial lymphocytes (IELs) were isolated from gut tissue according to published methods.16 In some experiments, spleen and liver were preserved in 10% neutral buffered formalin prior to routine histologic processing. Light micrographs were obtained in air using an Olympus CKX41 microscope equipped with a 4 ×/0.10 objective lens and an Olympus DP12 digital camera and software (Olympus America, Center Valley, PA). Adobe Photoshop 8.0 was used for image processing (Adobe Systems, San Jose, CA).
In vitro T-cell stimulation and intracellular flow cytometry
Ninety-six–well tissue-culture plates were coated overnight at 4°C with hamster anti–mouse CD3 and CD28 (50 μL per well, each antibody at 10 μg/mL; BD Pharmingen). Splenocytes were cultured at 2 × 106 cells/mL in complete medium in the presence of 20 μg/mL Golgiplug (Brefeldin A; BD Pharmingen) for 6 hours. After incubation, splenocytes were stained for surface markers, washed, and then fixed and permeabilized using the BD Intracellular Flow Cytometry kit according to the manufacturer's instructions. Intracellular staining was performed with PE-conjugated rat anti–mouse IFN-γ (IgG1) or control rat IgG1 (BD Pharmingen).
Measurement of cytokines
Mouse serum samples were diluted 1:2 and assayed for a panel of cytokines using the mouse Th1/Th2 Cytometric Bead Array Kit (BD Pharmingen). The kit was used according to the manufacturer's instructions.
Quantitative real-time PCR
RNA was purified from peripheral blood mononuclear cells (PBMCs) using the Qiagen Rneasy RNA extraction kit. RNA was reverse-transcribed using Invitrogen reagents and quantitative real-time polymerase chain reaction (PCR) was performed using an ABI 7600 Sequence Detector. Primers and a probe specific for murine T-bet and spanning an intron were designed as follows: forward 5′-CTAAGCAAGGACGGCGAATG-3′; reverse 5′-CAAACATCCTGTAATGGCTTGTG-3′; probe 5′-CTGTCCTTCACCGTGGCTGGGCT-3′. Amplification of 18s RNA served as an internal positive control in all samples.
Statistics
To compare the clinical evaluation and the percent of baseline weight data, a linear mixed effects model was fit to the data. The terms included group, time, and the group*time interaction. The model assumptions include normality and equal variances of the residuals. These assumptions were checked for each model. Survival data were compared using the logrank test and all other data were compared using the Student t test. P values equal to or less than .05 were considered statistically significant.
Results
Acute allogeneic GVHD is attenuated in the absence of endogenous IL-15 produced by donor bone marrow–derived cells
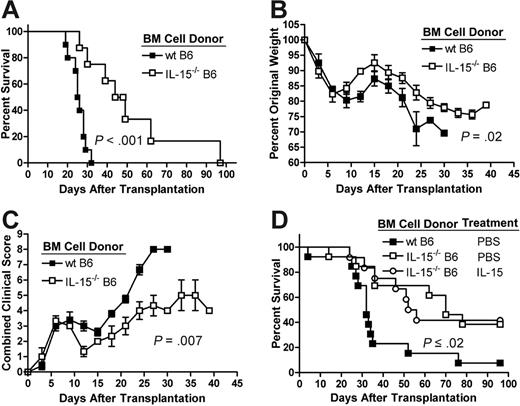
Allogeneic recipients of IL-15–/– B6 T-cell–depleted bone marrow (TCD BM) cells and highly purified (> 95% CD3+) wt B6 T cells demonstrated increased survival compared with recipients of wt B6 TCD BM cells and wt B6 T cells (median survival time [MST] 25.5 days versus 46.5 days, P < .001; Figure 1A). Weight loss (Figure 1B) and clinical evidence of acute GVHD (Figure 1C)17 were also significantly less severe in recipients of IL-15–/– B6 TCD BM cells compared with recipients of wt B6 TCD BM cells (P = .02 and P = .007, respectively). To prove that the increased death rate due to acute GVHD in recipients of wt B6 BM cells was a result of posttransplantation IL-15 production by these cells and not by differences in the cellular constituency of the wt and IL-15–/– allografts, IL-15–/– B6 donor mice were pretreated with rhIL-1512 or phosphate-buffered saline (PBS) as a control. Compared with wt B6 donors, control-treated IL-15–/– donors demonstrated significant decreases only in CD11c+B220+ plasmacytoid dendritic cells and NK1.1+CD3– natural killer (NK) cells. Treatment with IL-15 normalized plasmacytoid dendritic cell numbers and increased NK cell numbers (5.5% versus 1.47%, P < .001) compared with control-treated wt B6 donors (Table 1). However, recipients of wt B6 splenic T cells and TCD BM cells from IL-15–pretreated IL-15–/– mice showed significantly longer survival compared with recipients of wt B6 splenic T cells and wt B6 TCD BM cells (MST 32 days versus 54 days, P = .02; Figure 1D) and there was no difference in survival between recipients of BM cells from IL-15– and PBS-pretreated IL-15–/– B6 donors (Figure 1D).
Absence of IL-15 production by donor-derived BM cells decreases acute GVHD morbidity and mortality. Lethally irradiated wt B6D2F1 mice underwent transplantation with 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells from wt B6 (▪) or IL-15–/– B6 mice (□). Survival was monitored daily (A) and body weights (B) and clinical GVHD scores (C) were collected in a blinded fashion every 3 days after transplantation. Survival data are combined from 2 similar experiments; weight and clinical score data are from 1 of 2 similar experiments. N ≥ 8 mice per group. (D) IL-15–/– B6 donors were pretreated with 10 μg/d rhIL-15 or PBS as a control for 7 days prior to harvest of bone marrow. Survival of allogeneic recipients (N ≥ 12 mice per group) of 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells harvested from rhIL-15–treated IL-15–/– B6 donors (○), PBS-treated IL-15–/– B6 donors (□), or PBS-treated wt B6 donors (▪) was monitored daily after transplantation. All survival times were compared using the logrank test. Mean group weight and clinical GVHD data were compared using a linear mixed effects model as described in “Materials and methods.” Error bars represent standard error of the mean (SEM).
Absence of IL-15 production by donor-derived BM cells decreases acute GVHD morbidity and mortality. Lethally irradiated wt B6D2F1 mice underwent transplantation with 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells from wt B6 (▪) or IL-15–/– B6 mice (□). Survival was monitored daily (A) and body weights (B) and clinical GVHD scores (C) were collected in a blinded fashion every 3 days after transplantation. Survival data are combined from 2 similar experiments; weight and clinical score data are from 1 of 2 similar experiments. N ≥ 8 mice per group. (D) IL-15–/– B6 donors were pretreated with 10 μg/d rhIL-15 or PBS as a control for 7 days prior to harvest of bone marrow. Survival of allogeneic recipients (N ≥ 12 mice per group) of 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells harvested from rhIL-15–treated IL-15–/– B6 donors (○), PBS-treated IL-15–/– B6 donors (□), or PBS-treated wt B6 donors (▪) was monitored daily after transplantation. All survival times were compared using the logrank test. Mean group weight and clinical GVHD data were compared using a linear mixed effects model as described in “Materials and methods.” Error bars represent standard error of the mean (SEM).
Flow cytometric analysis of IL-15-pretreated IL-15–/– B6 BM cell donors
. | BM cell donor/pretreatment, percent bone marrow cells (P) . | . | . | ||
|---|---|---|---|---|---|
| Cell type . | WT B6/PBS . | IL-15-/- B6/PBS . | IL-15-/- B6/rhIL-15 . | ||
| Monocytes/granulocytes | 31.7 | 31.3 (.76) | 29.3 (.16) | ||
| Myeloid dendritic cells | 1.84 | 1.28 (.1) | 2.01 (.41) | ||
| Plasmacytoid dendritic cells | 2.72 | 2.26 (.009) | 2.63 (.74) | ||
| B lymphocytes | 22.3 | 25.9 (.12) | 18.6 (.04) | ||
| Natural killer cells | 1.47 | 0.16 (< .001) | 5.5 (< .001) | ||
. | BM cell donor/pretreatment, percent bone marrow cells (P) . | . | . | ||
|---|---|---|---|---|---|
| Cell type . | WT B6/PBS . | IL-15-/- B6/PBS . | IL-15-/- B6/rhIL-15 . | ||
| Monocytes/granulocytes | 31.7 | 31.3 (.76) | 29.3 (.16) | ||
| Myeloid dendritic cells | 1.84 | 1.28 (.1) | 2.01 (.41) | ||
| Plasmacytoid dendritic cells | 2.72 | 2.26 (.009) | 2.63 (.74) | ||
| B lymphocytes | 22.3 | 25.9 (.12) | 18.6 (.04) | ||
| Natural killer cells | 1.47 | 0.16 (< .001) | 5.5 (< .001) | ||
Donor mice were pretreated with rhIL-15 or PBS as described in “Results.” Values represent mean percent of bone marrow cells that were CD11b+GR-1+ monocytes/granulocytes, CD11c+B220+ plasmacytoid dendritic cells, CD11c+B220- myeloid dendritic cells, CD11c-B220+ B cells, or NK1.1+CD3- NK cells. P values (in parentheses) were calculated using the Student t test for comparisons between wt B6 and IL-15- or control-treated IL-15-/- B6 donors. N = 10 donors per group.
Coordinate expression of IL-15 with IL-15 receptor alpha (Rα) is required in acute GVHD
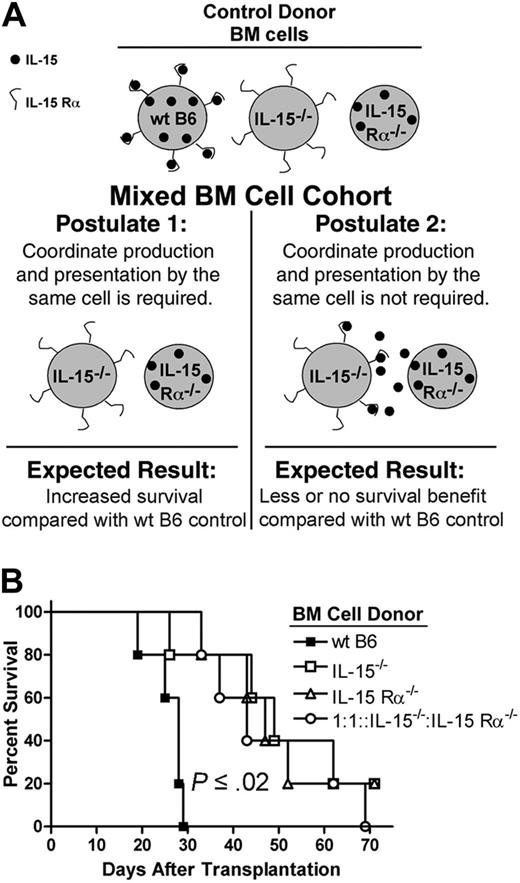
We hypothesized that expression of IL-15 in acute GVHD must occur by the same donor cell that presents IL-15 in trans via IL-15 Rα to responding lymphocytes; this so-called coordinate expression model has been described in immune-deficient mouse models18,19 but not in disease states. If this model holds in acute GVHD, then recipients of IL-15–/– and IL-15 Rα–/– BM cells should demonstrate similar survival advantages compared with recipients of wt BM cells. Further, because in the coordinate model IL-15 Rα–/– cells (which do express IL-15) cannot produce soluble IL-15 for capture and presentation by IL-15–/– cells (which do express IL-15 Rα), recipients of IL-15–/– and IL-15Rα–/– BM cells mixed 1:1 should also demonstrate a survival advantage. The rationale for the mixing experiment is presented in Figure 2A. Wild-type B6D2F1 mice underwent transplantation with 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells from either wt B6, IL-15–/– B6, or IL-15 Rα–/– B6 donors. A fourth cohort received 1 × 107 TCD BM cells from IL-15–/– and IL-15 Rα–/– B6 mice mixed 1:1. Recipients of IL-15–/– B6 or IL-15 Rα–/– B6 BM cells lived significantly longer after transplantation compared with recipients of wt B6 BM cells (IL-15–/– B6 versus wt B6: MST 49 days versus 28 days, P = .02; IL-15 Rα–/– B6 versus wt B6: MST 47 days versus 28 days, P = .002; Figure 2B). There was no difference in survival between recipients of IL-15–/– or IL-15 Rα–/– B6 BM cells (Figure 2B). In addition, recipients of the mixed BM cells demonstrated significantly longer survival after transplantation compared with recipients of wt BM cells (MST 43 days versus 28 days, P = .002; Figure 2B). There was no difference in survival between these mice and recipients of either IL-15–/– B6 or IL-15 Rα–/– B6 BM cells (Figure 2B).
IL-15 is coordinately expressed with IL-15 Rα in acute GVHD. (A) Rationale behind the mixed BM cell experiment. Wild-type B6 control donor BM cells can produce IL-15 for presentation by IL-15 Rα. IL-15–/– and IL-15 Rα–/– control donor BM cells are each genetically deficient in IL-15 signaling and each confers a survival benefit compared with wt B6 BM cells. In postulate 1, IL-15 Rα–/– donor BM cells produce IL-15 whereas IL-15–/– donor BM cells have IL-15 Rα. Because it is postulated that the same cell must produce and present IL-15, it is expected that survival will not be adversely affected in the mixed BM group. In postulate 2, coordinate production and presentation is not required. Thus, IL-15 Rα–/– donor BM cells produce IL-15, which is captured and presented by IL-15–/– donor BM cells, thereby restoring IL-15 signaling and reducing or eliminating any survival benefit in the mixed BM group compared with wt B6 control. (B) Lethally irradiated wt B6D2F1 mice (n = 5 per group) underwent transplantation with 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells from either wt B6 (▪), IL-15–/–B6 (□) or IL-15 Rα–/– B6 mice (▵). A fourth cohort of mice underwent transplantation with 1 × 107 TCD BM cells from IL-15–/– and IL-15 Rα–/– B6 mice mixed at a 1:1 ratio (○). Survival times were compared using the logrank test and results were consistent with postulate 1.
IL-15 is coordinately expressed with IL-15 Rα in acute GVHD. (A) Rationale behind the mixed BM cell experiment. Wild-type B6 control donor BM cells can produce IL-15 for presentation by IL-15 Rα. IL-15–/– and IL-15 Rα–/– control donor BM cells are each genetically deficient in IL-15 signaling and each confers a survival benefit compared with wt B6 BM cells. In postulate 1, IL-15 Rα–/– donor BM cells produce IL-15 whereas IL-15–/– donor BM cells have IL-15 Rα. Because it is postulated that the same cell must produce and present IL-15, it is expected that survival will not be adversely affected in the mixed BM group. In postulate 2, coordinate production and presentation is not required. Thus, IL-15 Rα–/– donor BM cells produce IL-15, which is captured and presented by IL-15–/– donor BM cells, thereby restoring IL-15 signaling and reducing or eliminating any survival benefit in the mixed BM group compared with wt B6 control. (B) Lethally irradiated wt B6D2F1 mice (n = 5 per group) underwent transplantation with 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells from either wt B6 (▪), IL-15–/–B6 (□) or IL-15 Rα–/– B6 mice (▵). A fourth cohort of mice underwent transplantation with 1 × 107 TCD BM cells from IL-15–/– and IL-15 Rα–/– B6 mice mixed at a 1:1 ratio (○). Survival times were compared using the logrank test and results were consistent with postulate 1.
Absence of donor-derived IL-15 does not decrease donor T-cell chimerism or memory CD8+ T-cell reconstitution
Splenocytes were harvested from allogeneic recipients of wt B6 splenic T cells and wt B6 TCD BM cells or IL-15–/– B6 TCD BM cells 5, 12, or 28 days after transplantation. There was no significant difference in donor CD4+ or CD8+ T-cell chimerism at any of these time points (Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article). Further, in the absence of donor-derived IL-15 there was no significant decrease in absolute numbers of donor-derived CD8+ CD122+ memory T cells (Figure S2), CD8+ CD44hi CD62Llo effector memory T cells (Figure S3), or CD8+ CD44hi CD62Lhi central memory T cells (Figure S4) harvested from the spleen. IL-15 is critical for the survival of NK cells12 and may contribute to the survival of monocytes and dendritic cells,20 yet there was no decrease in the absolute numbers of these cells in recipients of IL-15–/– B6 BM cells (Figures S5 and S6). Finally, IL-15 is reportedly critical for the survival of IELs21 ; IELs have been shown to contribute to intestinal epithelial cell apoptosis in acute GVHD.22 There was no difference in the percent of donor-derived TCR-β+ CD4+, TCR-β+ CD8+, TCR-γ+ CD4+, or TCR-γ+ CD8+ IELs in the presence or absence of donor-derived IL-15 (Figure S7).
Donor-derived IL-15 is necessary for optimal IFN-γ production and CXCR3 expression by T cells in acute GVHD
B6D2F1 mice were lethally irradiated and infused with wt B6 splenic T cells and TCD BM cells from either wt B6 or IL-15–/– B6 mice. Serum and splenocytes were harvested 5 and 12 days after transplantation. There was no difference in serum IFN-γ concentration between recipients of wt B6 and IL-15–/– B6 TCD BM cells at day 5 after transplantation (data not shown), whereas at day 12 after transplantation, recipients of wt B6 TCD BM cells demonstrated significantly higher serum IFN-γ compared with recipients of IL-15–/– B6 TCD BM cells (183 pg/mL ± 13.6 pg/mL versus 67 pg/mL ± 17.3 pg/mL, P = .002; Figure 3A). Further, in vitro restimulation of splenocytes harvested at day 12 using immobilized anti-CD3 and anti-CD28 crosslinking antibodies revealed increased percentages of IFN-γ–producing CD4+ and CD8+ T cells from recipients of wt B6 BM cells compared with CD4+ and CD8+ T cells from recipients of IL-15–/– B6 BM cells (data not shown). CXCR3 is a receptor for the chemokines CXCL9, CXCL10, and CXCL11 that is preferentially expressed on Th1 cells.23-25 Recipients of wt B6 splenic T cells and wt B6 BM cells demonstrated significantly higher expression of CXCR3 by donor-derived CD4+ T cells at day 5 after transplantation compared with recipients of wt B6 splenic T cells and IL-15–/– B6 BM cells (mean fluorescence intensity [MFI] 10.99 ± 0.18 versus 9.11 ± 0.60, P = .04; Figure 3B-C).
Donor-derived IL-15 is necessary for optimal IFN-γ production and CXCR3 expression by T cells in acute GVHD. (A) Wild-type B6D2F1 mice were lethally irradiated and underwent transplantation with 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells from either wt B6 or IL-15–/– B6 mice. Mice were killed 12 days after transplantation, serum harvested, and IFN-γ concentrations measured by cytometric bead array. Data represent mean plus or minus SEM from 1 of 2 similar experiments with a total of n = 8 mice per group. (B) Splenocytes were harvested from recipients of wt B6 or IL-15–/– B6 BM cells 5 days after transplantation, gated on CD4+ donor-derived (H-2Dd–) cells and costained for cell-surface expression of CXCR3. Data represent average (mean ± SEM) mean fluorescence intensity (MFI) values from n = 3 mice per group. The experiment was performed twice with similar results. (C) Representative flow cytometric plots from allogeneic or syngeneic recipients of wt B6 splenic T cells and either wt B6 BM cells or IL-15–/– B6 BM cells are shown.
Donor-derived IL-15 is necessary for optimal IFN-γ production and CXCR3 expression by T cells in acute GVHD. (A) Wild-type B6D2F1 mice were lethally irradiated and underwent transplantation with 5 × 106 wt B6 splenic T cells and 1 × 107 TCD BM cells from either wt B6 or IL-15–/– B6 mice. Mice were killed 12 days after transplantation, serum harvested, and IFN-γ concentrations measured by cytometric bead array. Data represent mean plus or minus SEM from 1 of 2 similar experiments with a total of n = 8 mice per group. (B) Splenocytes were harvested from recipients of wt B6 or IL-15–/– B6 BM cells 5 days after transplantation, gated on CD4+ donor-derived (H-2Dd–) cells and costained for cell-surface expression of CXCR3. Data represent average (mean ± SEM) mean fluorescence intensity (MFI) values from n = 3 mice per group. The experiment was performed twice with similar results. (C) Representative flow cytometric plots from allogeneic or syngeneic recipients of wt B6 splenic T cells and either wt B6 BM cells or IL-15–/– B6 BM cells are shown.
Expression of T-bet by donor splenocytes is necessary for IL-15–mediated acute GVHD
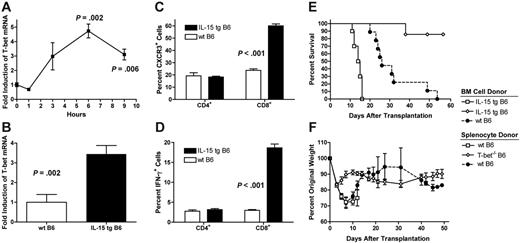
T-bet is a member of the T-box family of transcription factors that is necessary for type 1 T-cell lineage commitment, promotes IFN-γ production in both CD4+ and CD8+ T cells, and is necessary for optimal expression of CXCR3.26,27 Highly purified wt B6 splenic T cells cultured in the presence of rmIL-15 demonstrated significantly increased levels of T-bet mRNA beginning 6 hours after stimulation (Figure 4A). Further, peripheral blood leukocytes from IL-15 tg B6 mice that overexpress IL-15 using the H-2D promoter14 expressed T-bet mRNA at 3.4-fold higher levels compared with wt B6 mice (P = .002; Figure 4B). Accordingly, compared with wt B6 mice, IL-15 tg mice were found to have a greater percentage of CD8+ T cells constitutively expressing CXCR3 (60.0% ± 1.6% versus 23.7% ± 1.2%, P < .001, Figure 4C) and a greater percentage of CD8+ T cells that produced IFN-γ after anti-CD3/CD28 stimulation as measured by intracellular flow cytometry (18.7% ± 0.96% versus 3.0% ± 0.21%; P < .001; Figure 4D). Consistent with these in vitro findings, 86% of B6D2F1 recipients of 1 × 107 IL-15 tg TCD BM cells and whole T-bet–/– B6 splenocytes adjusted to contain 5 × 106 T cells survived more than 55 days after transplantation. In contrast, recipients of wt B6 splenocytes and either IL-15 tg B6 or wt B6 TCD BM cells demonstrated respective median survival times of 14.5 days or 28.5 days after transplantation (P < .001, P = .001 respectively, Figure 4E). Further, recipients of IL-15 tg B6 TCD BM cells and T-bet–/– splenocytes had significantly less severe weight loss over the course of the experiment when compared with recipients of IL-15 tg B6 BM cells and wt B6 splenocytes (P < .001 Figure 4F).
GVT activity is preserved in the absence of donor-derived IL-15
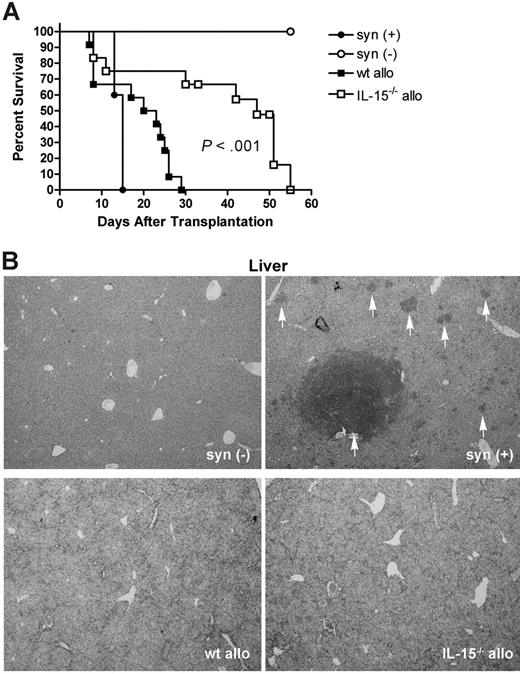
B6D2F1 mice were lethally irradiated and given 2000 P815 mastocytoma tumor cells (H-2d/d), 5 × 106 wt B6 (allogeneic) splenic T cells, and 1 × 107 TCD BM cells from either wt B6 or IL-15–/– B6 mice. For controls, identical recipients were given 5 × 106 wt B6D2F1 (syngeneic) T cells and 1 × 107 wt B6D2F1 TCD BM cells plus or minus 2000 P815 cells. Recipients of syngeneic T cells plus P815 demonstrated rapid death from tumor (MST 15 days; Figure 5A). Recipients of allogeneic IL-15–/– B6 TCD BM cells and wt T cells lived significantly longer compared with recipients of allogeneic wt B6 TCD BM cells and wt T cells (MST 47 days versus 21.5 days, P < .001; Figure 5A). Confirming death due to acute GVHD, all allogeneic recipients of wt or IL-15–/– B6 TCD BM cells demonstrated clinical signs of acute GVHD prior to death, and had no evidence of hindlimb paralysis and no evidence of circulating P815 cells in the peripheral blood at 1, 2, or 3 weeks after transplantation (data not shown). Representative mice were killed when moribund and liver and spleen harvested to examine for the presence of tumor cells. Only recipients of syngeneic TCD BM and syngeneic T cells showed evidence of tumor at these time points (Figure 5B). Taken together, these data show that not only is the GVT effect preserved in the absence of donor-derived IL-15, but overall survival after transplantation is dramatically increased as well.
Discussion
Using a well-described and clinically relevant murine model of allogeneic BMT, we have shown that IL-15 is produced and presented in trans by donor-derived cells in acute GVHD. Donor-derived IL-15 was necessary for full type 1 T-cell polarization and promoted acute GVHD lethality in a manner dependent on expression of T-bet in donor cells. Yet donor-derived IL-15 was dispensable for achieving full donor T-, NK, and myeloid cell reconstitution under myeloablative conditions. Finally, overall survival was substantially improved in the absence of donor-derived IL-15 in a commonly used model of acute GVHD and GVT activity. We believe that together these data provide the mechanism by which donor-derived IL-15 signals and impacts T-cell polarization and immune reconstitution in acute GVHD.
IL-15–mediated acute GVHD requires expression of T-bet by donor splenocytes. (A) Highly purified wt B6 splenic T cells were cultured in the presence of rmIL-15 (10 μg/mL) for the times indicated. Mean fold induction of T-bet relative to time 0 plus or minus SEM is shown. The experiment was performed 3 times with similar results. (B) Peripheral blood mononuclear cells were harvested from 4- to 6-week-old wt B6 or IL-15 tg B6 mice. Expression of T-bet was measured by quantitative real-time PCR. N ≥ 6 mice per group. Data represent mean fold induction of T-bet relative to wt B6 mice plus or minus SEM. (C, D) Splenocytes were harvested from wt B6 or IL-15 tg B6 mice (n = 4 per group) and stained for cell-surface expression of CXCR3 (C) or stimulated for 6 hours using immobilized anti-CD3/28 antibodies followed by fixation and staining for intracellular IFN-γ (D). Data represent mean percent positive cells plus or minus SEM. (E) Lethally irradiated wt B6D2F1 mice underwent transplantation with 1 × 107 wt B6 TCD BM cells and 5 × 106 wt B6 unpurified splenic T cells (•, n = 9), 1 × 107 IL-15 tg B6 TCD BM cells and 5 × 106 wt B6 unpurified splenic T cells (□, n = 10), or 1 × 107 IL-15 tg B6 TCD BM cells and 5 × 106 T-bet–/– B6 unpurified splenic T cells (⋄, n = 7). Survival was monitored daily and was compared using the logrank test. Data are combined from 2 similar experiments. (F) Body weights from animals in panel E were measured, normalized to day 0, and plotted as mean plus or minus SEM. Groups were compared as described in “Statistics.”
IL-15–mediated acute GVHD requires expression of T-bet by donor splenocytes. (A) Highly purified wt B6 splenic T cells were cultured in the presence of rmIL-15 (10 μg/mL) for the times indicated. Mean fold induction of T-bet relative to time 0 plus or minus SEM is shown. The experiment was performed 3 times with similar results. (B) Peripheral blood mononuclear cells were harvested from 4- to 6-week-old wt B6 or IL-15 tg B6 mice. Expression of T-bet was measured by quantitative real-time PCR. N ≥ 6 mice per group. Data represent mean fold induction of T-bet relative to wt B6 mice plus or minus SEM. (C, D) Splenocytes were harvested from wt B6 or IL-15 tg B6 mice (n = 4 per group) and stained for cell-surface expression of CXCR3 (C) or stimulated for 6 hours using immobilized anti-CD3/28 antibodies followed by fixation and staining for intracellular IFN-γ (D). Data represent mean percent positive cells plus or minus SEM. (E) Lethally irradiated wt B6D2F1 mice underwent transplantation with 1 × 107 wt B6 TCD BM cells and 5 × 106 wt B6 unpurified splenic T cells (•, n = 9), 1 × 107 IL-15 tg B6 TCD BM cells and 5 × 106 wt B6 unpurified splenic T cells (□, n = 10), or 1 × 107 IL-15 tg B6 TCD BM cells and 5 × 106 T-bet–/– B6 unpurified splenic T cells (⋄, n = 7). Survival was monitored daily and was compared using the logrank test. Data are combined from 2 similar experiments. (F) Body weights from animals in panel E were measured, normalized to day 0, and plotted as mean plus or minus SEM. Groups were compared as described in “Statistics.”
To rigorously prove that donor bone marrow–derived cells produce IL-15 in experimental acute GVHD with significant enhancement of morbidity and mortality, we have considered the possibility that the survival difference observed between recipients of wt B6 and IL-15–/– B6 BM cells was related not to bona fide differences in IL-15 production, but rather to differences in the cellular constituency of the bone marrow portion of the allografts.12 For example, there were significantly fewer CD11c+ B220+ plasmacytoid dendritic cells identified in control treated IL-15–/– B6 donors compared with control treated wt B6 donors. This shift could have contributed to the observed survival difference, irrespective of differences in IL-15 production after transplantation. However, our data show that administration of rhIL-15 to IL-15–/– B6 donors normalizes the cell population distribution in the donor bone marrow compartment, yet preserves a survival advantage for the recipients of these cells after transplantation. Notably, NK cells were nearly absent in control treated IL-15–/– B6 donors but increased to supraphysiologic levels by the administration of rhIL-15, consistent with their strict dependence on IL-15 for growth and survival.12 Because an increase in survival relative to recipients of wt donor cells remained in the presence or absence of donor NK cells in these experiments and because NK cell alloreactivity in our model has been demonstrated to be in the host-versus-graft direction,28 we believe that skewing of the donor NK cell compartment is not essential to the survival differences observed. Nevertheless, we cannot rule out the possibility that pretreatment with exogenous IL-15 specifically induced a tolerogenic NK cell subset (not present or not sufficiently active in wt B6 donors to confer a survival benefit compared with IL-15–/– B6 donors) that precisely neutralized any pathogenic effect of donor IL-15 pretreatment. We believe these data provide strong evidence that endogenous IL-15 production by donor-derived cells underlies the pathobiology of acute GVHD.
Studies in immunodeficient mouse models have linked production of IL-15 and its presentation in trans via IL-15 Rα to responding lymphocytes as events occurring coordinately by the same cell.18,19 Here, we have asked whether the dysregulation of proinflammatory cytokines and/or the rapid cell turnover (with potential release of IL-15 from dying cells) characteristic of acute GVHD are sufficient to circumvent this currently accepted mechanism of IL-15 signaling. Because recipients of mixed IL-15–/– and IL-15 Rα–/– BM cells demonstrate similar survival compared with recipients of either type of genetically deficient cells alone, it is unlikely that donor IL-15 Rα–/– cells can produce IL-15 for presentation by donor IL-15–/– cells. Thus the coordinate presentation model is not altered by the cytokine dysregulation in acute GVHD. Further, because recipients of IL-15–/– TCD BM cells (which could in theory scavenge IL-15 released by dying host or donor cells for presentation via IL-15 Rα) and IL-15Rα–/– TCD BM cells (which neither scavenge nor present native IL-15) demonstrate similar survival curves, there is no evidence that IL-15 released as a result of cellular turnover contributes to acute GVHD.
These observations have significant consequences given the cellular mechanisms of alloreactive T-cell activation. It is clear that the most important encounter by donor T cells with alloantigen occurs in the first 5 days after transplantation and is mediated by host-derived antigen presenting cells (APCs).29-31 Our data suggest that donor-derived IL-15 does not contribute to this T-cell–APC interaction because it remains bound to the surface of donor-derived cells. More likely, a donor-derived IL-15–producing and –presenting cell encounters alloreactive donor T cells some time after the initial contact with alloantigen. This conclusion is contrary to the proposed role of IL-15 trans-presentation as a third costimulatory signal between APCs and T cells in addition to allogeneic MHC-TCR and CD28-CD86.32 Alternatively, it is possible that the donor-derived IL-15–producing and –presenting cell has the ability also to present host alloantigens and contribute to acute GVHD via the indirect pathway, as has been described.33,34
GVT activity is preserved in the absence of donor-derived IL-15. (A) Lethally irradiated wt B6D2F1 mice received 2000 log-phase P815 cells, 5 × 106 wt B6 splenic T cells, and 1 × 107 TCD BM cells from either wt B6 (wt allo, ▪, n = 12) or IL-15–/– B6 (IL-15–/– allo, □, n = 12) mice. As controls, identical mice received 5 × 106 wt B6D2F1 (syngeneic) T cells and 1 × 107 wt B6D2F1 TCD BM cells plus (syn (+), •, n = 5) or minus (syn (-), ○, n = 5) 2000 P815 cells. Animals were monitored daily after transplantation and survival times were compared using the logrank test. Results are combined from 3 similar experiments. (B) Livers were harvested from representative moribund recipients. Paraffin sections were stained with toluidine blue. Slides were reviewed by a board-certified veterinary pathologist in a blinded fashion. Tumor rests are indicated by the white vertical arrows. Magnification = ×40.
GVT activity is preserved in the absence of donor-derived IL-15. (A) Lethally irradiated wt B6D2F1 mice received 2000 log-phase P815 cells, 5 × 106 wt B6 splenic T cells, and 1 × 107 TCD BM cells from either wt B6 (wt allo, ▪, n = 12) or IL-15–/– B6 (IL-15–/– allo, □, n = 12) mice. As controls, identical mice received 5 × 106 wt B6D2F1 (syngeneic) T cells and 1 × 107 wt B6D2F1 TCD BM cells plus (syn (+), •, n = 5) or minus (syn (-), ○, n = 5) 2000 P815 cells. Animals were monitored daily after transplantation and survival times were compared using the logrank test. Results are combined from 3 similar experiments. (B) Livers were harvested from representative moribund recipients. Paraffin sections were stained with toluidine blue. Slides were reviewed by a board-certified veterinary pathologist in a blinded fashion. Tumor rests are indicated by the white vertical arrows. Magnification = ×40.
Nevertheless, our data have shown that donor BM-derived IL-15 is necessary for maximal type 1 T-cell polarization. The role of IFN-γ, the prototypical type 1 cytokine, in acute GVHD remains controversial. IFN-γ is known to increase major histocompatibility complex class I (MHC-I), MHC-II, and costimulatory ligand expression by antigen presenting cells35-37 and to compromise the integrity of the intestinal epithelial lining, all of which are necessary for optimal alloreactive T-cell activation in acute GVHD.2,29,31 In early studies, IFN-γ was found to be elevated in humans with acute GVHD whereas murine IFN-γ–/– donor T cells were shown to confer a prolonged disease course, consistent with this picture.38-40 Yet studies using lethal conditioning regimens have demonstrated that a critically timed burst of IFN-γ production by donor-derived T cells in the first 2 days after transplantation can lead to apoptosis of donor T cells and protection from acute GVHD.41,42 Enhanced Fas/FasL-mediated clearance of alloreactive donor T cells likely explains these findings.43,44 Thus, donor-derived IFN-γ may play a bifunctional role in the pathogenesis of acute GVHD, promoting APC function and gut pathology while enhancing Fas-mediated apoptosis of alloreactive donor T cells.
Yet while IFN-γ production is the hallmark function of proinflammatory type 1 polarized T cells, it is by no means their only distinguishing characteristic. Expression of receptors for adhesion molecules and chemokines that promote T-cell migration to inflammatory sites such as PSGL-1 and CXCR3 are preferentially increased in type 1 T cells compared with type 2 T cells.23,45 T-bet has been identified as the master control gene for this plurality of functions: CD4+ T cells that overexpress T-bet produce more IFN-γ and express more cell-surface CXCR3 under both type 1 and type 2 polarizing conditions compared with CD4+ T cells from wt mice.26,27 Further, CD4+ T cells from T-bet–/– mice have severe defects in IFN-γ production and CXCR3 expression when cultured in type 1 polarizing conditions.26,27 Our observations that IL-15 tg mice demonstrate increased expression of IFN-γ, CXCR3, and T-bet and that purified T cells rapidly up-regulate T-bet in vitro in response to IL-15 identify T-bet as a downstream target of IL-15 in T cells. Although we cannot formally rule out an effect of IL-15 through T-bet on other donor-derived cells, the observations of decreased type 1 T-cell functions in allogeneic recipients of IL-15–/– B6 BM cells and a dramatic reduction in mortality in recipients of IL-15 tg B6 TCD BM and T-bet–/– splenocytes strongly suggest that enhancement of type 1 polarization is a primary, nonredundant role for donor-derived IL-15 in acute GVHD.
To understand the consequences of decreasing endogenous IL-15 production in terms of the GVT effect, we have used a model of tumor occurrence after challenge at the time of BMT that has been repeatedly validated in the literature.46-48 Here, we have shown for the first time that in the absence of donor-derived IL-15, GVT activity is maintained while GVHD mortality is substantially delayed. Because the vast majority of allogeneic hematopoietic stem cell transplantation in clinical practice is T-cell replete, these findings are an advancement on a previous study using T-cell–depleted transplants to show exogenously administered IL-15 enhanced GVT activity.11
While the absence of donor-derived IL-15 causes a substantial delay in acute GVHD mortality without loss of GVT activity, our experiments show that acute GVHD is not entirely eliminated. Thus, host-derived IL-15 and other cytokines including IL-2 from both donor- and host-derived tissues may also contribute to acute GVHD mortality observed in the absence of donor-derived IL-15. Although donor-derived IL-15 is but one of many proinflammatory cytokines that are likely involved in the pathogenesis of acute GVHD, these data do highlight the potential clinical benefit of neutralizing IL-15 through pharmacologic or immunotherapeutic means. Moreover, because our data strongly support the notion that IL-15 is expressed in a coordinate fashion with IL-15 Rα in acute GVHD, synergistic antagonism of IL-15 signaling may be possible by targeting both IL-15 and IL-15 Rα.
The timing, location, and specific cellular source of donor-derived IL-15 expression in acute GVHD still await discovery as does the impact of eliminating donor-derived IL-15 on chronic GVHD and long-term hematopoietic repopulation. Yet it is now clear that the absence of donor-derived IL-15 provides concrete benefits in experimental acute GVHD: systemic inflammation is reduced with a concomitant decrease in morbidity and mortality, while T-cell reconstitution and GVT effects are maintained. Further, our analysis of the signaling mechanism of IL-15 in acute GVHD has identified IL-15 Rα and T-bet as potential new therapeutic targets in the disease. Together, these findings compel further research into the role of IL-15 in transplantation biology at both the basic and clinical levels.
Prepublished online as Blood First Edition Paper, June 6, 2006; DOI 10.1182/blood-2006-04-019059.
Supported by National Institutes of Health grants R01 AI34 495 (B.R.B.), CA72 669 (B.R.B.), HL073 794 (B.R.B.), and CA068 458 (M.A.C.).
B.W.B. designed and performed experiments and wrote the manuscript; N.R.S., S.K., D.C., and S.S. acquired data and critically reviewed the manuscript; S.R. and B.B. substantially contributed to experimental design and critically reviewed the manuscript; A.F.K. and D.F.K. analyzed data and critically reviewed the manuscript; B.R.B. substantially contributed to experimental design and critically reviewed the manuscript; M.A.C. supported the work, substantially contributed to experimental design, and critically reviewed the manuscript.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Tamra Brooks and Donna Bucci for administrative support and preparation/submission of the manuscript. We also thank Amgen for provision of IL-15 and Averil Ma for IL-15 Rα–/– mice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal