Abstract
There is compelling evidence that circulating angiogenic cells exist that are able to home to sites of vascular injury and stimulate angiogenesis. However, the number of angiogenic cells in the blood is low, limiting their delivery to sites of ischemia. Treatment with certain cytokines may mobilize angiogenic cells into the blood, potentially circumventing this limitation. Herein, we show that treatment with granulocyte colony-stimulating factor (G-CSF) or AMD3100, a novel CXCR4 antagonist, significantly stimulated angiogenesis in a murine model of acute hindlimb ischemia. The kinetics of angiogenic-cell mobilization by these agents appears to be distinct, with more rapid revascularization observed in AMD3100-treated mice. Combination treatment with G-CSF and AMD3100 resulted in the earliest and most complete recovery in blood flow to the ischemic hindlimb. Adoptive transfer of mobilized blood mononuclear cells, while potently stimulating angiogenesis, did not result in the significant incorporation of donor cells into the neoendothelium. Cell-fractionation studies showed that it is the monocyte population in the blood that mediates angiogenesis in this model. Collectively, these data suggest that monocytes mobilized into the blood by G-CSF or AMD3100 stimulate angiogenesis at sites of ischemia through a paracrine mechanism.
Introduction
The endogenous response to tissue ischemia in the adult includes the up-regulation of angiogenic growth factors, migration of inflammatory cells, remodeling of the ischemic tissue environment, and the sprouting of new blood-vessel networks.1,2 This process, termed postnatal neovascularization, or neoangiogenesis, was originally thought to result exclusively from the proliferation and migration of mature endothelial cells from pre-existing blood vessels. This dogma was challenged in a pioneering study in which Asahara et al3 showed that CD34+ cells isolated from adult peripheral blood could differentiate in vitro into endothelial cells and contribute to neoangiogenesis in vivo. Further studies showed that these cells, termed endothelial progenitor cells (EPCs), were of bone marrow origin and were able to contribute to both physiologic and pathologic neovascularization.4-8 Recent studies, however, suggest that several angiogenic-cell populations, including monocytes and T lymphocytes, may exist in the blood in addition to EPCs.9-12 The relative importance of the different cell populations to angiogenesis in vivo is unclear.
The mechanism by which EPCs contribute to neoangiogenesis is controversial. The prevailing view is that these cells are recruited to sites of vascular injury where they directly incorporate into the neovasculature. Indeed, bone marrow transplantation studies have established that bone marrow–derived cells can incorporate into the neovasculature following vascular injury.5,6,13-15 Moreover, donor-cell incorporation into neovasculature has been observed after adoptive transfer of enriched populations of EPCs into animals following acute vascular injury.10,16 In contrast, recent studies have concluded that bone marrow–derived cells do not incorporate into the neovasculature.17,18 Based on these latter studies, it has been suggested that EPCs may contribute to neoangiogenesis through paracrine mechanisms instead of differentiating into functional endothelium.
At baseline, the number of EPCs in the blood is small, potentially limiting revascularization following acute vascular injury. Previous reports suggest that certain cytokines can mobilize angiogenic cells from the bone marrow to the blood, thereby circumventing this limitation.19-22 Cytokines known to mobilize EPCs include granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF), and placental growth factor (PlGF).19,23-25 Although these mobilizing agents offer a potential novel therapy for various vascular diseases, there has been no systematic study of the kinetics and magnitude of cytokine-induced EPC mobilization. Moreover, the mechanisms by which cytokine treatment stimulates angiogenesis are poorly understood.
To begin to address these issues, we characterized EPC mobilization by G-CSF, the prototypical mobilization cytokine. Although the kinetics of EPC mobilization by G-CSF are unknown, mobilization of hematopoietic progenitor cells (HPCs) by G-CSF is delayed, with peak levels of circulating HPCs achieved only after 4 to 5 days of treatment. We reasoned that a more rapid delivery of EPCs to sites of ischemia might be necessary to achieve maximal revascularization following acute vascular injury. Thus, we also studied the novel mobilizing agent AMD3100, a potent and highly selective antagonist of the CXCR4 receptor. Studies in humans and mice show that treatment with AMD3100 results in a rapid mobilization of HPCs, with peak levels achieved at 3 to 6 hours after a single injection and normalization of peripheral HPC levels after 24 hours. Furthermore, AMD3100 has been shown to act in synergy with G-CSF to increase the levels of HPC mobilization beyond those seen with G-CSF or AMD3100 alone.26 Herein, we show that treatment with G-CSF or AMD3100 significantly stimulates angiogenesis following surgical induction of hindlimb ischemia. Evidence is provided suggesting that the primary mechanism by which these agents act is through the mobilization of monocytes that in turn stimulate angiogenesis through a paracrine mechanism.
Materials and methods
Mice
Tie2–green fluorescence protein (GFP) mice on a FVB/NJ background (Stock Tg(Tie2GFP)287Sato/J) and control FVB/NJ mice were obtained from Jackson Laboratory (Bar Harbor, ME). G-CSFR–deficient mice (inbred 10 generations onto a C57BL/6 background) were generated in our laboratory as described previously.27 We used 8- to 10-week-old mice in all studies. Mice were housed in a specific pathogen-free environment. All experiments were approved by the Washington University Animal Studies Committee.
Mobilizing agents
Recombinant human G-CSF, a generous gift from Amgen (Thousand Oaks, CA), was given at a dose of 250 μg/kg per day for a period of 7 days by a miniosmotic pump (Alzet, Cupertino, CA) placed subcutaneously on the back of the mice. AMD3100 (Sigma, St Louis, MO) was given as a single 5-mg/kg subcutaneous injection once a day for 3 consecutive days.
For the adoptive transfer experiments, donor mice were given 250 μg/kg G-CSF per day by miniosmotic pump as previously described. After 5 days of G-CSF treatment, a single 5-mg/kg subcutaneous dose of AMD3100 was given. Three hours later, at the time of peak synergistic mobilization,26 peripheral blood was removed and the mononuclear-cell fraction obtained by centrifugation across a 1.011 density gradient (Histopaque; Sigma-Aldrich) at 1700g for 30 minutes.
Murine hindlimb ischemia model
The hindlimb ischemia surgical procedure was performed as previously described.28 In brief, an incision was made in the skin at the midportion of the right hindlimb overlying the femoral artery. The femoral artery and vein were then dissected free from the nerve and the proximal portion of the femoral artery and vein ligated with 6-0 silk sutures. The distal portion of the saphenous artery and vein and remaining arterial and venous side branches were ligated followed by their complete excision from the hindlimb. The overlying skin was then closed using Nexaband veterinary glue (Abbott Animal Health, Abbott Park, IL).
Laser Doppler perfusion imaging
Blood perfusion in the hindlimb was monitored by laser Doppler imaging (MoorLDI-2; Moor Instruments, Devon, United Kingdom). Before initiating scanning, mice were anesthetized and placed on a heating plate at 37°C to minimize temperature variations. For each time point, the laser Doppler image obtained was analyzed by averaging the perfusion, expressed as the relative unit of flux as determined by Moor Instruments, over the surface of both the ischemic and nonischemic foot. In order to control for ambient light and temperature, calculated perfusion was expressed as the flux ratio between the ischemic and nonischemic limbs.
Capillary density
Histologic sections, 5-μm thick, prepared from paraffin-embedded tissue samples of the ischemic adductor muscle dissected from mice at 14 days after surgery were stained with hematoxylin and eosin and observed under light microscopy. Twenty high-power fields (100 ×) per slide were obtained and counted for capillaries in a blinded fashion. Three slides were analyzed per animal for each treatment group and capillary density was expressed as number of capillaries/mm2.
Flow cytometry
The adductor muscle from both ischemic and nonischemic hindlimbs was surgically isolated 14 days after surgery. One half of the muscle mass was weighed, treated with 3 mg/mL type I collagenase (Worthington Biomedical, Lakewood, NJ) for 40 minutes at 37°C, and then filtered through a 50-μm cell strainer (Partec, Münster, Germany). Cells were incubated with Fc block (Miltenyi Biotec, Auburn, CA) for 10 minutes at 4°C followed by incubation with either phycoerythrin (PE)–conjugated CD146 or CD45, and 7-amino-actinomycin D (7-AAD) (Pharmingen, San Diego, CA). Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany).
Immunofluorescence
Muscle sections were fixed in a lysine-based fixative (0.02 M NaH2PO4, 0.08 M Na2HPO4, 0.1 M lysine, 0.01 M sodium metaperiodate in PBS mixed 3:1 with 4% paraformaldehyde) for 15 minutes at room temperature, washed 3 times with 5% sucrose in PBS, and then flash frozen in optimum cutting temperature (OCT) compound (Sakura, Torrance, CA). Frozen tissue sections were incubated at room temperature for 1 hour with primary antibodies to von Willebrand factor (VWF; DAKO Cytomation, Copenhagen, Denmark) and GFP (Chemicon, Temecula, CA), and were then incubated for 1 hour with either Alexa Fluor 488– or PE-conjugated secondary antibodies (Molecular Probes, Eugene, OR). Images were visualized using a Nikon Microphot-SA microscope (Nikon, Melville, NY) equipped with a Nikon Plan Apo 20×/0.45 numeric aperture objective. Images were obtained and analyzed using a ColorView camera and analySIS software version 3.1 (Soft Imaging Systems, Münster, Germany).
ELISA
Serum was isolated from peripheral blood and analyzed using a commercial enzyme-linked immunosorbent assay (ELISA) kit for murine G-CSF according to the manufacturer's instructions (R & D Systems, Minneapolis, MN).
CD11b+ cell isolation and cell sorting
Mobilized mononuclear cells were incubated at 4°C with PE-CD11b antibody (Pharmingen) followed by anti-PE microbeads (Miltenyi Biotec). CD11b+ cells were then isolated using the autoMACS cell sorter (Miltenyi Biotec). To isolate neutrophils (Gr-1+), monocytes (F4/80+), and natural killer (NK) cells (NK1.1+), CDllb+ blood mononuclear cells were incubated with FITC-F4/80 (Caltag Labs, Burlingame, CA), PE-Cy5.5-Gr-1, and APC-NK1.1 antibodies (Pharmingen). These cells were then sorted using a MoFlo high-speed flow cytometer (DAKO Cytomation, Fort Collins, CO).
Statistical analysis
Statistical significance was determined either by a 2-way ANOVA or by a 2-sided Student t test.
Results
G-CSF and AMD3100 treatment accelerates revascularization in a mouse model of acute hindlimb ischemia
To investigate the effect of cytokine treatment in the restoration of blood flow and subsequent repair of ischemic tissue in vivo, 4 groups of C57BL/6 mice (n = 7-10, each) were treated with saline alone, G-CSF, AMD3100, or G-CSF in combination with AMD3100 immediately after induction of unilateral hindlimb ischemia. Revascularization was monitored by laser Doppler imaging and histomorphometric analysis of capillary density. Following surgical induction of unilateral hindlimb ischemia, blood flow in the ischemic versus nonischemic limb was reduced to approximately 0.14 ± 0.01 in all treatment groups, indicating that a comparable level of ischemia was induced in all mice (Figure 1).
Serial laser Doppler perfusion analysis showed progressive recovery of limb perfusion over time in all groups (Figure 1). Mice that were treated with G-CSF alone showed a significant improvement in limb perfusion on days 10 and 14 after surgery (ratio of ischemic vs nonischemic limb perfusion ± SEM: 0.75 ± 0.04, P < .05, and 0.89 ± 0.05, P < .05, respectively) compared with saline-treated mice (0.58 ± 0.06 and 0.72 ± 0.05, respectively) (Figure 1A). On the other hand, mice that were treated with AMD3100 alone showed an early response with significant improvement in limb perfusion on days 5 and 7 after surgery (0.53 ± 0.04, P < .01, and 0.72 ± 0.06, P < .001, respectively) compared with saline-treated controls (0.32 ± 0.02 and 0.46 ± 0.04, respectively) (Figure 1B). These data show that while treatment with either G-CSF or AMD3100 improves blood perfusion following acute vascular injury, the kinetics of angiogenic recovery in these mice was distinct, suggesting that angiogenic-cell mobilization by AMD3100 is more rapid than G-CSF.
Cytokine treatment and neovascularization. C57BL/6 mice were treated with saline alone (PBS), G-CSF, AMD3100, or G-CSF in combination with AMD3100 (G + A), and perfusion of the ischemic hindlimb relative to the nonischemic hindlimb was measured. Representative laser Doppler images taken 14 days after surgery are shown. Low perfusion is displayed as blue, while the highest level of perfusion is displayed as red. (A) G-CSF versus PBS. (B) AMD-3100 versus PBS. (C) G-CSF + AMD-3100 versus PBS. Data represent the mean ± SEM.
Cytokine treatment and neovascularization. C57BL/6 mice were treated with saline alone (PBS), G-CSF, AMD3100, or G-CSF in combination with AMD3100 (G + A), and perfusion of the ischemic hindlimb relative to the nonischemic hindlimb was measured. Representative laser Doppler images taken 14 days after surgery are shown. Low perfusion is displayed as blue, while the highest level of perfusion is displayed as red. (A) G-CSF versus PBS. (B) AMD-3100 versus PBS. (C) G-CSF + AMD-3100 versus PBS. Data represent the mean ± SEM.
Capillary density. Quantitative analysis of capillary density (per mm2) of adductor muscles from ischemic hindlimbs at day 14 after surgery (n = 3). Data represent the mean ± SEM. (*P < .02; **P < .03.)
Capillary density. Quantitative analysis of capillary density (per mm2) of adductor muscles from ischemic hindlimbs at day 14 after surgery (n = 3). Data represent the mean ± SEM. (*P < .02; **P < .03.)
The apparent difference in kinetics of angiogenic-cell mobilization between G-CSF and AMD3100 and the known synergistic mobilization of HPCs by the combination of G-CSF and AMD3100 prompted us to test this combination in the hindlimb ischemia model. Mice were given G-CSF (250 μg/kg per day) by continuous subcutaneous infusion and AMD3100 (5 mg/kg per day) for the first 3 days starting immediately following surgery; this regimen is predicted to result in rapid and sustained mobilization. Indeed, a significant improvement in perfusion was observed as early as day 4 after the induction of hindlimb ischemia and persisted through day 14 compared with PBS-treated controls (Figure 1C). Moreover, this regimen resulted in significantly better hindlimb perfusion compared with mice treated with G-CSF alone (P = .014) or AMD3100 alone (P = .01). These data show that combination treatment with G-CSF and AMD3100 is a potent regimen to stimulate angiogenesis in mice.
To determine whether the improved recovery in hindlimb perfusion, as assessed by laser Doppler perfusion imaging, reflected increased angiogenesis at the site of ischemia, capillary density was measured in adductor muscles harvested from the ischemic hindlimb of mice at day 14 after surgery. As shown in Figure 2, capillary density was significantly increased in mice that were treated with G-CSF or G-CSF in combination with AMD3100 versus saline-treated controls. While capillary density measurements for AMD3100-treated mice showed a trend toward improvement, these values were not significant, perhaps reflecting the earlier mobilization obtained with AMD3100. These data suggest that the improved reperfusion observed with G-CSF and AMD3100 treatment is secondary to enhanced angiogenesis.
G-CSF signaling is not required for endogenous revascularization after hindlimb ischemia
The significant improvement in blood flow in saline-treated mice following induction of hindlimb ischemia suggested that endogenous repair mechanisms must exist. Since systemic levels of G-CSF are often elevated during inflammatory conditions,29 we asked whether endogenous production of G-CSF might contribute to this response. Indeed, systemic levels of G-CSF increased significantly following induction of hindlimb ischemia, with peak levels observed at 24 hours after surgery of 650 ± 83 pg/mL, a concentration that is above the dissociation constant (Kd, 100 pM) of the G-CSF receptor (G-CSFR)30 (Figure 3A). To assess the importance of G-CSF signaling to angiogenesis in vivo, we characterized the revascularization response of G-CSFR–/– mice following induction of hindlimb ischemia. Of importance, previous studies have demonstrated that the G-CSFR is the sole receptor for G-CSF; thus, G-CSF signaling is completely abrogated in these mice.31 As shown in Figure 3B, the endogenous recovery following the induction of acute hindlimb ischemia was similar in wild-type and G-CSFR–/– mice. In both groups of mice, an approximately 4-fold increase in blood flow was observed 2 weeks after surgery compared with immediately after surgery. These data show that G-CSF signals are not required for endogenous revascularization in this model of acute ischemia.
Role of G-CSF signaling in endogenous revascularization. (A) Serum G-CSF levels in C57BL/6 mice following induction of hindlimb ischemia. (B) Strain-matched wild-type or G-CSFR–/– (GRKO) mice underwent the hindlimb ischemia procedure and were monitored by laser Doppler perfusion imaging (P = NS). Data represent the mean ± SEM.
Role of G-CSF signaling in endogenous revascularization. (A) Serum G-CSF levels in C57BL/6 mice following induction of hindlimb ischemia. (B) Strain-matched wild-type or G-CSFR–/– (GRKO) mice underwent the hindlimb ischemia procedure and were monitored by laser Doppler perfusion imaging (P = NS). Data represent the mean ± SEM.
Adoptive transfer. (A) Blood mononuclear cells (1 × 107) were harvested from donor mice treated with G-CSF and AMD3100 and infused intravenously into recipient mice 24, 48, and 72 hours after induction of hindlimb ischemia. Blood flow was measured by laser Doppler perfusion imaging. (B) Capillary density of adductor muscle from ischemic hindlimbs at day 14 after surgery (*P = .001). (C) Mobilized or nonmobilized blood mononuclear cells (5 × 106) were infused intravenously 24 hours after induction of hindlimb ischemia. The difference between mice receiving mobilized or nonmobilized cells was not significant (P = .71). Data represent the mean ± SEM.
Adoptive transfer. (A) Blood mononuclear cells (1 × 107) were harvested from donor mice treated with G-CSF and AMD3100 and infused intravenously into recipient mice 24, 48, and 72 hours after induction of hindlimb ischemia. Blood flow was measured by laser Doppler perfusion imaging. (B) Capillary density of adductor muscle from ischemic hindlimbs at day 14 after surgery (*P = .001). (C) Mobilized or nonmobilized blood mononuclear cells (5 × 106) were infused intravenously 24 hours after induction of hindlimb ischemia. The difference between mice receiving mobilized or nonmobilized cells was not significant (P = .71). Data represent the mean ± SEM.
Adoptive transfer of mobilized peripheral-blood mononuclear cells increases revascularization after induction of hindlimb ischemia
The mechanism by which cytokines stimulate angiogenesis is largely unknown. To address this issue, we first determined whether the angiogenic effect of cytokine treatment could be reproduced by adoptive transfer of mobilized blood mononuclear cells. To mimic the increase in blood leukocytes seen in cytokine-treated mice, 1 × 107 G-CSF + AMD3100–mobilized mononuclear cells were injected into syngeneic recipient mice 24, 48, and 72 hours after surgical induction of hindlimb ischemia (Figure 4A). Revascularization as measured by Doppler blood flow was significantly improved in mice receiving mononuclear cells (P < .001). Moreover, capillary density in the ischemic tissue was significantly increased (Figure 4B). These data show that the angiogenic effect of G-CSF and AMD3100 can be adoptively transferred by infusing mobilized blood mononuclear cells.
We next asked whether G-CSF and AMD3100 mobilized a unique angiogenic-cell population or simply increased the number of angiogenic cells normally in the circulation. To answer this question, the same number of nonmobilized and mobilized blood mononuclear cells were adoptively transferred into mice following surgical induction of hindlimb ischemia. Of interest, a similar enhancement in perfusion was seen with mobilized and nonmobilized cells, suggesting that on a per-cell basis, the angiogenic effect of basal and mobilized blood mononuclear cells was similar (Figure 4C).
Adoptive transfer of monocytes increases revascularization after induction of hindlimb ischemia
The blood mononuclear-cell population is highly heterogeneous, containing both progenitor and mature cells. To begin to define the cell types mobilized by G-CSF and AMD3100 that are responsible for the increased angiogenic effect, we fractionated cells on the basis of CD11b (αmβ2-integrin) expression. CD11b is expressed at highest levels on mature leukocytes, including granulocytes, monocytes, natural killer (NK) cells, and a subset of T lymphocytes, whereas expression on CD34+ progenitor cells is low or absent.32,33 Based upon a CD11b+ cell frequency of approximately 36% in mobilized blood mononuclear cells and a minimum effective dose of 5 × 106 adoptively transferred mononuclear cells to induce a significant improvement in angiogenesis (data not shown), 1.8 × 106 CD11b+ or 3.2 × 106 CD11b– cells were injected separately into syngeneic recipient mice 24 hours after surgical induction of hindlimb ischemia. Revascularization as measured by Doppler blood flow was significantly improved in mice receiving CD11b+ cells (P = .04), and in fact, was similar to mice receiving unfractionated blood mononuclear cells (Figure 5A). In contrast, mice receiving CD11b– cells showed no significant improvement over PBS-injected controls. These data suggest that mobilization of CD11b+ cells by G-CSF and AMD3100 is responsible for the angiogenic response mediated by these agents.
Adoptive transfer of defined blood mononuclear-cell populations. (A) CD11b+ (1.8 × 106) or CD11b– (3.2 × 106) cells were infused intravenously into recipient mice 24 hours after induction of hindlimb ischemia. (B) Monocytes (F4/80+, 2.5 × 105), neutrophils (Gr-1+, 1 × 106), or NK cells (NK1.1+, 5.4 × 104) were infused intravenously into recipient mice 24 hours after induction of hindlimb ischemia. Blood flow was measured by laser Doppler perfusion imaging. Data represent the mean ± SEM.
Adoptive transfer of defined blood mononuclear-cell populations. (A) CD11b+ (1.8 × 106) or CD11b– (3.2 × 106) cells were infused intravenously into recipient mice 24 hours after induction of hindlimb ischemia. (B) Monocytes (F4/80+, 2.5 × 105), neutrophils (Gr-1+, 1 × 106), or NK cells (NK1.1+, 5.4 × 104) were infused intravenously into recipient mice 24 hours after induction of hindlimb ischemia. Blood flow was measured by laser Doppler perfusion imaging. Data represent the mean ± SEM.
The CD11b+ mononuclear-cell fraction is comprised mostly of neutrophils, with a smaller percentage of monocytes, and NK cells. To further define the cell population(s) responsible for the angiogenic effect, the CD11b+ cell fraction was sorted into monocytes, neutrophils, and NK-cell populations using a high-speed cell sorter and injected separately into syngeneic recipient mice 24 hours after the induction of hindlimb ischemia. The dose of each cell type was determined based upon their percentage within the CD11b+ cell fraction and upon a minimum effective cell dose of 1.8 × 106 CD11b+ cells. Mice treated with monocytes showed a significant improvement in revascularization compared with untreated controls, while mice treated with NK cells, or neutrophils, did not (Figure 5B). These data suggest that monocytes mediate the angiogenic response of G-CSF plus AMD3100 treatment.
Adoptively transferred blood mononuclear cells are not incorporated into the neoendothelium in significant numbers
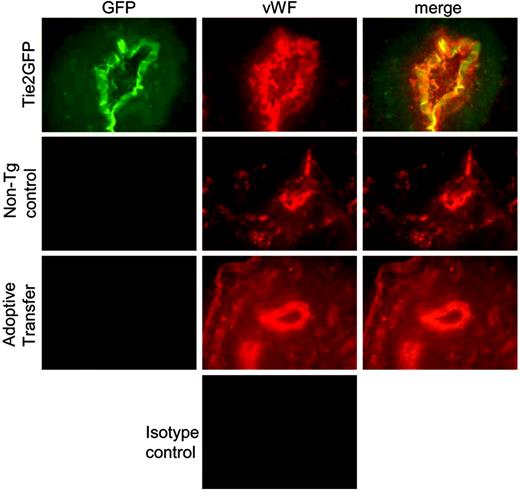
To determine whether mobilized blood mononuclear cells directly stimulate angiogenesis by incorporating into the neoendothelium, blood mononuclear cells from Tie2GFP transgenic mice were adoptively transferred into nontransgenic FVB mice following the induction of hindlimb ischemia. Of importance, GFP expression in Tie2GFP transgenic mice is mainly limited to endothelial cells.34 Thus, if adoptively transferred blood mononuclear cells directly incorporate into the neovasculature, GFP+ endothelial cells should be apparent in recipient (nontransgenic) mice. Endothelial-cell chimerism in the adductor muscle from the ischemic limb was analyzed 2 weeks after surgery by 2 methods. First, immunofluorescence was performed to look for coexpression of GFP and the endothelial marker von Willebrand factor (VWF). As controls, we first studied Tie2 transgenic and strain-matched nontransgenic mice on day 14 after surgery. As expected, in Tie2GFP transgenic mice, the VWF+ endothelial cells coexpressed GFP; whereas in nontransgenic mice, no GFP expression in VWF+ endothelial cells was observed (Figure 6). We next examined FVB mice that had received Tie2GFP blood mononuclear cells. Of interest, no GFP expression in VWF+ endothelial cells was observed, suggesting that the majority of neoendothelium was of recipient origin. To obtain a more quantitative assessment of endothelial-cell chimerism, a flow cytometry–based assay was developed. A portion of the ischemic adductor muscle was digested with collagenase to generate a single-cell suspension and the percent GFP+ endothelial cells quantified by flow cytometry (Figure 7). To validate this assay, we again studied Tie2GFP transgenic or nontransgenic mice. As expected, ischemic muscle taken from a Tie2GFP transgenic mouse 14 days after the hindlimb ischemia procedure shows a distinct GFP+ population of cells that are not present in the strain-matched nontransgenic controls (Figure 7A). This GFP+ population was negative for the pan leukocyte marker CD45 and positive for the endothelial marker CD146, confirming that the GFP+ cells were of endothelial origin (Figure 7B). Having validated this assay, we next examined the mice that had received Tie2GFP blood mononuclear cells. Consistent with the histologic studies, essentially no GFP+ cells were detected (Figure 7C). Collectively, these data show that the adoptively transferred blood mononuclear cells, while stimulating angiogenesis, do not appreciably incorporate into the endothelium after hindlimb ischemia.
Histologic assessment of endothelial-cell chimerism. Representative histologic sections from the ischemic muscle at 14 days after the induction of hindlimb ischemia were incubated with VWF or isotype control antibodies. Shown is GFP (green) and VWF (red) expression in Tie2GFP mice (n = 3), nontransgenic FVB mice (nontransgenic control) (n = 3), or FVB mice following adoptive transfer of Tie2GFP blood mononuclear cells (adoptive transfer) (n = 3). Original magnification (× 400).
Histologic assessment of endothelial-cell chimerism. Representative histologic sections from the ischemic muscle at 14 days after the induction of hindlimb ischemia were incubated with VWF or isotype control antibodies. Shown is GFP (green) and VWF (red) expression in Tie2GFP mice (n = 3), nontransgenic FVB mice (nontransgenic control) (n = 3), or FVB mice following adoptive transfer of Tie2GFP blood mononuclear cells (adoptive transfer) (n = 3). Original magnification (× 400).
Flow cytometric assessment of endothelial-cell chimerism. The adductor muscle from the ischemic limb was isolated 14 days after induction of hindlimb ischemia, digested with collagenase to generate a single-cell suspension, and then analyzed by flow cytometry. (A) Representative FACS plots of nontransgenic FVB (control) (n = 3) and Tie2GFP (n = 3) transgenic cells showing GFP expression; staining for 7-AAD was used to identify nonviable cells. (B) Expression of CD45 and CD146 (MUC18 antigen) by GFP+ cells from Tie2GFP transgenic mice is shown. Isotype controls are shown as dashed lines. (C) Representative histograms from 3 recipient mice in which Tie2GFP blood mononuclear cells had been adoptively transferred. Shown is the percentage of GFP-positive cells.
Flow cytometric assessment of endothelial-cell chimerism. The adductor muscle from the ischemic limb was isolated 14 days after induction of hindlimb ischemia, digested with collagenase to generate a single-cell suspension, and then analyzed by flow cytometry. (A) Representative FACS plots of nontransgenic FVB (control) (n = 3) and Tie2GFP (n = 3) transgenic cells showing GFP expression; staining for 7-AAD was used to identify nonviable cells. (B) Expression of CD45 and CD146 (MUC18 antigen) by GFP+ cells from Tie2GFP transgenic mice is shown. Isotype controls are shown as dashed lines. (C) Representative histograms from 3 recipient mice in which Tie2GFP blood mononuclear cells had been adoptively transferred. Shown is the percentage of GFP-positive cells.
Discussion
There is convincing evidence that a small number of angiogenic cells are present in the blood of most mammals, including humans. The number of circulating angiogenic cells is increased in response to stress, such as myocardial infarction, or after treatment with certain cytokines.19,23-25,35 Shi et al19 showed that treatment with G-CSF, the prototypical mobilizing cytokine, significantly enhanced endothelialization of surgically implanted vascular grafts in dogs. Likewise, re-endothelialization was enhanced following balloon angioplasty of the carotid artery in rats treated with G-CSF.21 Moreover, 2 studies have shown that recovery of cardiac function following myocardial infarction was significantly improved by treatment with regimens that included G-CSF.22,36 Finally, Hu et al37 showed that G-CSF mobilized EPCs in nonhuman primates. In the present study, we show that treatment with G-CSF significantly stimulated neoangiogenesis after induction of acute hindlimb ischemia. However, the angiogenic effect was delayed, with a peak response seen 10 to 14 days after surgery. In contrast, treatment with AMD3100 resulted in a more rapid angiogenic response, with peak effects seen on days 5 to 7. These data suggest that the kinetics of angiogenic-cell mobilization by these agents may mirror that observed for HPC mobilization; whereas peak mobilization of HPCs by G-CSF occurs after 4 to 5 days of treatment, peak HPC mobilization by AMD3100 occurs after 1 to 3 hours in mice.26 We reasoned that by combining an early-acting mobilizing agent (AMD3100) with a late-acting agent (G-CSF), maximal angiogenesis might be achieved. Indeed, combination treatment with G-CSF and AMD3100 resulted in the earliest and most robust angiogenic response in the hindlimb ischemia model. This regimen may warrant investigation in the clinical setting to improve revascularization following acute vascular injury.
The partial recovery of blood flow in control animals following induction of hindlimb ischemia indicates that endogenous mechanisms to induce neoangiogenesis must exist. One potential endogenous repair mechanism is the mobilization of angiogenic cells into the blood in response to the systemic release of inflammatory cytokines. Indeed, there is evidence that the level of EPCs is increased after certain stresses such as myocardial infarction and coronary artery bypass grafting.35,38 Additionally, circulating EPC levels in mice are increased after the induction of hindlimb ischemia.23 Angiogenic factors that are released from the ischemic tissue in response to the hindlimb ischemia procedure include PlGF and stromal-derived factor (SDF-1).8,39 In the present study, we show that serum levels of G-CSF also significantly increase following the induction of hindlimb ischemia. However, G-CSFR–deficient and wild-type mice display comparable blood flow recovery after hindlimb ischemia. Thus, G-CSF signaling is not required for endogenous revascularization in this model.
The mechanism by which cytokines stimulate angiogenesis is unclear. Previous studies have suggested that G-CSF is able to act directly upon endothelial cells to induce their proliferation and migration.40 Studies also have shown that G-CSF is able to act directly upon cardiomyocytes to promote their survival after myocardial infarction.41 Herein, we show that the angiogenic effect of G-CSF and AMD3100 can be adoptively transferred by blood mononuclear cells. Of interest, on a per-cell basis, blood mononuclear cells from untreated mice were equally as effective as G-CSF + AMD3100–mobilized blood mononuclear cells in inducing revascularization. Together, these observations suggest that these agents improve angiogenesis by increasing the basal number of circulating mononuclear cells rather than mobilizing a unique angiogenic-cell population.
Whether EPCs directly incorporate into neoendothelium following vascular injury is controversial. Kalka et al42 reported that infusion of cultured human EPCs following the induction of hindlimb ischemia resulted in their incorporation in 56% of newly formed vessels. Similarly, direct seeding of vascular grafts with cultured human EPCs markedly enhanced endothelialization of the graft, with approximately 60% of the endothelial cells derived from human EPCs.13 Several groups have also studied neoangiogenesis in bone marrow chimeras established by transplanting congenic hematopoietic cells into lethally irradiated recipient mice. Using the corneal micropocket and Matrigel angiogenesis assays, Murayama et al6 showed that 18% and 27%, respectively, of the neovasculature was composed of bone marrow–derived cells. Furthermore, Crosby et al14 demonstrated that 8% to 11% of endothelial cells in vessels that developed in sponge-induced granulation tissue were derived from bone marrow cells. On the other hand, Ziegelhoeffer et al,17 using thin-section confocal microscopy, showed no bone marrow–derived cell incorporation into the newly formed collateral arteries at both 7 and 21 days after hindlimb ischemia. Herein, we show that despite potently inducing angiogenesis, adoptively transferred blood mononuclear cells do not appreciably incorporate into the neovasculature in the hindlimb ischemia model. In contrast, Urbich et al10 reported that cultured human EPCs infused into mice following induction of hindlimb ischemia contributed significantly to the neoendothelium. Although the reason for the apparent discrepancy in the results is unknown, one obvious difference in these studies is the source of angiogenic cells. Whereas we used uncultured murine blood mononuclear cells, the study by Urbich et al10 used cultured human EPCs. Collectively, these data suggest that EPCs can contribute to angiogenesis through multiple mechanisms, perhaps dependent upon the type of angiogenic cell. While it is clear that cultured human EPCs can directly incorporate into the endothelium, mobilized EPCs appear to stimulate angiogenesis predominantly through a paracrine mechanism. These observations suggest the possibility that maximal therapeutic angiogenesis might be achieved by using multiple angiogenic-cell types.
The prevailing view in the field is that cytokines stimulate angiogenesis through the mobilization of endothelial progenitor cells (reviewed in Aicher et al43 ). In contrast, in the present study we provide evidence that monocytes are the major cell type mediating the angiogenic response of G-CSF and AMD3100 treatment. Recent studies have shown that monocytes are key contributors to angiogenesis at sites of ischemic injury. Urbich et al10 showed that cultured, but not freshly isolated, human CD14+ monocytes stimulate angiogenesis in a murine xenotransplantation model of hindlimb ischemia. Monocytes have also been implicated in tumor angiogenesis and corneal neovascularization.44,45 Furthermore, Heil et al46 showed that monocytes played a key role in endogenous collateral artery growth after acute vascular injury. Finally, Grunewald et al47 reported that monocytes are recruited to sites of neovascularization induced by VEGF expression. Consistent with our data, the latter 2 studies suggest that monocytes stimulate angiogenesis through paracrine mechanisms, largely based upon their lack of incorporation into the neoendothelium. The mechanism by which monocytes stimulate angiogenesis is not well understood. However, there is evidence that monocytes recruited to sites of ischemia may secrete proangiogenic cytokines, including VEGF, basic fibroblast growth factor (bFGF), and tumor necrosis factor-α (TNF-α).11,18,48,49
In summary, we show that treatment with G-CSF and AMD3100 rapidly stimulates angiogenesis following acute vascular injury. Our data suggest that these agents stimulate angiogenesis through the mobilization of monocytes into the blood with their subsequent recruitment to sites of ischemia and stimulation of angiogenesis through a paracrine mechanism. The rapid kinetics of angiogenic-cell mobilization by AMD3100 suggest that treatment with this agent, alone or in combination with G-CSF, might merit investigation as a method to stimulate revascularization following acute vascular injury, such as stroke or myocardial infarction.
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2006-04-013755.
Supported by a grant from the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) (RO1 HL 073762 [D.C.L.]) and by a grant from the Barnes-Jewish Hospital Foundation (D.C.L.). The Siteman Cancer Center is supported in part by National Cancer Institute (NCI) Cancer Center Support Grant no. P30 CA91842.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Amgen for supplying the G-CSF. We would also like to thank Dr David Hess for his help in developing the flow cytometry assay to measure donor endothelial cell chimerism. We thank William Eades and Jacqueline Hughes in the Siteman Cancer Center High Speed Sorter Core Facility for performing cell sorting segments of our experiments.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal