Abstract
Neoplastic T cells in mycosis fungoides (MF) are resistant to apoptotic agents, including galectin-1 that is abundant in skin. Although MF cells are typically CD7–, and thus galectin-1 resistant, CD7+ HH cells, derived from a patient with MF, were also resistant to galectin-1. HH cells demonstrate altered cell surface glycosylation, with loss of core 2 O-glycan ligands for galectin-1 created by core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT-I). Loss of core 2 O-glycans on tumor cells was also seen in primary CD7+ MF lesions. Surprisingly, HH cells are heterozygous for a C2GnT-I point mutation, yet this mutation resulted in a dramatic reduction in cellular glycosyltransferase activity. Expression of wild-type C2GnT-I in human HH cells, or murine lymphoma cells that lack C2GnT-I, restored core 2 O-glycan expression and susceptibility to galectin-1, whereas mutant enzyme lacked activity and did not restore core 2 O-glycan expression or susceptibility to galectin-1. Mutant enzyme did not have a dominant negative effect by affecting dimerization or activity of wild-type enzyme; rather, C2GnT-I haploinsufficiency is sufficient for loss of core 2 O-glycan expression and galectin-1 resistance. Thus, glycosyltransferase haploinsufficiency results in altered cellular glycosylation and resistance to cell death, identifying a new survival mechanism for T-lymphoma cells.
Introduction
Mycosis fungoides (MF) is the most common cutaneous T-cell lymphoma (CTCL). The disease progresses over years, evolving from focal plaques and tumors of neoplastic T cells confined to skin to the aggressive Sézary syndrome (SS) with involvement of peripheral blood.1,2 Neoplastic T cells in MF typically express a CD4+ Th2-type phenotype, with production of IL-4, IL-5, and IL-10.3-5 Like Th2 cells, MF T cells are resistant to stimuli that typically induce T-cell apoptosis, such as Fas and TNF receptor ligation.6-8 CTCL cells are protected from cell death by additional mechanisms, including increased expression of IL-7 and IL-15 that are produced in skin and maintain high levels of Bcl-2 in the tumor cells, and STAT3 activation.9-11 Although several therapeutic approaches are used in CTCL, including chemotherapy and radiotherapy, these approaches only achieve disease remission and are only effective in early stages of the disease.12-14 Thus, understanding factors that contribute to apoptosis resistance in MF will allow development of more effective therapies.
Several endogenous apoptotic triggers for T cells are expressed in skin, including galectin-1 and galectin-7, members of the galectin family of immunoregulatory lectins. Galectins bind oligosaccharide ligands on cell surface glycoproteins and glycolipids to regulate cell growth, adhesion, and death.15-18 Galectin-1 induces T-cell apoptosis via a Fas-, steroid-, and CD3-independent pathway.19-22 Galectin-1 is secreted from cells but remains associated with the cell surface or surrounding extracellular matrix by binding to saccharide ligands in the local milieu.23,24 Galectin-1 binds several T-cell surface glycoproteins, including CD43, CD45, and CD7.25 Although CD43 and CD45 modulate T-cell susceptibility to galectin-1,26 CD7 expression is required for susceptibility to galectin-1–induced cell death. HUT78 T cells derived from a patient with SS are CD7– and resistant to galectin-1, and expression of CD7 in HUT78 cells rendered the cells susceptible to galectin-1–induced death.27
Importantly, loss of CD7 expression is a hallmark of neoplastic T cells in MF.28-30 Prior work demonstrated that loss of CD7 expression by MF cells in primary patient samples correlated with resistance to galectin-1–induced death.30 However, we found that HH, a CD7+ T-cell line derived from a patient with cutaneous T-cell lymphoma, was also resistant to galectin-1–induced cell death.31 Unlike other T-cell lines, this cell line lacked specific O-glycans that can decorate CD43 and CD45 and that confers susceptibility to galectin-1,26,32 and it did not bind the lectin peanut agglutinin (PNA) that recognizes asialo core 1 structures on O-glycans. Primary MF lesions also demonstrated a lack of PNA binding by neoplastic T cells.31 The asialo core 1 O-glycan recognized by PNA is the acceptor substrate for core 2 β1,6-N-acetylglucosaminyl-transferases (C2GnT),33 a family of enzymes that add core 2 branches bearing lactosamine ligands recognized by galectin-1 to O-glycans.32 One mechanism for loss of PNA binding to T cells is masking of the asialo core 1 O-glycans by sialic acid; the sialyltransferases that add the masking sialic acid compete with C2GnT for acceptor glycoprotein substrates during O-glycan synthesis in the Golgi.34,35 Addition of sialic acid to the termini of asialo core 1 O-glycans blocks core 2 branching and blocks PNA binding.35 Because the addition of core 2 O-glycans by C2GnT is essential for galectin-1–induced death of T cells expressing CD45,26 this suggested that lack of core 2 O-glycans on CD7+ HH cells was responsible for resistance to galectin-1.
As mentioned in the previous paragraph, C2GnT acts in the Golgi to modify O-glycans on appropriate acceptor glycoprotein substrates. The enzyme requires precise localization in the cis-to-medial Golgi for optimal production of core 2–branched O-glycans, indicating the importance of the order of glycosylation during glycoprotein modification.34 Like many glycosyltransferases, C2GnTs can dimerize in the Golgi via interchain disulfide bonds, and dimerization may help retain the enzyme in the proper Golgi domain and protect the enzyme from proteolysis.36
In this study, we examined expression and activity of C2GnT in the CD7+ SS T-cell line, HH, to determine whether altered glycosyltransferase activity contributes to galectin-1 resistance in these cells. HH cells were heterozygous for a single point mutation in C2GnT-I that resulted in a dramatic reduction in enzyme activity, whereas expression of wild-type (WT) C2GnT-I in HH cells conferred susceptibility to galectin-1–induced cell death. Although altered expression of glycosyltransferase enzymes and altered cell surface glycosylation are known to contribute to tumor cell invasion and metastasis, this is the first example of a glycosyltransferase that modifies cell surface glycoproteins regulating lymphoma cell susceptibility to death. In addition, these findings demonstrate that a point mutation resulting in haploinsufficiency of a glycosyltransferase has a profound impact on the phenotype and biology of lymphoma cells.
Materials and methods
Cells and reagents
HUT78 and CEM T cells (American Type Culture Collection, Gaithersburg, MD), and HH and MyLa2059 MF cells (gift of Dr G. Burg, University of Zurich, Switzerland) were maintained as described.31 Murine BW5147 and PhaR2.1 T-cell lines were obtained and cultured as described.26 Human recombinant galectin-1 was prepared as described.37
Antibodies and reagents were as follows: anti–human CD7 monoclonal antibody (mAb) LT-7 (Advanced Immunochemical, Long Beach, CA), anti–human CD43 mAbs DFT1 (DAKO, Carpinteria, CA) and 1D4 that specifically recognize CD43-bearing core 2 O-glycans (Medical & Biological Laboratories, Woburn, MA), anti–human CD45 mAbs LCA PD7/26 + 2B11 (DAKO), anti–mouse CD43 mAbs S7 and 1B11, anti-EGFP mAb JL-8, anti–mouse CD45 mAb 69, mouse IgG1 and IgG2a, and PE-rat IgG2a (BD Biosciences, Palo Alto, CA), fluorescein isothiocyanate (FITC)–goat anti–mouse Ig (DAKO), horseradish peroxidase (HRP)–goat anti–rabbit IgG, HRP-goat anti–mouse IgG (Bio-Rad, Hercules, CA), HRP-streptavidin (Zymed, San Francisco, CA), FITC- and allophycocyanin-annexin V (Invitrogen, Carlsbad, CA), propidium iodide (BD Biosciences), ImmunoPure Immobilized Streptavidin and ImmunoPure Immobilized Protein G (Pierce, Rockford, IL), and Enhanced Chemiluminescence (ECL) Detection Kit (Amersham, Piscataway, NJ).
Phenotypic analysis
Cells (2 × 105) were incubated with 0.2 μg antibody, mouse IgG1, IgG2a,or PE-rat IgG2a in 50 μL, 45 minutes at 4°C, followed by FITC-secondary antibody, 45 minutes at 4°C. Antibody binding was analyzed by flow cytometry on a FACScan flow cytometer using CELLQuest software (BD Biosciences).
Immunohistochemistry
Archival mycosis fungoides biopsy specimens were obtained from the Surgical Pathology Division, University of California, Los Angeles (UCLA) Department of Pathology. Sections (3-5 μm) of formalin-fixed skin biopsies were stained with anti-CD7 mAb as described.31 Serial sections of samples that had CD7 reactivity of neoplastic T cells were analyzed with 1D4 mAb to detect C2GnT modification of human CD43 on neoplastic cells, followed by HRP-goat anti–mouse IgG and counterstained with hematoxylin. Samples were analyzed by using a BX51 microscope equipped with PlanApo objective lenses (20 ×/0.70 NA, 40 ×/0.95 NA, and 100 ×/1.30 oil-immersion), a DP11 camera (Olympus, Melville, NY), and Image Pro Plus software (Media Cybernetics, Silver Spring, MD). The study protocol for use of human tissue was approved by the University of California at Los Angeles (UCLA) Institutional Review Board.
RT-PCR
Total RNA was extracted from 107 cells using RNAeasy Mini kit (Qiagen, Valencia, CA). Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed according to the protocol in the Super Script One-Step RT-PCR Kit with Platinum Taq (Invitrogen), using 1 μg RNA and the following primers: human C2GnT-I, 5′-TTATTGTTTGAAATGCTGAGGACG-3′ (sense) and 5′-TAATGGTCAGTGTTTTAATGTCT-3′ (antisense); human actin, 5′-GCTCGTCGTCGACAACGGCTC-3′ (sense) and 5′-CAAACATGATCTGGGTCATCTTCTC-3′ (antisense). Amplification was performed with an iCycler Thermal Cycler (Bio-Rad): 50°C for 30 minutes, 94°C 2 minutes (reverse transcription), 35 cycles at 94°C for 15 seconds, 54°C for 30 seconds, 72°C for 1 minute, 72°C for 10-minute extension. PCR products were purified with the Qiaquick PCR Purification kit (Qiagen) and sequenced at UC Davis Automated DNA Sequencing Facility (Davis, CA). Quantitative real-time RT-PCR was performed using QuantiTect Probe RT-PCR kit (Qiagen) with the following primers and Taqman TAMRA probes: human C2GnT-I, 5′-CTACCCGCCCTGCGATG-3′ (sense primer), 5′-CATCCAGTTCAAGTCACCAGCTC-3′ (antisense primer), 5′-TCCATGTGCGCTCAGTGTGCATTT-3′ (probe); mouse actin, 5′-AGAGGGAAATCGTGCGTGAC-3′ (sense), 5′-CAATAGTGATGCCTGGCCGT-3′(antisense), 5′-CACTGCCGCATCCTCTTCCTCCCT-3′ (probe). Analysis was performed with a DNA Engine Opticon real-time PCR machine (MJ Research, Waltham, MA): 50°C for 30 minutes (reverse transcription), 95°C for 15 minutes (initial activation step), 45 cycles of 94°C for 15 seconds, 60°C for 60 seconds. Results were expressed as C2GnT-actin ratios of interpolated CT values for each sample.
Western blot
Cells (3 × 107) were lysed in hypotonic buffer (20 mM HEPES, 5 mM sodium pyrophosphate, 5 mM EGTA, 1 mM MgCl2, 1 mM PMSF, 10 μg/mL leupeptin, 5 μg/mL aprotinin), Dounce homogenized, and centrifuged at 3000g. Supernatants were centrifuged at 100 000g at 4°C for 1 hour, and membrane pellets were resuspended in 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA pH 8.0, 1% NP-40, 1 mM PMSF, 10 μg/mL leupeptin, and 5 μg/mL aprotinin. For analysis of C2GnT monomers and dimers in total membrane extracts, 20 μg protein was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing or nonreducing conditions. Iodoacetamide (100 mM final concentration) was added to samples to prevent disulfide bond formation during sample preparation. After transfer to nitrocellulose, blots were probed with rabbit polyclonal anti-C2GnT antibody,34 and bound antibody was detected with HRP-anti–rabbit IgG and ECL.
To analyze dimerization of C2GnT-Enhanced Green Fluorescent Protein (EGFP), enzyme was precipitated with anti-EGFP mAb JL-8 and ImmunoPure Immobilized Protein G and separated by 10% SDS-PAGE under nonreducing conditions. Blots were probed with anti-C2GnT polyclonal antibody and bound antibody detected as described in the previous paragraph.
For CD45 analysis, whole-cell lysates of 3 × 107 murine BW5147 cells transfected with plasmid alone, WT C2GnT, or mutant C2GnT were prepared as described38 and precipitated with anti–mouse CD45 mAb 69 and Immunopure Immobilized Protein G. Precipitates were separated in 3% to 8% Tris-Acetate NuPAGE gels under reducing conditions. After transfer to nitrocellulose, blots were probed with anti–mouse CD45 mAb 69, and bound antibody was detected as described in the previous paragraph. For CD43 analysis, whole-cell lysates of the same cells were prepared as described above and precipitated with anti–mouse CD43 mAbs S7 or 1B11 and Immunopure Immobilized Protein G. Precipitates were separated and transferred to nitrocellulose as described for CD45, blots were probed with the respective anti–murine CD43 mAbs, and bound antibody was detected as for CD45.
Core 2 GnT enzyme activity assays
C2GnT activity was assayed as described.39,40 Briefly, equal volumes of cell lysates were incubated in a final volume of 20 μL with 50 mM HEPES-NaOH pH 7.0 containing 1 mM UDP-N-acetyl-d-glucosamine (GlcNAc), 3.7 kBq UDP-[6-3H]GlcNAc (1.5 TBq/mmol; PerkinElmer Life Science, Boston, MA), 1 mM galactoseβ1,3N-acetyl-galactosamine-p-nitrophenol (Toronto Research Chemicals, Ontario, Canada), 100 mM N-acetyl-glucosamine, 50 mM galactono-1,5-lactone, 5 mM dithiothreitol, and 10 mM EDTA at 37 °C for 2 hours. The product was purified on a C-18 Extract-Clean column (Alltech, Nicholasville, KY), washed extensively, eluted with methanol, and subjected to liquid scintillation counting on a Beckman LS6000SC scintillation counter (Beckman Coulter, Fullerton, CA). Picomoles of GlcNAc transferred per hour and per milligram of protein were calculated based on specific activity of 3.8 cpm/pM UDP-[6-3H]GlcNAc.
Cell-death assays
Galectin-1 death assays were performed as described.37 Briefly, 2 × 105 cells were treated with 20 μM galectin-1, rocking, in buffer containing 1.0 mM dithiothreitol, or 1.0 mM dithiothreitol buffer alone, for 4 hours, 37°C in 5% or 10% CO2. β-Lactose (0.1 M; final concentration) was added to dissociate galectin-1, and cell death was measured by annexin V binding and propidium iodide or 7-aminoactinomycin D uptake, analyzed on FACScan or FACSCalibur flow cytometers with CellQuest software (BD Biosciences). The percentage of cell death was determined as 100 – [(% annexin V – propidium iodide – (galectin-1)/% annexin V – propidium iodide – (control)]. Statistical analysis was done by analysis of variance and post hoc analysis by Newman-Keuls multiple comparison test using Prism 3.0 software (Graphpad, San Diego, CA).
Loss of core 2 O-glycan expression by neoplastic CD7+ T cells. (A) HUT78, MyLa2059, and HH cell lines derived from patients with SS were analyzed for expression of CD7, CD43, and CD45, the major glycoprotein receptors for galectin-1, and for reactivity with 1D4 mAb that recognizes core 2 O-glycans on human CD43. CEM T-acute lymphoblastic leukemia cells were used as a positive control. (B) Representative patch stage MF lesion with neoplastic Sézary T cells infiltrating the dermis and epidermis. (Bi) Immunohistochemistry with anti-CD7 demonstrates atypical expression of CD7 by Sézary cells seen singly in the dermis (bottom left) and in characteristic microabscesses in the epidermis (center). (Bii) Tumor cells in a microabscess demonstrate cell surface reactivity with anti-CD7 (arrowheads). (Biii) Immunohistochemistry with 1D4 shows that both 1D4+ cells (arrowhead) and 1D4– cells (arrow) are present in a microabscess. (Biv) Characteristic Sézary cells with large, highly convoluted nuclei demonstrate lack of 1D4 reactivity at the cell surface (arrow). Original magnification × 200 (Bi), × 400 (Bii-iii) and × 1000 (Biv).
Loss of core 2 O-glycan expression by neoplastic CD7+ T cells. (A) HUT78, MyLa2059, and HH cell lines derived from patients with SS were analyzed for expression of CD7, CD43, and CD45, the major glycoprotein receptors for galectin-1, and for reactivity with 1D4 mAb that recognizes core 2 O-glycans on human CD43. CEM T-acute lymphoblastic leukemia cells were used as a positive control. (B) Representative patch stage MF lesion with neoplastic Sézary T cells infiltrating the dermis and epidermis. (Bi) Immunohistochemistry with anti-CD7 demonstrates atypical expression of CD7 by Sézary cells seen singly in the dermis (bottom left) and in characteristic microabscesses in the epidermis (center). (Bii) Tumor cells in a microabscess demonstrate cell surface reactivity with anti-CD7 (arrowheads). (Biii) Immunohistochemistry with 1D4 shows that both 1D4+ cells (arrowhead) and 1D4– cells (arrow) are present in a microabscess. (Biv) Characteristic Sézary cells with large, highly convoluted nuclei demonstrate lack of 1D4 reactivity at the cell surface (arrow). Original magnification × 200 (Bi), × 400 (Bii-iii) and × 1000 (Biv).
Site-directed mutagenesis
Cloning of C2GnT-I and C2GnT-I–EGFPin pcDNA3.1/Zeo (+) and pcDNA3.1+ vectors, respectively, was previously described (Invitrogen).40,41 Both C2GnT-I and EGFP–C2GnT-I were subjected to site-directed mutagenesis to introduce a point mutation (C/G) at position 692 using Quick Change Site Directed Mutagenesis Kit according to the manufacturer's instructions (Stratagene, La Jolla, CA) and primers with the mutation (sense, 5′-GCATTCATGTGGACACAAAATGCGAGGATTCCTATTTAGCTG-3′; antisense, 5′-CAGCTAAATAGGAATCCTCGCATTTTG-3′). Mutated plasmid was purified using Qiagen Plasmid Midi Kit (Qiagen) and sequenced at the University of California (UC)–Davis Automated DNA Sequencing Facility.
Generation of transfectants
HH and BW5147 cells were transfected with either wild-type or mutant human C2GnT-I cDNA or C2GnT-I–EGFP cDNA cloned in pcDNA3.1/Zeo(+) or pcDNA3.1+, respectively, or with vector alone, using the Nucleofection Kit V (Amaxa Biosystems, Cologne, Germany). Plasmids were linearized by PvuI digestion (Invitrogen), purified with Qiaquick PCR Purification kit, and transfected using 5 × 106 cells and Amaxa instrument program A23 for BW5147 or O12 for HH. Cells were cultured in 300 μg/mL Zeocin or 800 μg/mL G418 (Calbiochem, San Diego, CA) for 15 days and cloned by limiting dilution. Clones were screened by flow cytometry to detect EGFP intensity and/or 1B11-PE staining.
Results
Loss of core 2 O-glycan expression on MF T cells
Prior work has shown that CD7– SS T-cell lines and CD7– neoplastic cells isolated directly from patients with SS are resistant to galectin-1–induced cell death.27,30,31 However, we also observed that a CD7+ SS cell line, HH, was resistant to galectin-1–induced death.31 Resistance to galectin-1 suggested that HH cells either had altered expression of another glycoprotein receptor for galectin-1, or altered cellular glycosylation, as suggested by the PNA– phenotype of HH cells. We analyzed 3 MF cell lines, HUT78, MyLa2059, and HH, to characterize the pattern of expression of the 3 primary glycoprotein receptors for galectin-1 on T cells, CD7, CD43, and CD45. In addition, we used the 1D4 mAb to determine whether the cells expressed CD43 modified by core 2 O-glycans.
HH cells express CD7, whereas HUT78 and MyLa2059 cells do not express CD7, as previously observed (Figure 1A).31 HH cells also express CD43 and CD45, the other major glycoprotein receptors for galectin-1, although neither CD43 nor CD45 expression is absolutely required for T-cell susceptibility to galectin-1. Thus, resistance of HH cells to galectin-1 death cannot be accounted for by loss of the 3 primary glycoprotein receptors for galectin-1. As mentioned in “Introduction,” expression of core 2 O-glycans is required for CD45+ T cells to be susceptible to galectin-1. Although no reagents exist that specifically detect core 2 O-glycans on CD45, the 1D4 mAb specifically recognizes human CD43 only when CD43 is modified by core 2 O-glycans, so that 1D4 reactivity indicates addition of core 2 O-glycans to CD43.42 HH cells demonstrated minimal reactivity with the 1D4 mAb, compared with the positive 1D4 reactivity observed for HUT78 and MyLa2059 cells and for CEM T cells used as a positive control. These data suggested that lack of core 2 O-glycan expression was responsible for resistance of CD45+ HH cells to galectin-1 cell death.
To determine whether loss of core 2 O-glycan expression occurs in primary MF lesions, we examined skin biopsies from a patient with MF in whom the transformed T cells had an atypical CD7+ phenotype. Numerous neoplastic T Sézary cells, large irregularly shaped lymphoid cells with convoluted nuclei, were seen infiltrating the dermis and epidermis (Figure 1B). These cells all stained positively for CD7. However, although some neoplastic cells were 1D4+, we observed frequent 1D4– Sézary cells throughout the dermis and epidermis. Thus, loss of core 2 O-glycan expression by neoplastic T cells can occur in primary MF lesions.
HH cells have minimal C2GnT activity
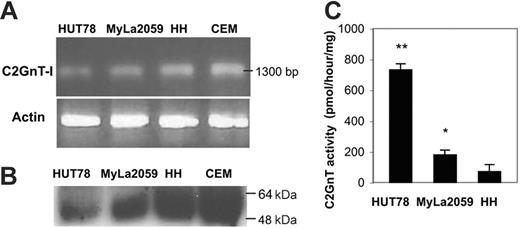
Lack of 1D4 binding to HH cells (Figure 1A) suggested that these cells had a defect in initiation or elongation of core 2 O-glycans. Initiation of core 2 O-glycans is regulated by C2GnT enzymes that attach a branching GlcNAc residue to core 1 O-glycans; this allows sequential addition of multiple lactosamine sequences to the core 2 branch, creating polylactosamine sequences that are preferred saccharide ligands for galectin-1.32 We examined C2GnT expression in HH cells by RT-PCR and immunoblot. C2GnT-I mRNA was present in 1D4– HH cells at roughly the same level as in the 1D4+ cell lines HUT78 and MyLa2059 (Figure 2A). Similarly, HH cells had abundant C2GnT protein expression, using a polyclonal rabbit antiserum to C2GnT (Figure 2B). However, HH cells had minimal C2GnT enzyme activity, whereas specific enzyme activity was observed in extracts of HUT78 and MyLa2059 cells (Figure 2C). Thus, although HH cells expressed C2GnT-I mRNA and protein, the cells had minimal C2GnT enzymatic activity, accounting for the lack of 1D4 binding to HH cells and suggesting that loss of core 2 O-glycans was responsible for resistance of HH cells to galectin-1 death.
C2GnT-I is expressed in 1D4– HH cells but has minimal enzyme activity. (A). RT-PCR (for C2GnT and β-actin) and (B) immunoblotting (for C2GnT) demonstrate abundant expression of C2GnT mRNA and protein in all cell lines. (C) Extracts of HUT78, MyLa2059, and HH cells were assayed for C2GnT enzyme activity. **P < .001, HUT78 compared with HH; *P < .01, Myla2059 compared with HH. Values are means ± SD of duplicates from 1 of 5 independent experiments.
C2GnT-I is expressed in 1D4– HH cells but has minimal enzyme activity. (A). RT-PCR (for C2GnT and β-actin) and (B) immunoblotting (for C2GnT) demonstrate abundant expression of C2GnT mRNA and protein in all cell lines. (C) Extracts of HUT78, MyLa2059, and HH cells were assayed for C2GnT enzyme activity. **P < .001, HUT78 compared with HH; *P < .01, Myla2059 compared with HH. Values are means ± SD of duplicates from 1 of 5 independent experiments.
HH cells are heterozygous for a missense mutation in C2GnT-I
Because HH cells had minimal C2GnT activity, despite expressing abundant C2GnT mRNA and protein, we sequenced C2GnT-I cDNA from HH cells to look for mutations that would affect C2GnT function. Analysis of C2GnT-I cDNA revealed a single point mutation, C→Gat position 692 (Figure 3A). This mutation was not present in HUT78, MyLa2059, or CEM cells (data not shown). The single nucleotide change causes a missense mutation, with an amino acid substitution of Ser to Cys at position 158, designated S158C (Figure 3C). Surprisingly, HH cells were heterozygous for the S158C mutation (Figure 3B), suggesting that either the mutant enzyme acts in a dominant-negative fashion, or that 1 copy of a functional gene does not produce sufficient enzyme to properly modify glycoproteins passing through the Golgi in these cells.
Expression of C2GnT-I makes HH cells susceptible to galectin-1
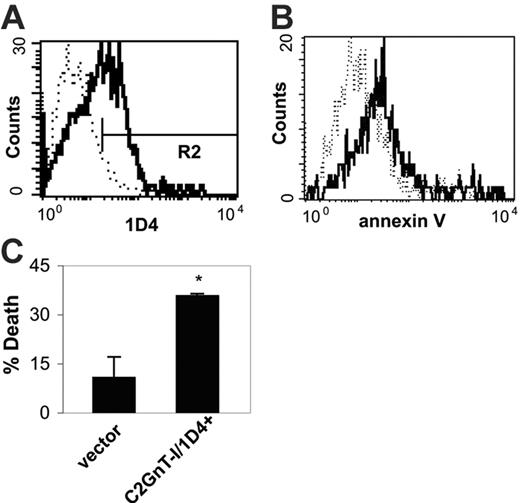
To determine whether loss of C2GnT-I activity was responsible for galectin-1 resistance of HH cells, we overexpressed wild-type (WT) C2GnT-I in HH cells. Transfected cells were analyzed with the 1D4 mAb as a measure of C2GnT-I activity (Figure 4A). Expression of C2GnT-I in HH cells conferred susceptibility to galectin-1–induced death (Figure 4B). Thirty-five percent of 1D4+ cells treated with galectin-1 bound annexin V, compared with 10% of cells transfected with vector alone (Figure 4C). Because expression of exogenous C2GnT-I was sufficient to restore galectin-1 susceptibility to HH cells, the reduction of C2GnT activity in HH cells likely accounted for resistance to galectin-1 death.
S158C mutation reduces C2GnT activity but does not affect substrate specificity or enzyme dimerization
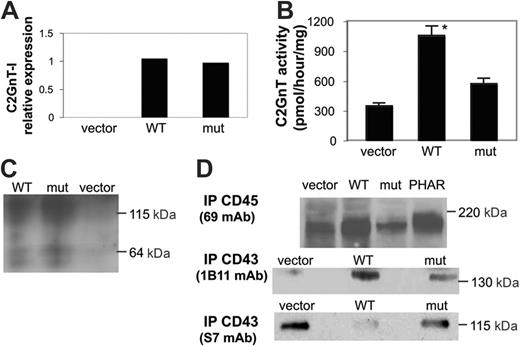
To understand the effects of the S158C mutation on the C2GnT-I enzyme, we used murine BW5147 cells that do not express core 2 O-glycans.32 Site-directed mutagenesis was used to introduce the C→G at position 692 in human C2GnT-I. WT C2GnT-I or mutant C2GnT-I was expressed in BW5147 cells, and stably transfected clones were isolated. Real-time quantitative RT-PCR analysis demonstrated high levels of human C2GnT-I mRNA in clones transfected with either WT or mutant C2GnT-I, whereas no human C2GnT-I mRNA was observed in vector-transfected cells (Figure 5A).
We analyzed C2GnT activity of clones expressing comparable levels of WT or mutant C2GnT-I mRNA (Figure 5B). BW5147 cells transfected with WT C2GnT-I had a 3-fold increase in enzyme activity compared with cells transfected with vector alone, demonstrating that WT human enzyme was expressed in an active form. In contrast, cells transfected with mutant C2GnT-I had no increase in enzyme activity compared with cells transfected with vector alone. These data demonstrated that the S158C mutation was sufficient to reduce C2GnT-I enzyme activity.
How could a point mutation in C2GnT-I reduce enzyme activity? Introduction of an additional Cys residue in this enzyme could have effects on conserved paired Cys residues or on the unpaired Cys residue near the enzyme active site.43,44 Altered disulfide bonding could cause enzyme misfolding or could affect enzyme structure and function (eg, by altering enzyme dimerization that promotes proper enzyme localization and retention in the Golgi,36 or by altering acceptor substrate specificity). To investigate enzyme dimerization, WT or mutant C2GnT was immunoprecipitated from transfected cells and analyzed by nondenaturing SDS-PAGE and immunoblotting. We detected dimeric C2GnT enzyme, with Mr of approximately 120 kDa, in cells expressing either WT or mutant C2GnT-I, in addition to a small fraction of monomeric C2GnT (60 kDa) in both cells (Figure 5C). Thus, the S158C mutation did not prevent enzyme dimerization.
HH cells are heterozygous for a point mutation in C2GnT-I. (A) Partial sequence alignment of C2GnT-I cDNA from HH cells (underlined) with human C2GnT-I cDNA sequence derived from NCBI Nucleotide Database, accession number BC074885. The 692 C/G mutation is indicated in bold (arrow). Sequences were aligned using NCBI Blast 2 sequences. (B) Chromatogram from the C2GnT-I cDNA forward strand sequence from HH cells, demonstrating the 692 C/G mutation. The asterisk (*) denotes overlapping peaks, indicating that the cells are heterozygous for the mutation. (C) Partial amino acid sequence alignment of the predicted C2GnT-I nucleotide sequences from HH cells, translated with the ExPASy Translate Tool. The 692 C/G mutation results in a substitution of Cys for Ser at position 158 (arrow). Boxes indicate Cys residues that are highly conserved in the β1,6-N-acetylglucosaminyltransferase family.
HH cells are heterozygous for a point mutation in C2GnT-I. (A) Partial sequence alignment of C2GnT-I cDNA from HH cells (underlined) with human C2GnT-I cDNA sequence derived from NCBI Nucleotide Database, accession number BC074885. The 692 C/G mutation is indicated in bold (arrow). Sequences were aligned using NCBI Blast 2 sequences. (B) Chromatogram from the C2GnT-I cDNA forward strand sequence from HH cells, demonstrating the 692 C/G mutation. The asterisk (*) denotes overlapping peaks, indicating that the cells are heterozygous for the mutation. (C) Partial amino acid sequence alignment of the predicted C2GnT-I nucleotide sequences from HH cells, translated with the ExPASy Translate Tool. The 692 C/G mutation results in a substitution of Cys for Ser at position 158 (arrow). Boxes indicate Cys residues that are highly conserved in the β1,6-N-acetylglucosaminyltransferase family.
Expression of wild-type C2GnT-I in HH cells renders the cells susceptible to galectin-1 cell death. (A) HH cells were transfected with wild-type C2GnT-I. Pooled transfected cells were analyzed by staining with 1D4. Cells transfected with C2GnT-I (solid line) demonstrated increased reactivity with 1D4 compared with cells transfected with vector alone (dotted line). Marker shows 1D4+ region (R2). (B) Pooled HH cells transfected with wild-type C2GnT-I or vector alone were treated with galectin-1 for 4 hours and analyzed for binding of annexin V. C2GnT-I–transfected cells (1D4+ cells gated in R2, solid line) demonstrated increased annexin V binding after galectin-1 treatment compared with cells transfected with vector alone (dotted line). (C) Galectin-1 cell death was determined for cells transfected with vector alone versus 1D4+ cells transfected with C2GnT-I. 1D4+ cells demonstrated significantly greater cell death compared with cells transfected with vector alone (*P < .01). Values are mean ± SD for triplicates and are from 1 of 3 independent experiments.
Expression of wild-type C2GnT-I in HH cells renders the cells susceptible to galectin-1 cell death. (A) HH cells were transfected with wild-type C2GnT-I. Pooled transfected cells were analyzed by staining with 1D4. Cells transfected with C2GnT-I (solid line) demonstrated increased reactivity with 1D4 compared with cells transfected with vector alone (dotted line). Marker shows 1D4+ region (R2). (B) Pooled HH cells transfected with wild-type C2GnT-I or vector alone were treated with galectin-1 for 4 hours and analyzed for binding of annexin V. C2GnT-I–transfected cells (1D4+ cells gated in R2, solid line) demonstrated increased annexin V binding after galectin-1 treatment compared with cells transfected with vector alone (dotted line). (C) Galectin-1 cell death was determined for cells transfected with vector alone versus 1D4+ cells transfected with C2GnT-I. 1D4+ cells demonstrated significantly greater cell death compared with cells transfected with vector alone (*P < .01). Values are mean ± SD for triplicates and are from 1 of 3 independent experiments.
The S158C mutation in C2GnT-I reduces enzyme activity. BW5147 murine T cells lack C2GnT-I. BW5147 cells were transfected with wild-type C2GnT-I (WT), mutant C2GnT-I with the S158C mutation (mut), or vector alone. (A) Relative expression of WT or mut C2GnT-I mRNA in transfected cells was analyzed by real-time RT-PCR. Clones with similar C2GnT-I mRNA expression, calculated as C2GnT-actin ratios, were selected for further analysis; these were designated WT clone 6 and mut clone 2. (B) Total membrane extracts of selected clones were analyzed for C2GnT activity. Cells expressing mutant C2GnT-I or vector alone had comparable activity, whereas cells expressing WT C2GnT-I had increased enzyme activity (*P < .001 for WT compared with vector or mut). Values are mean ± SD for duplicates and are from 1 of 6 independent experiments. (C) The S158C mutation does not affect enzyme dimerization. For analysis of C2GnT, total membrane extracts of the clones in panel A were separated by 10% SDS-PAGE under nonreducing conditions, blotted to nitrocellulose, and probed with rabbit polyclonal antiserum to C2GnT. C2GnT dimers (∼ 115 kDa) were observed in cells expressing WT or mutant C2GnT-I. A minor fraction of monomeric enzyme (∼ 64 kDa) was detected in both samples. (D) Glycosylation of the primary C2GnT acceptor substrates, CD43 and CD45. (Top) CD45 was precipitated from BW5147 cells transfected with vector alone, WT C2GnT-I, or mutant C2GnT-I, or PhaR2.1 cells that express endogenous C2GnT. BW5147 cells expressing WT C2GnT-I demonstrated increased CD45 heterogeneity comparable to that seen for CD45 from PhaR2.1 cells, indicating that WT C2GnT-I expression increased CD45 glycosylation. BW5147 cells expressing mutant C2GnT-I demonstrated minimal CD45 heterogeneity, comparable to that seen from cells transfected with vector alone. (Bottom) CD43 was precipitated with the 1B11 mAb that recognizes murine CD43 modified with core 2 O-glycans or the S7 mAb that recognizes murine CD43 modified with core 1 O-glycans, and immunoblotted with the same mAb used for precipitation. 1B11-reactive CD43 was detected in cells transfected with C2GnT-I, whereas S7-reactive CD43 was detected in control cells and was the primary band detected in cells transfected with mutant C2GnT-I.
The S158C mutation in C2GnT-I reduces enzyme activity. BW5147 murine T cells lack C2GnT-I. BW5147 cells were transfected with wild-type C2GnT-I (WT), mutant C2GnT-I with the S158C mutation (mut), or vector alone. (A) Relative expression of WT or mut C2GnT-I mRNA in transfected cells was analyzed by real-time RT-PCR. Clones with similar C2GnT-I mRNA expression, calculated as C2GnT-actin ratios, were selected for further analysis; these were designated WT clone 6 and mut clone 2. (B) Total membrane extracts of selected clones were analyzed for C2GnT activity. Cells expressing mutant C2GnT-I or vector alone had comparable activity, whereas cells expressing WT C2GnT-I had increased enzyme activity (*P < .001 for WT compared with vector or mut). Values are mean ± SD for duplicates and are from 1 of 6 independent experiments. (C) The S158C mutation does not affect enzyme dimerization. For analysis of C2GnT, total membrane extracts of the clones in panel A were separated by 10% SDS-PAGE under nonreducing conditions, blotted to nitrocellulose, and probed with rabbit polyclonal antiserum to C2GnT. C2GnT dimers (∼ 115 kDa) were observed in cells expressing WT or mutant C2GnT-I. A minor fraction of monomeric enzyme (∼ 64 kDa) was detected in both samples. (D) Glycosylation of the primary C2GnT acceptor substrates, CD43 and CD45. (Top) CD45 was precipitated from BW5147 cells transfected with vector alone, WT C2GnT-I, or mutant C2GnT-I, or PhaR2.1 cells that express endogenous C2GnT. BW5147 cells expressing WT C2GnT-I demonstrated increased CD45 heterogeneity comparable to that seen for CD45 from PhaR2.1 cells, indicating that WT C2GnT-I expression increased CD45 glycosylation. BW5147 cells expressing mutant C2GnT-I demonstrated minimal CD45 heterogeneity, comparable to that seen from cells transfected with vector alone. (Bottom) CD43 was precipitated with the 1B11 mAb that recognizes murine CD43 modified with core 2 O-glycans or the S7 mAb that recognizes murine CD43 modified with core 1 O-glycans, and immunoblotted with the same mAb used for precipitation. 1B11-reactive CD43 was detected in cells transfected with C2GnT-I, whereas S7-reactive CD43 was detected in control cells and was the primary band detected in cells transfected with mutant C2GnT-I.
We also investigated whether mutant C2GnT-I had altered substrate specificity. CD45 and CD43 are the most abundant acceptor substrates on T cells for C2GnT modification. Modification of CD45, but not CD43, by core 2 O-glycans is essential for susceptibility to galectin-1.26 There are no specific reagents that detect core 2 O-glycan–modified CD45; to determine whether CD45 was differentially modified in cells expressing WT versus mutant C2GnT-I, we examined CD45 from BW5147 cells transfected with vector alone, WT C2GnT-I, or mutant C2GnT-I, and compared this with CD45 from PhaR2.1 cells, a derivative of BW5147 that expresses endogenous C2GnT.32 CD45 from BW5147 cells expressing WT C2GnT-I ran as a diffuse band, similar to CD45 from PhaR2.1 cells that express C2GnT (Figure 5D). In contrast, CD45 from BW5147 cells transfected with mutant C2GnT-I or vector alone ran as a narrow band, suggesting relatively little glycosylation-related heterogeneity. Thus, mutant C2GnT-I did not appear to modify CD45 to the degree observed with WT C2GnT-I.
Similarly, expression of mutant C2GnT-I resulted in reduced modification of CD43, compared with BW5147 cells transfected with wild-type C2GnT-I. The 1B11 mAb recognizes murine CD43 modified with core 2 O-glycans, analogous to 1D4 mAb recognition of human CD43 with core 2 O-glycans, whereas the S7 mAb recognizes murine CD43 modified with core 1 O-glycans.32 CD43 was immunoprecipitated with 1B11 and S7 from cells transfected with vector alone, WT C2GnT-I, or mutant C2GnT-1. BW5147 cells transfected with WT C2GnT-I had only a 1B11 reactive band and no S7 reactive band, indicating that CD43 was modified with core 2 O-glycans in these cells (Figure 5D). In contrast, BW5147 cells transfected with vector alone had no 1B11 reactive band, whereas S7 precipitated CD43 from these cells. BW5147 cells transfected with mutant C2GnT-1 had primarily S7-reactive CD43, although a small amount of 1B11-reactive CD43 was detected. Thus, the single point mutation in C2GnT-I appeared to cause a global reduction in enzyme activity, with reduced modification of both CD45 and CD43.
C2GnT-I with the S158C mutation does not confer galectin-1 susceptibility
Overexpression of WT C2GnT-I in HH cells that were heterozygous for the S158C mutation was sufficient to restore susceptibility to galectin-1 (Figure 4). To firmly establish that the mutant C2GnT-I could not restore susceptibility to galectin-1, we examined death of BW5147 cells expressing WT or mutant C2GnT-I. Several clones expressing WT or mutant enzyme were examined. Expression of WT C2GnT-I in BW5147 cells rendered the cells susceptible to galectin-1, with approximately 50% cell death of galectin-1–treated cells, a level of cell death comparable to that observed for PhaR2.1 cells that express endogenous C2GnT (Figure 6). In contrast, expression of mutant C2GnT-I, although confirmed by RT-PCR analysis (see Figure 5), was not sufficient to make the cells susceptible to galectin-1, consistent with low C2GnT activity in cells expressing mutant enzyme (Figure 5).
S158C mutation appears to result in haploinsufficiency of C2GnT activity
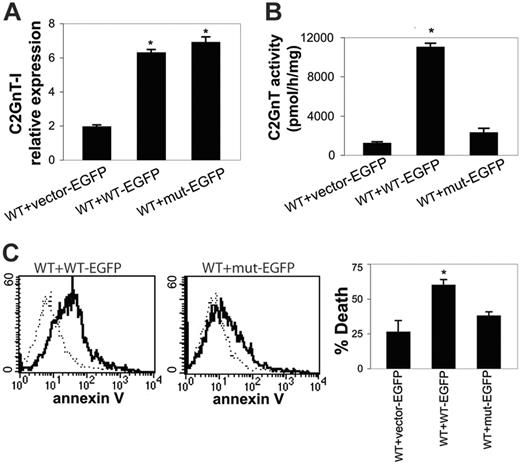
The S158C mutation in HH cells was heterozygous (Figure 3), so expression of a single copy of WT C2GnT enzyme was not sufficient to create the 1D4 epitope on the cell surface or to make HH cells susceptible to galectin-1. This suggested that either the mutant enzyme had a dominant-negative effect on WT enzyme or that haploinsufficiency of C2GnT activity was sufficient to result in resistance to galectin-1 death. To determine whether the S158C mutation in C2GnT-I had a dominant-negative effect, we used BW5147 cells stably expressing WT C2GnT-I (Figures 5, 6). We chose a clone expressing a moderate amount of C2GnT activity (WT clone 6; see Figure 5A) to examine the effect of expressing additional mutant or wild-type C2GnT. These cells were transfected again with either WT or mutant C2GnT-I tagged with EGFP, or with EGFP plasmid alone as a control, to examine the effect of mutant C2GnT-I on WT enzyme activity. Transfected cells were screened by EGFP expression (data not shown). We examined pairs of clones expressing equivalent amounts of WT or mutant C2GnT-I–EGFP, as determined by real-time quantitative RT-PCR and normalization to actin (Figure 7A).
Expression of mutant C2GnT-I does not confer galectin-1 susceptibility. (A) Cells expressing WT clone 6 or mut clone 2 enzyme (from Figure 5A) were analyzed for annexin V binding after treatment with galectin-1 (dark line) and compared with annexin V binding of cells transfected with vector alone after treatment with galectin-1 (dotted line). (B) Cell death determined by annexin V binding and propidium iodide uptake of BW5147 cells transfected with vector alone, WT C2GnT-I (WT clone 6), or mutant C2GnT-I (mut clone 2). PhaR2.1 cells that express endogenous C2GnT and are susceptible to galectin-1 were used as a positive control. *P < .001 for cells expressing WT C2GnT-I compared with vector or mutant C2GnT-I. Values are mean ± SD of triplicates from 1 of 3 independent experiments.
Expression of mutant C2GnT-I does not confer galectin-1 susceptibility. (A) Cells expressing WT clone 6 or mut clone 2 enzyme (from Figure 5A) were analyzed for annexin V binding after treatment with galectin-1 (dark line) and compared with annexin V binding of cells transfected with vector alone after treatment with galectin-1 (dotted line). (B) Cell death determined by annexin V binding and propidium iodide uptake of BW5147 cells transfected with vector alone, WT C2GnT-I (WT clone 6), or mutant C2GnT-I (mut clone 2). PhaR2.1 cells that express endogenous C2GnT and are susceptible to galectin-1 were used as a positive control. *P < .001 for cells expressing WT C2GnT-I compared with vector or mutant C2GnT-I. Values are mean ± SD of triplicates from 1 of 3 independent experiments.
The S158C mutant C2GnT-I does not act in a dominant-negative fashion. BW5147 cells expressing WT C2GnT-I (WT clone 6, from Figure 5A) were transfected again with EGFP alone (WT + vector-EGFP), WT C2GnT-I–EGFP (WT + WT-EGFP), or mutant C2GnT-I–EGFP (WT + mut-EGFP). (A) Relative expression of C2GnT-I in WT or mutant C2GnT-I–EGFP analyzed by real-time RT-PCR. Clones with similar levels of C2GnT-I mRNA, calculated as C2GnT-I–actin ratios, were selected for further analysis. *P < .001 for WT or mutant C2GnT-I–EGFP compared with vector alone. Values are mean ± SD of triplicates from 1 of 3 independent experiments. (B) Mutant C2GnT-I–EGFP does not decrease enzyme activity in WT clone 6 cells. Cells expressing WT C2GnT-I–EGFP had increased activity compared with WT clone 6 cells transfected with EGFP-vector alone or mutant C2GnT-I–EGFP. *P < .01. Values are mean ± SD of duplicates from 1 of 5 independent experiments. (C) Mutant C2GnT-I–EGFP does not reduce the susceptibility of WT clone 6 cells to galectin-1–induced death. (Left) Annexin V binding of WT clone 6 cells (dotted line) versus WT clone 6 cells transfected with WT C2GnT-I–EGFP (solid line). (Center) Annexin V binding of WT clone 6 cells (dotted line) versus WT clone 6 cells expressing mutant C2GnT-I–EGFP (solid line). (Right) Cell death was determined by annexin-V–allophycocyanin binding and 7-aminoactinomycin D uptake. Percentage of cell death was similar for WT clone 6 cells expressing mutant C2GnT-I–EGFP and EGFP-vector alone (P, NS), indicating that the mutant C2GnT-I–EGFP did not reduce the susceptibility to galectin-1. Cell death was significantly higher for WT clone 6 cells expressing WT C2GnT-I–EGFP (*P < .01). Values are mean ± SD for triplicates from 1 of 6 independent experiments.
The S158C mutant C2GnT-I does not act in a dominant-negative fashion. BW5147 cells expressing WT C2GnT-I (WT clone 6, from Figure 5A) were transfected again with EGFP alone (WT + vector-EGFP), WT C2GnT-I–EGFP (WT + WT-EGFP), or mutant C2GnT-I–EGFP (WT + mut-EGFP). (A) Relative expression of C2GnT-I in WT or mutant C2GnT-I–EGFP analyzed by real-time RT-PCR. Clones with similar levels of C2GnT-I mRNA, calculated as C2GnT-I–actin ratios, were selected for further analysis. *P < .001 for WT or mutant C2GnT-I–EGFP compared with vector alone. Values are mean ± SD of triplicates from 1 of 3 independent experiments. (B) Mutant C2GnT-I–EGFP does not decrease enzyme activity in WT clone 6 cells. Cells expressing WT C2GnT-I–EGFP had increased activity compared with WT clone 6 cells transfected with EGFP-vector alone or mutant C2GnT-I–EGFP. *P < .01. Values are mean ± SD of duplicates from 1 of 5 independent experiments. (C) Mutant C2GnT-I–EGFP does not reduce the susceptibility of WT clone 6 cells to galectin-1–induced death. (Left) Annexin V binding of WT clone 6 cells (dotted line) versus WT clone 6 cells transfected with WT C2GnT-I–EGFP (solid line). (Center) Annexin V binding of WT clone 6 cells (dotted line) versus WT clone 6 cells expressing mutant C2GnT-I–EGFP (solid line). (Right) Cell death was determined by annexin-V–allophycocyanin binding and 7-aminoactinomycin D uptake. Percentage of cell death was similar for WT clone 6 cells expressing mutant C2GnT-I–EGFP and EGFP-vector alone (P, NS), indicating that the mutant C2GnT-I–EGFP did not reduce the susceptibility to galectin-1. Cell death was significantly higher for WT clone 6 cells expressing WT C2GnT-I–EGFP (*P < .01). Values are mean ± SD for triplicates from 1 of 6 independent experiments.
WT clone 6 cells transfected with plasmid alone or with EGFP-tagged mutant C2GnT-I had approximately equal C2GnT enzyme activity, whereas WT clone 6 cells transfected with EGFP-tagged WT C2GnT-I had the expected increase in enzyme activity (Figure 7B). Thus, the mutant enzyme was not acting in a dominant-negative fashion to reduce the activity of WT enzyme. Similarly, 1B11 reactivity of WT clone 6 cells expressing mutant enzyme was not decreased compared with WT clone 6 cells transfected with EGFP plasmid alone, whereas additional overexpression of EGFP-tagged WT C2GnT-I in the WT clone 6 cells increased 1B11 reactivity (data not shown), confirming that mutant enzyme did not prevent CD43 modification by the WT enzyme present in WT clone 6 cells.
We examined the cells for susceptibility to galectin-1. WT clone 6 cells were susceptible to galectin-1, as in Figure 6. Expression of mutant C2GnT-I did not reduce cell death induced by galectin-1, again indicating that mutant enzyme did not have a dominant-negative effect (Figure 7C). Conversely, expression of additional WT C2GnT-I resulted in increased galectin-1 death. Thus, the S158C mutation in C2GnT-I did not appear to have a dominant-negative effect on WT enzyme activity. Rather, these data indicate that the heterozygous point mutation in C2GnT-I in HH cells resulted in haploinsufficiency of the enzyme in these cells.
Discussion
Resistance of neoplastic T cells in CTCL to agents that induce apoptosis presents a major obstacle in development of effective therapies for this disease. We have identified a novel mechanism of apoptosis resistance in cells derived from a patient with CTCL (ie, altered glycosylation resulting in loss of oligosaccharide ligands required for galectin-1–induced cell death). Galectin-1, as well as galectin-7 that also triggers T-cell death,45 is highly abundant in skin.15,16,31 Thus, T-lymphoma cells lacking saccharide ligands that promote galectin-mediated cell death would have an increased ability to survive and proliferate in skin. Moreover, lymphoma cells resistant to galectin-1 or galectin-7 cell death could exploit the metastasis-promoting activities of these 2 galectins.46,47
This study demonstrates that haploinsufficiency of a glycosyl-transferase enzyme, C2GnT-I, is responsible for galectin-1 resistance of HH cells. This suggests that C2GnT may act in the manner of tumor suppressor genes, in that loss of function resulted in resistance to apoptosis. Haploinsufficiency of some tumor suppressor genes have been described in MF/SS, including p15, p16,48 and NAV3.49 In contrast to tumor suppressors that act in the cytosol to regulate cell growth and survival, C2GnT-I is an enzyme that acts in the Golgi to modify glycoproteins destined for export to the cell surface, where glycoproteins interact with the extracellular milieu. Protein glycosylation is a complex process that is affected by glycosyltransferase abundance and activity, glycosyltransferase localization within the Golgi, and rate of glycoprotein transport through the Golgi.34-36,50 Moreover, a single glycosyltransferase can modify many different acceptor glycoproteins.51,52 Thus, modest changes in enzyme activity or localization can profoundly disrupt normal cellular glycosylation. Indeed, 30% reduction in sialyltransferase activity in activated CD8 T cells was sufficient to abolish the addition of terminal sialic acid to CD45.53 Altered expression of other glycosyltransferases, including other GlcNAc transferase enzymes,35,54-57 have been associated with resistance or susceptibility to apoptosis induced by a variety of stimuli.
Asingle missense mutation in C2GnT-I in HH cells, S158C, markedly reduced enzyme activity. How could this point mutation affect enzyme activity? C2GnT-I has 9 conserved cysteine residues, 8 of which are paired in intrachain disulfide bonds.43,44 The S158C mutation did not grossly affect the level of C2GnT-I mRNA or protein (Figure 2), nor did the mutation prevent interchain disulfide bonding required for enzyme dimerization (Figure 5). Moreover, in WT C2F cells transfected with EGFP-tagged wild-type or mutant enzyme (Figure 7), both mutant and wild-type EGFP-tagged enzyme formed dimers with wild-type enzyme (data not shown), suggesting that loss of activity was not due to altered dimerization or mislocalization in the Golgi.34,36 However, cells expressing the mutant enzyme had minimal C2GnT activity. It is possible that a cysteine at position 158, which is near the enzyme active site based on modeling studies,43 could pair with Cys217 to disrupt protein conformation near the active site. Alternatively, gross changes in enzyme conformation could occur if a tenth cysteine residue altered some or all of the other conserved disulfide bonds critical for enzyme activity. In addition, altered C2GnT activity could affect glycosylation by other enzymes in the O-glycan biosynthetic pathway.35,50 Further studies are in progress to determine the precise nature of the defect in the S158C mutant enzyme.
Our previous studies indicated that C2GnT modification of CD45 is specifically required to render cells susceptible to galectin-1, because C2GnT expression is not required for galectin-1 susceptibility of CD45– cells.26 As shown in Figure 1, HH cells express abundant CD45; thus, loss of C2GnT-I expression by CD45+ HH cells would be expected to result in resistance to galectin-1 death.
CTCL is a complex disease, with numerous factors proposed to contribute to transformation and progression.2,6-8 Mutations in several genes have been described in CTCL, so that different genotypic alterations could result in the common phenotype of apoptosis resistance. However, in 2 CTCL cell lines that we characterized, HUT78 and HH, resistance to galectin-1 could be explained by loss of CD7 expression or loss of C2GnT-I expression, respectively, and restoration of CD7 or C2GnT-I expression was sufficient to restore susceptibility to galectin-1. These findings indicate that all other components of the galectin-1 death pathway were intact in these cells and suggest that restoring CD7 expression and proper glycosylation could promote apoptotic elimination of tumor cells. Although glycosylation is proposed to control trafficking of neoplastic T cells to the skin in CTCL,58 this is the first report that altered glycosylation results in apoptosis resistance of CTCL-derived cells. In addition, this same phenotype of altered cellular glycosylation was demonstrated in primary tumor cells from another patient with CD7+ MF (Figure 1). Understanding regulation of glycosyl-transferase expression in neoplastic T cells and developing novel strategies to modify cellular glycosylation58,59 may provide new therapeutic approaches to overcome tumor cell resistance to apoptosis in this disease. Moreover, as altered glycosylation and resistance to apoptosis are hallmarks of many types of tumors, these findings may be relevant for understanding how subtle changes in glycosylation can profoundly alter tumor cell biology.
Prepublished online as Blood First Edition Paper, June 15, 2006; DOI 10.1182/blood-2006-04-018556.
Supported by grants from the Lymphoma Research Foundation (M.A. and J.S.) and the National Institutes of Health (GM63281 [L.G.B.], CA33000, and CA48737 [M.F.]).
P.V.C. and M.A. designed and performed the majority of the experiments, and P.V.C. contributed to manuscript preparation; J.M. performed all enzyme assays; J.C. was involved in patient sample selection and image analysis; J.S. identified patient samples and assisted with data collection and image analysis;M.F. participated in the experimental design and provided intellectual input; and L.G.B. directed the study, participated in the experimental design and data analysis, and contributed to manuscript preparation.
P.V.C. and M.A. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Joseph Hernandez for advice, Mabel Pang and Dorina Gui for technical assistance, Udo Dobbeling and Gunter Burg for MF cell lines, and the UCLA Jonsson Comprehensive Cancer Center Flow Cytometry Facility (National Institutes of Health [NIH] CA16042 and AI28697).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal