Abstract
Mice lacking interleukin-7 (IL-7–/– mice) have no signs of autoimmune disease, contrary to other models of lymphopenia. We investigated whether the absence of disease was due to the fact that IL-7 is dispensable for the ontogeny, function, and homeostasis of regulatory CD4+ T cells. We show here that the establishment of the peripheral pool of Foxp3-expressing regulatory cells is IL-7 independent, and the premature involution of the thymus in IL-7–/– mice does not change the representation of the CD4+CD25+ T-cell compartment. In addition, CD4+CD25+ T cells expand in the absence of IL-7, without losing Foxp3 expression. The frequency of activated peripheral CD4+ T cells increases with age in both the CD25– and CD25+ compartments, with the CD4+CD25+ T cells displaying signs of constant activation. IL-7–/– CD4+CD25+ T cells control inflammatory bowel disease induced by IL-7–/– T cells even in hosts lacking IL-7. Depletion of the CD25+ T-cell subset after thymic involution results in a mild form of inflammatory bowel disease (IBD), which resolves concomitantly with the regeneration of this subset. This study shows for the first time that IL-7–/– mice have a robust regulatory Foxp3-expressing CD4+ T-cell compartment that controls T-cell–mediated disease. It also highlights the potential of the regulatory Foxp3-expressing CD4+CD25– T-cell population to restore a functional CD4+CD25+ T-cell compartment through an IL-7–independent pathway.
Introduction
In healthy individuals, the number of peripheral T lymphocytes is tightly regulated and remains stable throughout life. The mechanisms responsible for this homeostatic control of T-cell numbers are multiple and not yet entirely understood. Survival, expansion, and death of lymphocytes are dependent on both cell-mediated signals and cell-mediated cytokines (for a review, see Freitas and Rocha1 ). Among the latter, γ chain–dependent cytokines are involved in the development and homeostasis of both naive2,3 and memory T-cell pools.4,5 In particular, the lifespan of naive T cells is drastically reduced in the absence of IL-7,6,7 and this interleukin is involved in the generation and survival of memory CD4+ T cells.8 IL-7 is produced by bone marrow and thymic stromal cells and is essential for murine T and B lymphopoiesis.9,10 The thymic cellularity of IL-7–/– mice is drastically reduced (20-fold) due to a blockage at the DN2 to DN3 transition,11 although the distribution of the major thymocyte subsets is normal.9 In the periphery, the number of splenic T and B cells is 10- and 20-fold reduced, respectively, compared with wild-type animals.9
It is known that severe lymphopenia can be associated with lymphoproliferation of both naive and memory T cells.2,12,13 Lymphopenia can also lead to immune dysregulation resulting in autoimmune disorders14,15 and other inflammatory diseases (eg, inflammatory bowel disease [IBD]).16,17 It is also well documented that in some such cases, disease is due to a deficit in numbers or in the function of natural regulatory Foxp3-expressing CD4+CD25+ T cells.18 This specialized subset of lymphocytes is essential for the maintenance of peripheral tolerance to self antigen,19,20 in the regulation of adaptative21,22 and innate immune response against pathogens23 and in the control of peripheral lymphocyte numbers.24
Despite severe lymphopenia, IL-7–/– mice are healthy and display no overt signs of autoimmune disease. One possible explanation is that IL-7 is dispensable for the development, homeostasis, and function of regulatory Foxp3-expressing CD4+ T cells. Another, nonexclusive possibility is that IL-7 is required for the development of efficient immune responses.
We show here that the development and the establishment of the peripheral pool of Foxp3-expressing regulatory CD4+ T cells is IL-7 independent. In spite of lymphopenia and early thymic involution, the size of the splenic CD4+ T-cell compartment and the relative frequency of Foxp3-expressing CD4+CD25+ T cells remain constant up to 28 weeks of age. At this time point essentially all CD4+ T cells are activated in both the CD25– and the CD25+ compartments. Of interest, depletion of the CD4+CD25+ T-cell subset in IL-7–/– mice, after thymic involution, results in mild IBD, which resolved concomitantly with the regeneration of this subset. This indicates that there is a peripheral, IL-7–independent pathway that is able to generate regulatory CD25+ T cells from CD25– precursors (possibly Foxp3-expressing regulatory CD4+CD25– T cells that are well represented in IL-7–/– mice). This study shows for the first time that IL-7–/– mice have a robust regulatory Foxp3-expressing CD4+ T-cell compartment that controls T-cell–mediated disease. Furthermore, it also highlights the potential of the regulatory CD4+CD25– T-cell population to restore a functional CD4+CD25+ T-cell compartment.
Materials and methods
Mice
C57Bl/6 mice were obtained from Charles River (L'Arbresle, France) and Rag-2–deficient (Rag-2–/–) C57/Bl6 mice from CDTA (Orléans, France). IL-7–/– mice were bred as described before9 and Rag–/– IL-7–/– mice came from our own production. Mice were bred in isolators and maintained in microisolator cages with filtered air in our animal facilities under specific pathogen-free conditions. All animal experiments were done in accordance with the guidelines of the Institut Pasteur, which are approved by the French Ministry of Agriculture.
Antibodies and flow cytometric analysis
The following mAbs (Pharmingen, San Diego, CA) were used: anti-αβ–FITC or -biotin (H57); anti-CD25–PE (PC61 or 7D4); anti-CD25–FITC or -PE-Cy7 or -biotin (7D4); anti-CD4–APC, –PE, –PE-Cy7, or –FITC (L3T4); anti-CD8–APC; anti-CD69–biotine (H1.2F3); anti-CD45RB–PE (16A); anti-CD62L–biotin (MEL-14); anti-CD44–FITC (IM7); anti-CD103–biotin (M290); and anti-Ly5.1–FITC. Biotinylated Abs were revealed with streptavidin (Sav)–FITC, –PE, –APC, –PE-Cy7, or –cychrome. PE anti-FOXP3 antibody (clone FJK-16s) was purchased from Bioscience (San Diego, CA) and used according to the manufacturer's protocol. Cell suspensions were analyzed using an LSR flow cytometer and FlowJo software (Treestar, Ashland, OR).
Cell preparations
For cell transfers, splenocytes were first enriched for CD4+ T cells by magnetic sorting using L3T4 MicroBeads (Miltenyi Biotec, Bergisch-Gladsbach, Germany) for 20 minutes at 4°C in PBS–1% FCS. The cells were passed through a magnetic-activated cell sorting (MACS) separation column (Miltenyi Biotech). The positive fraction was stained with anti-αβ –FITC, anti-CD4–APC, anti-CD25–PE, and anti-CD103-biotine, revealed with Sav-PE-Cy7. The different subsets of interest were then sorted on a MOFLO (Cytomation, Fort Collins, CO): CD4+CD25+ as well as CD4+CD25–CD103+ T cells. The purity of the cells was always more than 95%.
Cell transfers
Rag-2–/– mice and Rag-2–/– IL-7–/– mice were injected intravenously with 3 × 105 CD4+CD25–CD45RBhighCD103–C57Bl/6 donor cells alone or with either 3 × 105 CD4+CD25+ or CD4+CD25–CD103– donor cells from C57Bl/6 or IL-7–/– origin. Weight of recipient mice was scored twice a week, and the animals were analyzed when they had lost at least 20% of the initial body weight or when severe signs of inflammation (diarrhea, prolapsus) were apparent. All the other mice were analyzed 2 or 3 months after injection. The 2 independent experiments used donors and recipients that were kept in the same animal facilities.
In vivo depletion of CD25+ cells
Mice received 100 μg monoclonal antibody to CD25 (clone PC61) injected daily during 10 days as has been already published.25 Blood was obtained from mice and analyzed to confirm elimination of CD4+CD25+ T cells. All mice used in the depletion experiments were older than 16 weeks of age.
Microscopic examination
Colons and intestines were removed from mice and fixed in AFA (750 mL absolute alcohol, 200 mL 40%-formalin, and 50 mL acetic acid). Paraffin-embedded sections (5-μm each) were cut and stained with hematoxylin and eosin. Stained sections were analyzed in a Leitz DMRD 301 microscope (Leitz, Clichy, France) at a resolution of 0.15 and a magnification of 50× (Figures 6F and 7) or a resolution of 0.30 and a magnification of 100× (Figure 6B-E). Pictures were taken with a Sony DONPISHA XC-003P camera (Sony, New York, NY) and analyzed with Samba TPS version 5.03 software (Samba Technology, Meylan, France). Tissues were graded semiquantitatively from 0 to 5 in a blinded fashion by one of us (F.M.). A grade of 0 was given when no changes were observed. Changes typically associated with other grades are as follows: grade 1, minimal scattered mucosal inflammatory cell infiltrates, with or without minimal epithelial hyperplasia; grade 2, mild scattered to diffuse inflammatory cell infiltrates, sometimes extending into the submucosa and associated with erosions, with minimal to mild epithelial hyperplasia and minimal to mild mucin depletion from goblet cells; grade 3, mild to moderate inflammatory cell infiltrates that were sometimes transmural, often associated with ulceration, with moderate epithelial hyperplasia and mucin depletion; grade 4, marked inflammatory cell infiltrates that were often transmural and associated with ulceration, with marked epithelial hyperplasia and mucin depletion; and grade 5, marked transmural inflammation with severe ulceration and loss of intestinal glands.
Foxp3-expression analysis by real-time reverse-transcriptase–polymerase chain reaction (RT-PCR)
RNA from sorted cells was extracted using RNAPlus extraction solution (QBiogen, Illkirch, France) according to the manufacturer's instructions. Extracted RNA was then incubated at 70°C for 10 minutes with oligodT (Pharmacia Biotech, Piscataway, NY), and the first-strand cDNA was synthesized using SuperScriptII RNaseH– Reverse Transcriptase (Invitrogen, Carlsbad, CA) at 37°C for 50 minutes. The reaction was stopped by incubation at 90°C for 5 minutes. The relative Foxp3 expression was determined as already described.26 In brief, Foxp3 mRNA was measured using an Applied Biosystems 7000 Sequence detection System (PE Applied Biosystems, Foster City, CA) with the primers 5′-GGCCCTTCTCCAGGACAGA-3′ and 5′-GCTGATCATGGCTGGGTTGT-3′ (300 nM) and the internal TaqMan probe 5′-FAM-ACTTCATGCATCAGCTCTCCACTGTGGAT-TAMRA-3′ (100 nM). Dad1 was used as endogenous reference and the mRNA measured using the primers 5′-CCTCTCTGGCTTCATCTCTTGTGT-3′ and 5′-CCGGAGAGATGCCTTGGAA-3′ (50 nM) and the internal TaqMan probe 5′-FAM-AGCTTCATCCTAGCGGTTTGCCTGAGAATAC-TAMRA-3′ (100 nM). The TaqMan Universal Master Mix (PE Applied Biosystems) was used and PCR cycling conditions were 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. The reference cDNA sample used to make standard curves was obtained from sorted αβ+CD25+CD4+ splenocytes. The relative expression of Foxp3 was obtained by dividing the relative quantity of Foxp3 mRNA by the relative quantity of Dad1 mRNA and multiplying the result by 100.
In vitro suppression assay
Cultures were set up in triplicates in 96-well round-bottom plates (Costar, Cambridge, MA) in a total volume of 200 μL. Cells were cultured in RPMI 1640 medium with 10% FCS, 10 mM Hepes, 1% nonessential amino acids, 2mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (all from GIBCO BRL, Grand Island, NY), and 5 × 10–5 M 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO). Responder cells (2.5 × 104; sorted CD4+CD25–CD45RBhigh) and 105 irradiated stimulator cells were mixed with variable numbers of sorted CD4+CD25+ T cells (2.5 to 0.32 × 104) to obtain the indicated ratios. Cells were stimulated with 0.5 μg/mL anti-CD3 (145-2C11; BD Biosciences, San Jose, CA). Proliferation was assessed after 3 days by pulsing the cells with 1 μCi (0.037 MBq)/well [3H] thymidine (Amersham Biosciences, Freiburg, Germany) for the last 16 hours. Cells were harvested onto filter membranes using a Wallac harvester (Perkin-Elmer, Shelton, CT), and the amount of incorporated [3H] thymidine was measured with a Wallac Betaplate counter (Perkin-Elmer).
Statistical analysis
Analyses were performed using the unpaired Student t test. Differences were considered significant when P values were less than .05.
Results
Foxp3 expression and regulatory function in CD4+CD25+ cells in the thymus and spleen of IL-7–/– mice
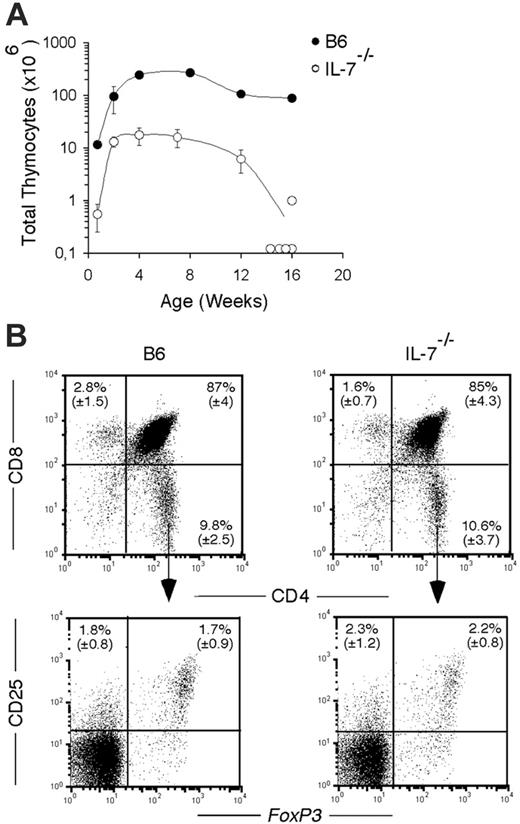
We investigated the consequences of the absence of IL-7 on the development of the thymic and splenic CD4+ Foxp3+ T-cell populations. In agreement with previous observations,9 we found 15-fold fewer thymocytes in IL-7–/–, from birth to 12 weeks, compared with control B6 mice (Figure 1A). Thymic involution was already apparent by 12 weeks in this mouse, and was complete in all but 1 of 5 animals analyzed at 16 weeks (Figure 1A). As is the case for the major thymocyte subsets (DN, DP, CD4+ SP, and CD8+ SP cells), the frequency of CD25+ T cells, as well as the frequency of Foxp3+ T cells, within the CD4 SP subset was similar between IL-7–/– mice and controls (Figure 1B). Thus, the absence of IL-7 has no particular effect on the development of CD4+ FOXP3+ thymocytes.
In the spleen of IL-7–/– mice, the number of CD4+ T cells is 7-fold reduced from day 5 after birth to 28 weeks of age, compared with age-matched wild-type mice, and reaches a plateau at 7 to 8 weeks of age (data not shown). At all time points analyzed, the proportion of CD25+ Foxp3+ T cells, within the CD4+ T-cell compartment, was comparable between IL-7–/– and wild-type mice (Figure 2A). This was confirmed by the analysis of the expression of Foxp3 mRNA in sorted thymic and splenic CD4+CD25+ T cells. The splenic CD25+ subset of both wild-type and IL-7–/– mice expressed comparable amounts of Foxp3 mRNA (not shown).
Thymic development in IL-7–/– mice. (A) Total number of thymocytes in B6 (•) and IL-7–/– (○) mice at the indicated ages after birth. Mean (± SD) from groups of 3 to 6 mice per time point is shown, except at 16 weeks where individual mice are depicted. (B) Flow cytometric analysis of thymocytes from B6 (left panels) and IL-7–/– (right panels) mice stained with CD8 and CD4 (top panels) at 8 weeks of age. Bottom panels show the coexpression of CD25 and FOXP3 in gated CD4+ SP subset. The mean (± SD) of groups of 3 to 6 mice is shown in the indicated quadrants.
Thymic development in IL-7–/– mice. (A) Total number of thymocytes in B6 (•) and IL-7–/– (○) mice at the indicated ages after birth. Mean (± SD) from groups of 3 to 6 mice per time point is shown, except at 16 weeks where individual mice are depicted. (B) Flow cytometric analysis of thymocytes from B6 (left panels) and IL-7–/– (right panels) mice stained with CD8 and CD4 (top panels) at 8 weeks of age. Bottom panels show the coexpression of CD25 and FOXP3 in gated CD4+ SP subset. The mean (± SD) of groups of 3 to 6 mice is shown in the indicated quadrants.
Splenic CD4+CD25+ T cells in IL-7–/– mice. (A) Flow cytometric analysis of CD25 and FOXP3 expression on gated CD4+ T cells in splenic B6 (left panel) and IL-7–/– (right panel) mice at 8 weeks of age. Mean (± SD) from groups of 3 to 6 mice is indicated. (B) Total number of splenic CD4+CD25+ T cells in B6 (•) and IL-7–/– (○) mice is represented at the indicated ages. Diamonds represent the number of CD4+ FOXP3+ T cells. Mean (± SD) from groups of 3 to 6 mice per time point is shown.
Splenic CD4+CD25+ T cells in IL-7–/– mice. (A) Flow cytometric analysis of CD25 and FOXP3 expression on gated CD4+ T cells in splenic B6 (left panel) and IL-7–/– (right panel) mice at 8 weeks of age. Mean (± SD) from groups of 3 to 6 mice is indicated. (B) Total number of splenic CD4+CD25+ T cells in B6 (•) and IL-7–/– (○) mice is represented at the indicated ages. Diamonds represent the number of CD4+ FOXP3+ T cells. Mean (± SD) from groups of 3 to 6 mice per time point is shown.
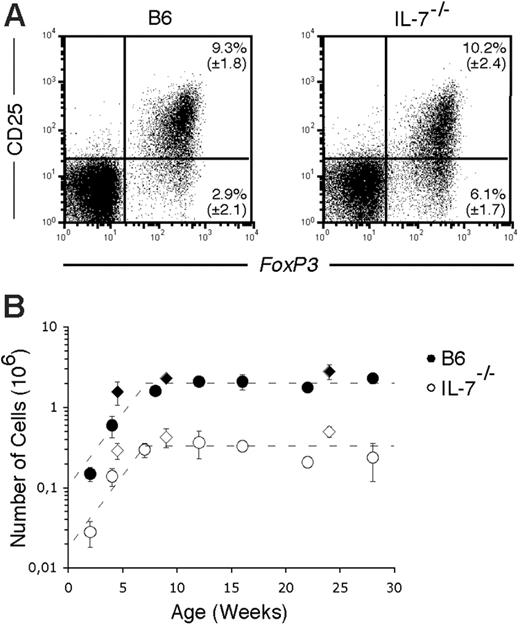
In consequence, numbers of splenic CD4+CD25+ and Foxp3+ CD4+ T cells in IL-7–/– mice are constant from 7 weeks of age onward, although always reduced compared with age-matched wild-type mice (Figure 2B).
Wild-type CD4+CD25+ T cells transferred into nonirradiated Rag-2–/– recipients can survive and expand in the absence of IL-7 (not shown). In addition, as can be seen in Figure 3, transfer of CD4+CD25+ from wild-type mice (of which more than 98% express Foxp3; Figure 2A) into mice lacking IL-7 results in the expansion of a population composed of more than 99% Foxp3+ cells (Figure 3).
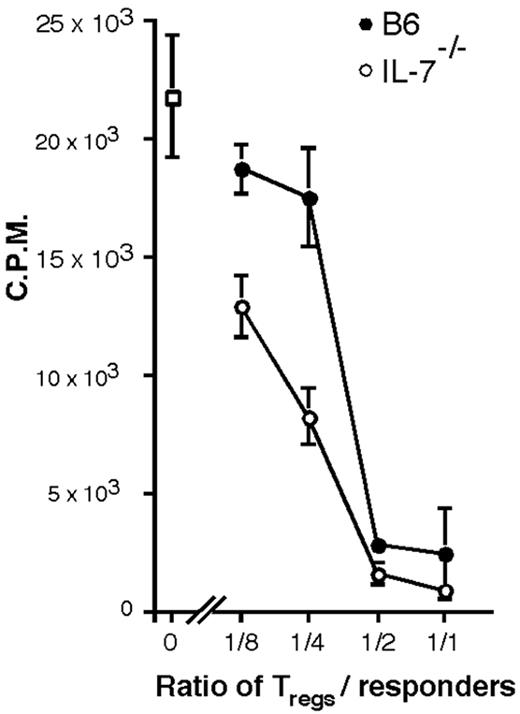
The regulatory function of the splenic CD4+CD25+ T-cell compartment from IL-7–/– mice was confirmed by their ability to efficiently suppress the in vitro proliferation of naive T cells (Figure 4) and to control T-cell–mediated IBD in transfer experiments using syngeneic RAG–/– mice as recipients (Figure 5).
In conclusion, the absence of IL-7 does not specifically affect the peripheral CD4+CD25+ T-cell compartment. The CD4+CD25+ T cells that develop in the absence of IL-7 are indeed regulatory T cells, as shown both by Foxp3 expression and by their regulatory function in vitro and in vivo.
Maintenance of FoxP3 expression in the absence of IL-7. Flow cytometric analysis of CD25 and FOXP3 expression in gated Ly5.1+ cells, 2.5 weeks after transfer of sorted splenic CD4+CD25+ cells into irradiated Rag-2–/– (left panel) and IL-7–/– (right panel) mice. A representative flow cytometric analysis of a group of 4 mice is shown.
Maintenance of FoxP3 expression in the absence of IL-7. Flow cytometric analysis of CD25 and FOXP3 expression in gated Ly5.1+ cells, 2.5 weeks after transfer of sorted splenic CD4+CD25+ cells into irradiated Rag-2–/– (left panel) and IL-7–/– (right panel) mice. A representative flow cytometric analysis of a group of 4 mice is shown.
In vitro suppression assay. Inhibition of proliferation of naive T cells stimulated with α-CD3 and APC, by CD4+CD25+ T cells isolated from B6 (•) or IL-7–/– (○) mice, at the indicated ratios. (□) indicates naive T cells alone. Bars indicate the standard deviation of triplicate values.
In vitro suppression assay. Inhibition of proliferation of naive T cells stimulated with α-CD3 and APC, by CD4+CD25+ T cells isolated from B6 (•) or IL-7–/– (○) mice, at the indicated ratios. (□) indicates naive T cells alone. Bars indicate the standard deviation of triplicate values.
The pool of activated splenic CD4+CD25– and CD25+ T cells increases with age in IL-7–/– mice
IL-7–/– mice are lymphopenic,9 a situation that is usually accompanied by activation and homeostatic expansion of the peripheral T cells.27 Moreover, IL-7 is necessary for the survival of naive T cells.6 One would expect therefore that the CD4+ T-cell compartment in IL-7–/– mice would be composed mainly of activated/memory T cells. We compared the proportions of splenic naive and activated/memory CD4+ T cells in IL-7–/– and wild-type animals at distinct time points and simultaneously analyzed the phenotype of the CD4+CD25+ T cells.
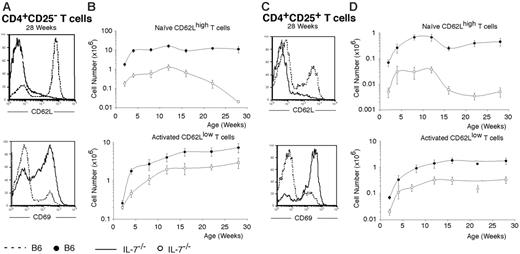
Expression of activation markers in IL-7–/– T cells. (A) Representative flow cytometric analysis of the expression of CD62L (top panel) and CD69 (bottom panel) in splenic CD4+CD25– T cells of B6 (dashed line) and IL-7–/– (bold line) mice at 28 weeks of age. (B) Absolute numbers of naive (top panel) and activated (bottom panel) splenic CD4+CD25– T cells in B6 (•) and IL-7–/– (○) mice at the indicated ages. Mean (± SD) from groups of 3 to 6 mice per time point is shown. (C) Representative flow cytometric analysis for CD62L (top panel) and CD69 (bottom panel) of splenic CD4+CD25+ T cells in B6 (dashed line) and IL-7–/– (bold line) mice at 28 weeks of age. (D) Absolute numbers of resting (top panel) and activated (bottom panel) splenic CD4+CD25+ T cells in B6 (•) and IL-7–/– (○) mice are represented at the indicated ages. Mean (± SD) of groups of 3 to 6 mice per time point is shown. The differences between the number of control and IL-7–/– cells were statistically significant (P < .05) for all age groups, in all cases.
Expression of activation markers in IL-7–/– T cells. (A) Representative flow cytometric analysis of the expression of CD62L (top panel) and CD69 (bottom panel) in splenic CD4+CD25– T cells of B6 (dashed line) and IL-7–/– (bold line) mice at 28 weeks of age. (B) Absolute numbers of naive (top panel) and activated (bottom panel) splenic CD4+CD25– T cells in B6 (•) and IL-7–/– (○) mice at the indicated ages. Mean (± SD) from groups of 3 to 6 mice per time point is shown. (C) Representative flow cytometric analysis for CD62L (top panel) and CD69 (bottom panel) of splenic CD4+CD25+ T cells in B6 (dashed line) and IL-7–/– (bold line) mice at 28 weeks of age. (D) Absolute numbers of resting (top panel) and activated (bottom panel) splenic CD4+CD25+ T cells in B6 (•) and IL-7–/– (○) mice are represented at the indicated ages. Mean (± SD) of groups of 3 to 6 mice per time point is shown. The differences between the number of control and IL-7–/– cells were statistically significant (P < .05) for all age groups, in all cases.
The percentage of activated CD4+CD25– T cells increases markedly with age in IL-7–/– mice. At 28 weeks, more than 90% of the CD4+CD25– cells are CD62Llow in IL-7–/– mice, compared with only 30% in wild-type mice (Figure 5A). The high frequency of CD69+ cells (66% in IL-7–/– versus 16% in wild-type mice) indicates that the majority of these cells are continuously activated. The absolute number of naive (CD62Lhigh) CD4+CD25– T cells is always 10-fold reduced in IL-7–/– mice until 12 to 15 weeks. After this time point, when thymus involution is essentially complete (Figure 1), the number of naive T cells decreases progressively (Figure 5B). By contrast, the number of activated/memory T cells increases continuously with age and IL-7–/– mice have only 3-fold fewer of these cells than wild-type controls by the age of 28 weeks.
It has been recently described that in healthy adults, resting CD4+CD25+ T cells are CD62Lhigh, CD45RBint/low, and CD69– and, upon activation, acquire CD69, up-regulate CD44, and down-regulate CD62L.28 In IL-7–/– mice, we observed that more than 80% of CD4+CD25+ T cells express low levels of CD62L but only 60% in wild-type mice at 28 weeks of age (Figure 5C). As for CD4+CD25– cells, a much higher frequency of CD4+CD25+ T cells express CD69 in IL-7–/– mice than in wild-type mice (80% versus 22%, respectively). These results indicate that a vast majority of CD4+CD25+ T cells are constantly activated in IL-7–/– mice. In absolute numbers, “resting” (CD62Lhigh) CD4+CD25+ T cells were 10-fold reduced in IL-7–/– mice compared with wild type until 12 weeks (data not shown) and decreased sharply after thymic involution (Figure 5D). In contrast, the number of “activated” (CD62Llow) CD4+CD25+ T cells increased steadily from birth to 12 weeks, reaching a plateau at this age (Figure 5D), corresponding to a 6-fold reduction, in number, compared with wild-type animals.
Taken together, the results above indicate that the frequency of activated T cells increases with age in both the CD25– and CD25+CD4+ T-cell compartment in the absence of IL-7. It also highlights the high level of activation of CD4+CD25+ T cells, this compartment being constantly activated in this mouse.
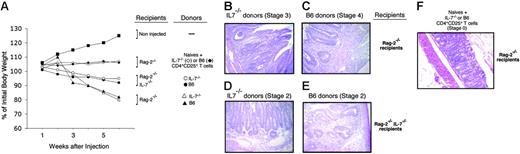
IBD-inducing potential from naive IL-7–/– CD4+ T cells. (A) Weight loss expressed as a percentage of initial body weight at the indicated times after injection of naive CD4+ T cells (groups of 3-5 mice). (▪) indicates noninjected mice; (♦, ⋄), Rag-2–/– recipients of mixtures of naive CD4 cells with B6 or IL-7–/– CD4+CD25+ T cells, respectively; (•, ○), Rag-2–/– IL-7–/– recipients of naive wild-type or IL-7–/– mice, respectively; (▴, ▵) Rag-2–/– recipients of naive wild-type or IL-7–/– mice, respectively. (B-E) H&E histologic analysis of the colon of recipient mice. (B) Naive IL-7–/– T cells transferred into Rag-2–/– recipients. Inflammatory cells largely infiltrate into the submucosa and partially into the muscularis with microabscess and mucin depletion (stage 3). (C) Naive wild-type T cells transferred into Rag-2–/– recipients. Transmural infiltration with marked inflammatory cells, epithelial erosion, and mucin depletion (stage 4). (D) Naive IL-7–/– wild-type T cells transferred into Rag-2–/– IL-7–/– recipients. Diffuse inflammatory infiltration into the submucosa with moderate mucine depletion (stage 2). (E) Naive wild-type T cells transferred into Rag-2–/– IL-7–/– recipients. Diffuse inflammatory infiltration into the submucosa with moderate mucine depletion (stage 2). The lesions are less severe with wild-type donors (E). (F) Naive wild-type CD4+ T cells transferred with IL-7–/– CD4+CD25+ T cells. No infiltration is observed (stage 0).
IBD-inducing potential from naive IL-7–/– CD4+ T cells. (A) Weight loss expressed as a percentage of initial body weight at the indicated times after injection of naive CD4+ T cells (groups of 3-5 mice). (▪) indicates noninjected mice; (♦, ⋄), Rag-2–/– recipients of mixtures of naive CD4 cells with B6 or IL-7–/– CD4+CD25+ T cells, respectively; (•, ○), Rag-2–/– IL-7–/– recipients of naive wild-type or IL-7–/– mice, respectively; (▴, ▵) Rag-2–/– recipients of naive wild-type or IL-7–/– mice, respectively. (B-E) H&E histologic analysis of the colon of recipient mice. (B) Naive IL-7–/– T cells transferred into Rag-2–/– recipients. Inflammatory cells largely infiltrate into the submucosa and partially into the muscularis with microabscess and mucin depletion (stage 3). (C) Naive wild-type T cells transferred into Rag-2–/– recipients. Transmural infiltration with marked inflammatory cells, epithelial erosion, and mucin depletion (stage 4). (D) Naive IL-7–/– wild-type T cells transferred into Rag-2–/– IL-7–/– recipients. Diffuse inflammatory infiltration into the submucosa with moderate mucine depletion (stage 2). (E) Naive wild-type T cells transferred into Rag-2–/– IL-7–/– recipients. Diffuse inflammatory infiltration into the submucosa with moderate mucine depletion (stage 2). The lesions are less severe with wild-type donors (E). (F) Naive wild-type CD4+ T cells transferred with IL-7–/– CD4+CD25+ T cells. No infiltration is observed (stage 0).
CD4+ T cells from IL-7–/– mice have the potential to induce IBD
IL-7–/– mice remain healthy although their peripheral CD4+ T-cell compartments are fully activated. This suggests that ongoing immune responses, either to self or to environmental antigens, are controlled to prevent immunopathology. Another nonexclusive possibility is that pathogenic T cells might be unable to induce disease in the IL-7–deficient situation.
We thus assessed whether IL-7–/– mice contained cells capable of inducing IBD and to what extent the severity of this disease would be dependent on IL-7. Sorted IL-7–/– or wild-type naive (CD45RBhigh) CD4+ T cells were injected into syngeneic Rag-2–/– or Rag-2–/– IL-7–/– recipients. Six weeks later, IL-7–/– and wild-type donor CD4+ T-cell populations had expanded in both hosts, although to a significantly lower extent in IL-7–/––deficient recipients (data not shown).
All recipients developed signs of wasting disease, as illustrated by weight loss (Figure 6A). Histologic analysis of the colon of the hosts revealed a severe disease in Rag-2–/– recipients (stages 3 and 4 in Figure 6B and 6C, respectively), and milder signs of IBD in Rag-2–/– IL-7–/– recipients (never more severe than stage 2, Figure 6D-E). For comparison, histologic analysis of Rag2–/– recipients of B6 naive CD4+ cells with IL-7–/– CD4+CD25+ T cells (stage 0) is represented (Figure 6F).
In conclusion, naive T cells from IL-7–/– mice are able to induce disease even in the absence of IL-7, although in this case, the severity of IBD is reduced. Furthermore, we also show in Figure 5 that regulatory T cells from IL-7–/– and wild-type mice inhibit IBD equally well.
Depletion of CD25+ T cells causes transient IBD in IL-7–/– mice
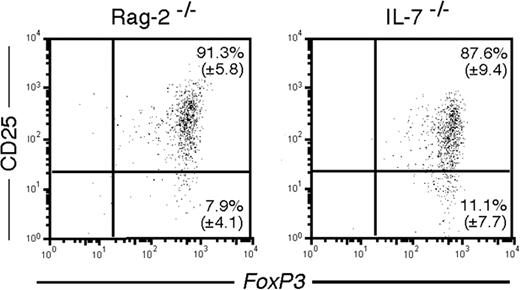
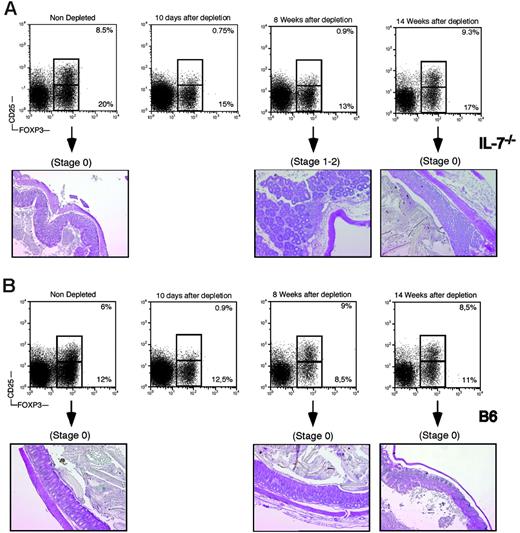
To directly assess the role of the CD4+CD25+ regulatory T cells in the control of immunopathology in the IL-7–/– mice, we depleted this compartment in animals more than 16 weeks old by 10 daily injections of PC61mAb.25 Ten days after the last injection, all animals had less than 1% of circulating (not shown) and splenic CD4+CD25+ T cells and were essentially devoid of CD25high T cells (Figure 7), as previously reported.25 Four weeks after the last injection, circulating CD4+CD25+ T cells could be detected in wild-type mice, and their frequency and absolute number were back to control levels 8 weeks after treatment (Figure 7B), as also observed by others.29 However, in IL-7–/– mice, the number of circulating CD4+CD25+ T cells stayed below 1% in the blood (not shown) or spleen for at least 8 weeks after the last Ab injection (Figure 7A). At this time point, depleted IL-7–/– mice had no more than 3 × 104 CD4+CD25+ T cells in the spleen, whereas nondepleted IL-7–/– animals had 16 × 104 (data not shown). CD4+CD25+ T cells could first be detected in IL-7–/– mice at 10 weeks after treatment (data not shown), and a full recovery of this compartment was observed only after 14 weeks (Figure 7A).
None of the depleted animals suffered weight loss, rectal prolapse, diarrhea, or cutaneous manifestations of inflammation (data not shown), signs usually associated with lack of regulatory T cells.30-32 However, the colons of all depleted IL-7–/– mice were enlarged and histologic analysis revealed typical signs of mild IBD (Figure 7A, 8 weeks after depletion). None of the age-matched, nondepleted IL-7–/– mice or the depleted wild-type control mice had any histologic signs of disease (Figure 7). Fourteen weeks after depletion, the number of CD4+CD25+ T cells was back to normal and histologic analysis of the colon and of the intestine did not reveal any lesions (Figure 7A).
In conclusion, the CD4+CD25+ T-cell compartment of IL-7–/– mice actively prevents the development of inflammatory disease in the gut of IL-7–/– mice. Of importance, the peripheral CD4+ pool is able to regenerate a CD4+CD25high T-cell compartment as seen before,29 in a context of lymphopenia and in the absence of IL-7.
Discussion
In this work, we show that IL-7 is dispensable for the development of CD4+CD25+ Foxp3-expressing regulatory T cells. In IL-7–/– mice, there is a 10- to 20-fold reduction in the number of thymocytes9 and the same reduction in the number of CD4+CD25+ Foxp3+ T cells. As a consequence, the proportion of CD4+CD25+ Foxp3+ T cells is maintained, relative to the other major thymocyte subsets. After 12 to 15 weeks, there is a premature involution of the thymus in IL-7–/– mice, while in control mice such involution is observed only much later. In the IL-7–/– spleen, we found a normal proportion of CD25+ Foxp3-expressing T cells within the CD4+ compartment. This indicates that CD4+CD25+ T cells are produced and exported from the thymus and shows that they are maintained in the periphery in the absence of IL-7. Indeed, CD4+CD25+ T cells survive and expand when transferred into IL-7–/– lymphopenic mice, maintaining Foxp3 expression. Moreover, they control T-cell–mediated IBD in vivo in cotransfer experiments, and their depletion, in otherwise intact IL-7–/– animals, leads to the appearance of an inflammatory bowel syndrome. Therefore, we conclude that the absence of IL-7 does not interfere with the regulatory function of Foxp3-expressing cells. Thus, the γc dependency of regulatory T cells33 is due to cytokines other than IL-7.
In vivo depletion of CD25+ T cells in IL-7–/– and wild-type mice. (A) IL-7–/– mice were depleted of CD25+ T cells by 10 daily injections of anti-CD25 antibodies. (Top panels) Flow cytometric analysis of CD25 and Foxp3 expression in gated CD4 cells in the spleen of depleted mice. (Bottom panels) Histologic analysis of the colon of mice at the indicated times before or after depletion. (B) B6 mice were depleted of CD25+ T cells by 10 daily injections of anti-CD25 antibodies. (Top panels) Flow cytometric analysis of CD25 and Foxp3 expression in gated CD4 cells in the spleen of depleted mice. (Bottom panels) Histologic analysis of the colon of mice at the indicated times before or after depletion.
In vivo depletion of CD25+ T cells in IL-7–/– and wild-type mice. (A) IL-7–/– mice were depleted of CD25+ T cells by 10 daily injections of anti-CD25 antibodies. (Top panels) Flow cytometric analysis of CD25 and Foxp3 expression in gated CD4 cells in the spleen of depleted mice. (Bottom panels) Histologic analysis of the colon of mice at the indicated times before or after depletion. (B) B6 mice were depleted of CD25+ T cells by 10 daily injections of anti-CD25 antibodies. (Top panels) Flow cytometric analysis of CD25 and Foxp3 expression in gated CD4 cells in the spleen of depleted mice. (Bottom panels) Histologic analysis of the colon of mice at the indicated times before or after depletion.
We show for the first time that the phenotype of the CD4+CD25+ T cells found in IL-7–/– mice is markedly different from those of wild-type mice. In IL-7–/– mice, at 28 weeks of age, the vast majority of CD4+CD25+ T cells express low levels of CD62L and, an indication of constant activation, they are CD69+. This phenotype is the result of activation and proliferation of the regulatory CD4+ T cells and was proposed to correspond to an “effector/memory” phenotype in the regulatory CD4+ T-cell compartment.28 The number of resting CD4+CD25+ T cells decreases substantially after 12 weeks of age, coinciding with the involution of the thymus. This indicates that in the absence of continuous thymic output, resting CD4+CD25+ T cells need IL-7 for survival, similarly to what was observed with all naive T cells.6 It is striking to note the parallel in the disappearance of naive and resting CD4+CD25+ T cells after thymic involution. In contrast, the number of activated CD4+CD25+ T cells progressively increases with age, again with the same kinetic of the activated/memory nonregulatory CD4+ T cells. Since previous studies have shown that the suppressive function of CD4+CD25+ T cells requires activation,34 we suggest that the peripheral compartment of CD4+CD25+ T cells in IL-7–/– mice is highly active. This would readily explain the 2- to 3-fold increase in the capacity of IL-7–/– CD4+CD25+ T cells to suppress the in vitro proliferation of naive CD4+ T cells.
Contrasting with the continuous activation of peripheral CD4+ T cells, IL-7–/– mice remain healthy in the context of lymphopenia. It is known that, in mice, IL-7 can exacerbate chronic colitis with expansion of IL-7Rhigh memory CD4+ mucosal T cells in mice.35 It is also known that IL-7 deficiency prevents the development of a Helicobacter hepaticus–dependent, non-T-cell–mediated, non-B-cell–mediated colitis.36 It is important to note in this context that our mice are colonized by H hepaticus, a bacteria known to be associated with colitis in immunodeficient mice.37 The absence of signs of wasting disease or IBD in our IL-7–/– mice could thus be explained by a crucial role of IL-7 in the development of the disease, by the absence of pathogenic T cells, or by the presence of sufficient numbers of regulatory T cells (Tregs) to control the disease. We show here that IL-7–/– donor T cells are able to induce disease in a transfer system, even when the recipient mice lack IL-7. Thus, IL-7–/– animals contain potentially pathogenic T cells, although the lesions are less severe when the recipients lack IL-7. This shows that the absence of IL-7 does not affect the development of the disease but has an impact on its severity. It also underlines the presence of an active regulatory T-cell compartment to prevent immune-mediated disease in the context of lymphopenia in IL-7–/– mice.
Treatment of IL-7–/– mice with a CD25-depleting mAbs for 10 days leads to the appearance of typical, albeit mild, signs of IBD. This demonstrates the active role played by CD4+CD25+ T cells in the lymphopenic environment of the IL-7–/– mouse, since nondepleted animals never develop disease. A normal representation of CD4+CD25+ T cells is observed, coinciding with the disappearance of the signs of IBD, 3 to 4 months after depletion. This is not due to thymic output because the depletion was performed after thymic involution, although thymic output can play a role in the recovery of CD4+CD25+ T cells since its kinetics are accelerated in wild-type mice. At any rate, the CD4+CD25– T-cell compartment contains Foxp3-expressing regulatory cells, and it has been recently shown that these cells are enriched in the CD103+ fraction.38 CD4+CD25+ T cells expressing CTLA-4, GITR, and CD103—markers associated with regulatory T cells—can be obtained during lymphopenia-driven expansion of normal CD25– T cells.29,39 In IL-7–/– mice, there is a sizeable population of Foxp3-expressing cells within the compartment of CD4+CD25– T cells. Therefore, it is likely that CD25+ cells are regenerated from the CD25– Foxp3-expressing cells and are responsible for the cure of the IBD, although we cannot exclude a contribution from the small number of CD25low Tregs remaining after depletion.
Regulatory T cells play a role in peripheral lymphocyte homeostasis.24,40 The maintenance of the number of peripheral T cells during the depletion period may be due on the one hand to the absence of IL-7 and on the other hand to the regulatory activity of the remaining CD25–Foxp3+ T cells. Different regulatory activities may be dependent to different extents on Foxp3-expressing cells.41 It is thus possible that the CD25– regulatory T cells, remaining under depletion, are able to control cell numbers but not inflammatory disease.
In conclusion, we show in this work that IL-7–/– mice have a robust regulatory CD25+CD4+ T-cell compartment, which is fully activated by 28 weeks of age. CD4+CD25+ T cells control inflammatory disease and actively prevent the development of IBD in lymphopenic IL-7–/– mice. We also show that, in the absence of further thymic export, the CD4+CD25+ T-cell compartment can be regenerated from CD4+CD25– Foxp3-expressing T cells in an IL-7–deficient environment.
Prepublished online as Blood First Edition Paper, June 8, 2006; DOI 10.1182/blood-2006-04-017947.
Supported by the Institut Pasteur, INSERM, Association Francaise contre les Myopathies (AFM), and the Juvenile Diabetes Research Fund (JDRF) through an Adult Stem Cell program grant. R.P.L. and J.Z. are supported by the Caisse Nationale d'Assurance Maladie—Assistance Publique Hôpitaux de Paris (CANAM-APHP). H.C.D. is supported by the Ministere de la Recherche et de la Technologie.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Anne O'Garra for critical reading of the paper and Monique Peffault de Latour for help with the histologic analysis. We also thank Fabrice Lemaître and Aude Thiriot for help with the experiments and discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal