Abstract
The platelet procoagulant response requires a sustained elevation of the intracellular Ca2+ concentration, [Ca2+]i, causing exposure of phosphatidylserine (PS) at the outer surface of the plasma membrane. An increased [Ca2+]i also activates Ca2+-dependent K+ channels. Here, we investigated the contribution of the efflux of K+ ions on the platelet procoagulant response in collagen-thrombin–activated platelets using selective K+ channel blockers. The Gardos channel blockers clotrimazol, charybdotoxin, and quinine caused a similar decrease in prothrombinase activity as well as in the number of PS-exposing platelets detected by fluorescence-conjugated annexin A5. Apamin and iberiotoxin, inhibitors of other K+ channels, were without effect. Only clotrimazol showed a significant inhibition of the collagen-plus-thrombin–induced intracellular calcium response. Clotrimazol and charybdotoxin did not inhibit aggregation and release under the conditions used. Inhibition by Gardos channel blockers was reversed by valinomycin, a selective K+ ionophore. The impaired procoagulant response of platelets from a patient with Scott syndrome was partially restored by pretreatment with valinomycin, suggesting a possible defect of the Gardos channel in this syndrome. Collectively, these results provide evidence for the involvement of efflux of K+ ions through Ca2+-activated K+ channels in the procoagulant response of platelets, opening potential strategies for therapeutic interventions.
Introduction
Blood platelets fulfill a dual role in the hemostatic process. Their adhesive and aggregating properties affirm the formation of a physical barrier required for the primary arrest of a bleeding. In addition, platelets provide a catalytic surface for the assembly of enzyme complexes of the coagulation cascade to ensure an accelerated fibrin formation. The latter function is referred to as the platelet procoagulant response, which involves a remodeling of the platelet plasma membrane.1 In quiescent platelets, phosphatidylserine (PS) is maintained in the inner leaflet of the plasma membrane mainly through the action of an aminophospholipid translocase.2,3 Stimulation of platelets causes aminophospholipid translocase activity to shut down, while simultaneously switching on the activity of a phospholipid scramblase. These events lead within minutes to a collapse of the normal asymmetric lipid distribution, resulting in surface exposure of negatively charged PS required for the binding of coagulation factor complexes. Despite several studies revealing various properties of the scramblase mechanism, the identity of the membrane protein(s) involved remains unresolved.2,4,5
A prerequisite for PS exposure in platelets appears to be a persistent elevation of intracellular Ca2+ concentration ([Ca2+]i), a condition that is particularly accomplished using a combination of the physiologic agonists collagen and thrombin.6 A prolonged rise in [Ca2+]i may also cause a collapse of lipid asymmetry in cells other than platelets. In the presence of extracellular Ca2+, Ca2+ ionophores such as ionomycin or A23187 have been shown to induce PS exposure in a variety of cells, including erythrocytes. Studies by Lang and coworkers7 have demonstrated that Ca2+, entering erythrocytes, not only activates the scramblase, but also stimulates the Ca2+-sensitive “Gardos” K+ channel in these cells. Subsequent loss of K+ ions together with efflux of Cl– ions induces cell shrinkage, accompanied by shedding of microvesicles from the plasma membrane. It was further demonstrated that these events might be linked, as both could be inhibited by either an increase of the extracellular K+ concentration or selective Gardos K+ channel blockers. Impaired cell shrinkage, microvesicle production, and PS exposure upon treatment with Ca2+ ionophore are also observed in erythrocytes from patients with Scott syndrome.8 This rare hereditary bleeding disorder is caused by a defective scramblase mechanism that appears to be present in all cells of the hematologic lineage. Hence, platelets from patients with Scott syndrome exhibit impaired PS exposure and microvesicle production in response to Ca2+ ionophore or collagen plus thrombin, despite a normal increase in [Ca2+]i.
Here, we have investigated the role of the Gardos K+ channel in the procoagulant response of platelets to physiologic agonists such as the combined action of collagen and thrombin. To this end, we have used selective Gardos channel blockers as well as an increased extracellular K+ concentration. In addition we have used valinomycin, a K+ ionophore, to abolish the effect of the K+ channel blockers. We demonstrate that Gardos channel blockers, but not other K+ channel blockers, inhibit the platelet procoagulant response evoked by collagen and thrombin without having an appreciable effect on other platelet responses involved in the primary arrest of bleeding, such as aggregation or release. Furthermore, we present evidence that the impaired PS exposure in Scott syndrome platelets upon activation with collagen and thrombin may be partially attributed to an impaired efflux of K+ ions.
Materials and methods
Reagents
Calcium ionophore ionomycin, bovine serum albumin (BSA; essentially fatty acid free), clotrimazol, quinine hydrochloride, charybdotoxin, apamin, iberiotoxin, and valinomycin were obtained from Sigma (St Louis, MO). Human thrombin was purified as previously described,9 and collagen was from Horm (Nycomed, Münich, Germany). Tirofiban was obtained from MSD (Haarlem, the Netherlands). The coagulation proteins prothrombin, factor Xa and factor Va were purified from bovine blood as described before.9 Thrombin-specific chromogenic substrate S2238 was obtained from Chromogenix (Mölndal, Sweden). Fluorescein isothiocyanate (FITC)–conjugated annexin A5 and Fura-2 were from Invitrogen (Leiden, the Netherlands). ATP Bioluminescence Assay Kit HS II was from Boehringer Mannheim (Mannheim, Germany).
Platelet isolation and activation
Platelet isolation was performed as described.10 Briefly, 10 mL blood was collected in 2 mL anticoagulant ACD (80 mM trisodium citrate, 52 mM citric acid, and 180 mM glucose). Platelet-rich plasma was obtained by centrifuging whole blood for 15 minutes at 200g. Platelets were spun down at 11500g for 2 minutes using a microfuge. The platelet pellet was resuspended and washed twice with 10 mM HEPES, 137 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 5 mM glucose, and 0.5 mg/mL BSA, adjusted at pH 6.6; before each centrifugation step, 100 μL ACD was added to each milliliter of resuspended platelets. Finally, platelets were resuspended in HEPES buffer at pH 7.4. Platelet count was adjusted at 2 × 108 platelets/mL. For measurement of the [Ca2+]i, platelets were loaded with Fura-2 (3 μM) for 45 minutes at 37°C, prior to isolation.
Platelet activation was performed at 37°C at a concentration of 107 platelets/mL in a volume of 0.5 mL under continuous stirring in the presence of 3 mM CaCl2. Platelet agonists used were thrombin (5 nM) plus collagen (10 μg/mL) or ionomycin (1 μM). The effect of different inhibitors was studied by pretreatment of platelets with the various agents for 30 minutes before starting the activation procedure. For the effects of high extracellular [K+], platelet suspensions were diluted 20-fold to a concentration of 107 platelets/mL in a modified HEPES buffer, referred to as HEPES/KCl buffer (10 mM HEPES, 19.7 mM NaCl, 120 mM KCl, 2 mM MgCl2, 5 mM glucose, and 0.5 mg/mL BSA [pH 7.4]), activated, and assayed for prothrombinase activity. In some experiments, HEPES buffer was used in which 120 mM NaCl was replaced by 120 mM choline chloride (10 mM HEPES, 17 mM NaCl, 120 mM choline chloride, 2.7 mM KCl, 2 mM MgCl2, 5 mM glucose, and 0.5 mg/mL BSA [pH 7.4]), referred to as HEPES/choline buffer.
For experiments with platelets from a patient with Scott syndrome, freshly obtained citrated blood from the patient and a control was shipped by airmail, and platelets were isolated and used for experiments immediately upon arrival (approximately 24 hours after blood donation).
Platelet prothrombinase activity
Platelet procoagulant activity was assayed as described in more detail elsewhere.9 Briefly, after activation of 107 platelets/mL with collagen (10 μg/mL) and thrombin (4 nM) for 10 minutes, samples were taken and diluted to 106 platelets/mL in HEPES buffer containing 3 mM CaCl2 and 0.5 mg/mL BSA. The samples were incubated with 0.2 nM factor Xa and 2 nM factor Va for 1 minute at 37°C. Thrombin formation was initiated by addition of 1 μM prothrombin and arrested after 2 minutes by addition of 10 mM EDTA. Thrombin was measured using the chromogenic substrate S2238.
Separation of microparticles from remnant platelets after activation
Platelet activation has been demonstrated to be accompanied by shedding of microparticles from the plasma membrane.11,12 To evaluate the contribution of microparticles, we have used a previously described method to separate microparticles from remnant cells.13 Briefly, after activation, platelet suspensions were centrifuged at 11500g; both the prothrombinase activity remaining in the supernatant and the lipid content of the supernatant approached constant values after 3 minutes of centrifugation. This prothrombinase activity is operationally defined as derived from microparticles. The absence of platelets or remnant cells in the supernatant was confirmed by flow cytometry.
Flow cytometry
Samples for flow cytometry were taken simultaneously with samples for the prothrombinase assay from the platelet incubations after 10 minutes of activation. To measure PS exposure, samples were diluted 10-fold in HEPES buffer containing 3 mM CaCl2 and incubated with FITC-conjugated annexin A5 (final concentration, 10 nM) for 5 minutes and analyzed for light scatter and fluorescence by a Coulter Epics XL-MLC flow cytometer (Beckman Coulter, Mÿdrecht, the Netherlands). Light scatter and fluorescence channels were set at logarithmic gain. Scatter parameters and fluorescence intensities were obtained from 10 000 platelets and analyzed offline using WinMDI version 2.8 software (http://facs.scripps.edu/software.html). Ionomycin-activated platelets were used to set a marker for fluorescence intensity of annexin-positive platelets to measure the percentage of annexin-positive platelets after the various incubation procedures.
Measurement of [Ca2+]i of platelets in suspension
Platelets (5 × 107/mL) were incubated with K+ channel inhibitors for 5 minutes, followed by the addition of tirofiban (5 μg/mL) and Ca2+ (3 mM), and incubated for an additional 5 minutes under stirring conditions. Collagen (10 μg/mL) plus thrombin (5 nM) were then added simultaneously. Changes in [Ca2+]i of Fura-2–loaded platelets were measured under continuous stirring by dual excitation fluorometry in an SLM-Aminco 8100 spectrofluorometer (SLM Instruments, Abcoude, the Netherlands). Ratio values of fluorescence at 340- and 380-nm excitation, obtained 5 minutes after activation with collagen plus thrombin, were converted to levels of [Ca2+]i as described.14 Experiments were performed in triplicate at 37°C.
Measurement of platelet aggregation and release.
Aggregation was measured at 37°C in 450-μL samples of a platelet suspension of 2 × 108/mL using an automated model 400 Chronolog aggregometer (Chronolog Corporation, Chicago, IL). After stirring for 2 minutes, CaCl2 (3 mM) was added, followed 1 minute later by simultaneous addition of 10 μg/mL collagen and 4 nM thrombin. The resulting aggregation was recorded for 5 minutes. after which 50 μLATP reagent (Boehringer Mannheim ATP Bioluminescence Assay Kit HS II) was added to quantify the release reaction.
Statistics
Results
The influence of high extracellular [K+] on the generation of procoagulant activity
Initial experiments to demonstrate the importance of an efflux of K+ ions during platelet stimulation on the procoagulant response were designed by changing the cation composition of the buffer. In these experiments, high extracellular [K+] in the HEPES/KCl buffer was compensated by reducing [Na+] in order to maintain osmolarity. It should be emphasized, however, that a decreased [Na+] may have 2 major consequences: it reduces the catalytic efficiency of thrombin,15 and it likely causes inhibition of the Na+/H+ exchanger, which may be involved in PS exposure during platelet activation.16 We therefore compared the procoagulant response induced by collagen plus thrombin in low [Na+] solutions (ie, HEPES/choline buffer and HEPES/KCl buffer) with the response evoked in high [Na+] solution (ie, HEPES buffer). In these initial experiments, the procoagulant response was determined by the number of annexin-binding platelets measured by flow cytometry. As shown in Figure 1, the response in HEPES/choline buffer decreased to 45.3% ± 5.6% (n = 4) of that induced in HEPES buffer, whereas in HEPES/KCl buffer the response dropped to 31.9% ± 4.8% (n = 4). Thus, although the low [Na+] caused a decreased procoagulant response in both buffers, there appeared to be a significant additional inhibitory effect of the presence of high extracellular K+, supporting the notion that efflux of K+ ions contributes to the procoagulant response. When platelets were stimulated with collagen alone, the procoagulant activity induced in HEPES/choline buffer was similar to that induced in HEPES (data not shown). This suggests that the lower response with collagen plus thrombin in HEPES/choline is more likely to be caused by a decreased activity of thrombin in low-sodium buffers rather than a nonfunctional Na+/H+ exchanger.
Since microvesicle formation and PS exposure are closely associated events in the procoagulant response,11-13 we also studied the effect of a high [K+] on microvesicle formation in collagen-and-thrombin–activated platelets. Because of their small size, flow cytometry is not particularly suitable for quantifying platelet microparticles. Therefore, formation of microvesicles was operationally defined as residual procoagulant activity in the supernatant of a platelet suspension that was centrifuged at 11 500g for 3 minutes as was described previously.13,17 In HEPES/KCl, a 50% reduction in prothrombinase activity of both the supernatant and the total platelet incubation was found, indicating that a reduced PS exposure was accompanied by a reduced formation of microvesicles.
Effect of high extracellular [K+] on collagen-plus-thrombin–induced platelet procoagulant response. Platelets at 107 mL–1 were activated with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes in HEPES, HEPES/choline, or HEPES/KCl, and the procoagulant response was measured as the binding of FITC-conjugated annexin A5. Data are expressed as a percentage of the response obtained in HEPES. We emphasize that the 100% value in HEPES corresponds to approximately 20% to 25% of the total cell population that becomes positive for annexin binding, depending on the donor. Data are mean values ± 1SD(n = 4).
Effect of high extracellular [K+] on collagen-plus-thrombin–induced platelet procoagulant response. Platelets at 107 mL–1 were activated with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes in HEPES, HEPES/choline, or HEPES/KCl, and the procoagulant response was measured as the binding of FITC-conjugated annexin A5. Data are expressed as a percentage of the response obtained in HEPES. We emphasize that the 100% value in HEPES corresponds to approximately 20% to 25% of the total cell population that becomes positive for annexin binding, depending on the donor. Data are mean values ± 1SD(n = 4).
Effect of potassium channel inhibitors on collagen-plus-thrombin–induced platelet procoagulant activity. Platelets at 107 mL–1 were preincubated with various inhibitors for 30 minutes followed by activation with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes. Samples from these incubations were diluted 10-fold in HEPES buffer containing 3 mM CaCl2 and subsequently analyzed for prothrombinase activity (A) or percentage of annexin-positive cells (B) as described in “Materials and methods.” Valinomycin was added 5 minutes prior to activation of the platelets (to avoid long-term effects of this ionophore). ▪ indicates absence of valinomycin; and ▦, presence of valinomycin. Concentrations used: clotrimazol, 10 μM; charybdotoxin, 20 nM; quinine, 0.4 mM; apamin, 500 nM; iberiotoxin, 200 nM; and valinomycin, 3 μM. Data are expressed as percentage of control (ie, platelets in absence of inhibitor [mean ± 1 SD] panel A, n > 6; panel B, n > 3]). Student t test was used to compare data in the presence and absence of valinomycin.
Effect of potassium channel inhibitors on collagen-plus-thrombin–induced platelet procoagulant activity. Platelets at 107 mL–1 were preincubated with various inhibitors for 30 minutes followed by activation with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes. Samples from these incubations were diluted 10-fold in HEPES buffer containing 3 mM CaCl2 and subsequently analyzed for prothrombinase activity (A) or percentage of annexin-positive cells (B) as described in “Materials and methods.” Valinomycin was added 5 minutes prior to activation of the platelets (to avoid long-term effects of this ionophore). ▪ indicates absence of valinomycin; and ▦, presence of valinomycin. Concentrations used: clotrimazol, 10 μM; charybdotoxin, 20 nM; quinine, 0.4 mM; apamin, 500 nM; iberiotoxin, 200 nM; and valinomycin, 3 μM. Data are expressed as percentage of control (ie, platelets in absence of inhibitor [mean ± 1 SD] panel A, n > 6; panel B, n > 3]). Student t test was used to compare data in the presence and absence of valinomycin.
Gardos channel blockers inhibit platelet procoagulant response
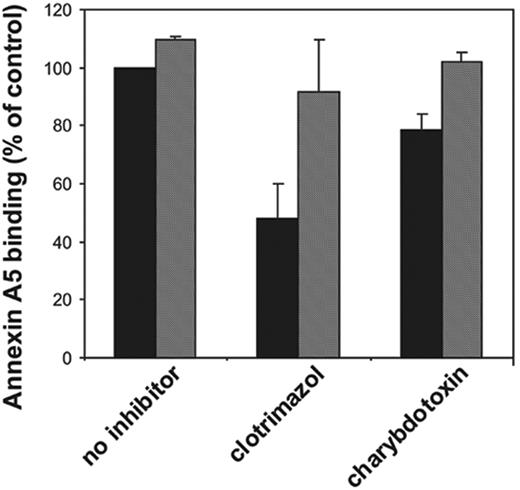
We have tested various K+ channel inhibitors for their ability to affect the collagen-plus-thrombin–induced platelet prothrombinase activity. The results are depicted in Figure 2A. The Gardos channel inhibitors clotrimazol (10 μM) and charybdotoxin (20 nM) caused 46% ± 13% (n = 12) and 31% ± 14% (n = 9) inhibition of the prothrombinase activity of collagen-and-thrombin–activated platelets, respectively. The estimated inhibitory concentration at 50% (IC50) for these 2 inhibitors on the platelet procoagulant response are 100 nM and 5 nM, respectively, in good agreement with the estimated potencies to inhibit K+ fluxes.18 Quinine, one of the first compounds demonstrated to block K+ efflux in erythrocytes,19-21 appeared to be the most potent inhibitor, being able to decrease the collagen-plus-thrombin–induced procoagulant activity by 60% ± 10% (n = 6). Two other K+ channel inhibitors, iberiotoxin and apamin, were without effect. Very similar findings with these inhibitors were obtained when the procoagulant response was measured as the binding of annexin A5 (Figure 2B). We could not demonstrate inhibition of clotrimazol, charybdotoxin, or quinine on the procoagulant response induced by the Ca2+ ionophore, ionomycin (5 μM).
To investigate whether inhibition of the procoagulant response by clotrimazol, charybdotoxin, and quinine was caused by a preferential effect of these inhibitors on the process of microvesicle formation, we compared the prothrombinase activity of a total suspension of activated platelets with that of its corresponding 11 500g supernatant. For each inhibitor, the decrease in prothrombinase activity of the supernatant was similar to that of the total platelet suspension (ie, the prothrombinase activity of the supernatant varied between 30%-35% of that of the total suspension, irrespective the presence of an inhibitor).
Gardos channel blockers decrease the fraction of PS-exposing platelets after collagen-plus-thrombin stimulation
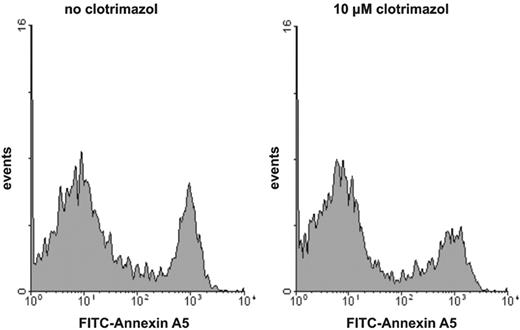
It has been demonstrated that the procoagulant activity of platelets in suspension is caused by a fraction of the platelets showing maximal PS exposure.6,17,22,23 The level of procoagulant activity induced by various agonists is determined by the size of the subpopulation of platelets that have maximal externalized PS. To investigate whether the inhibition of procoagulant activity by the K+ channel blockers is caused by a decrease in the fraction of PS-exposing platelets rather than by a decrease in the extent of PS exposure of the procoagulant platelet fraction, we used flow cytometry to measure the binding of FITC-conjugated annexin A5 on single-cell level. Figure 3 shows a typical example of the effect of a K+ channel inhibitor, clotrimazol, on the number of annexin-positive platelets after activation with collagen plus thrombin. The number of PS-exposing platelets is decreased in the presence of clotrimazol, but the extent of annexin binding (mean fluorescence intensity) of the residual PS-positive platelets remains at the same level as found in the absence of inhibitor. Identical findings were obtained for the other Gardos channel blockers that inhibited the procoagulant response (data not shown).
Besides a role in supporting blood coagulation, surface-exposed PS is a hallmark of the apoptotic process, serving as “eat me” signals for phagocyting cells. As shown previously, local anesthetics can cause PS exposure in platelets, a phenomenon associated with mitochondrial-related apoptotic-like events.17,24 Neither clotrimazol nor charybdotoxin could inhibit tetracaine-induced prothrombinase activity or binding of FITC-conjugated annexin A5 (data not shown).
No effect of Gardos channel inhibitors on collagen-plus-thrombin–induced aggregation and release
To investigate whether or not other platelet functions were affected by the presence of K+ channel inhibitors under these conditions of platelet stimulation, we studied their effect on aggregation and release of ATP. Neither high extracellular [K+] nor the presence of clotrimazol or charybdotoxin showed an appreciable effect on aggregation or release (Table 1). (The diminished ATP release observed for platelets in HEPES/KCl appeared to be caused by a reduced activity of the ATP reagent [luciferine/luciferase] in this buffer.) Quinine caused approximately 60% inhibition of the aggregation, but had only a minor effect on the release. It should be noted in this respect that quinine has also been observed to inhibit phospholipase A2, which may result in a decreased thromboxane A2 production, a secondary stimulator of platelet aggregation.25 Since we were exclusively interested in inhibitory effects under conditions of platelet stimulation that evoke a procoagulant response, we did not perform dose-response curves for each activator separately. Hence, it cannot be excluded that these K+ channel blockers may affect the aggregation and/or release induced by collagen or thrombin at lower dosages.
Effect of Gardos channel inhibitors on platelet aggregation and release
. | Aggregation, % control ± SD . | Release (ATP), % control ± SD . |
|---|---|---|
| 120 mM high K+ | 100.8 ± 6.0 | 79.4 ± 3.3 |
| 10 μM clotrimazol | 90.0 ± 2.8 | 91.3 ± 5.0 |
| 20 nM charybdotoxin | 108.3 ± 10.2 | 98.7 ± 9.0 |
| 0.4 mM quinine | 40.0 ± 3.0 | 90.1 ± 4.1 |
. | Aggregation, % control ± SD . | Release (ATP), % control ± SD . |
|---|---|---|
| 120 mM high K+ | 100.8 ± 6.0 | 79.4 ± 3.3 |
| 10 μM clotrimazol | 90.0 ± 2.8 | 91.3 ± 5.0 |
| 20 nM charybdotoxin | 108.3 ± 10.2 | 98.7 ± 9.0 |
| 0.4 mM quinine | 40.0 ± 3.0 | 90.1 ± 4.1 |
Histogram of the binding of annexin A5 to collagen-plus-thrombin–activated platelets: effect of clotrimazol. Platelets were preincubated with (right histogram) or without (left histogram) clotrimazol (10 μM) prior to activation with collagen plus thrombin. Following activation, platelet samples were diluted and FITC-conjugated annexin A5 was added. For experimental details, consult “Materials and methods.” Similar results were obtained for charybdotoxin- or quinine-treated platelets.
Histogram of the binding of annexin A5 to collagen-plus-thrombin–activated platelets: effect of clotrimazol. Platelets were preincubated with (right histogram) or without (left histogram) clotrimazol (10 μM) prior to activation with collagen plus thrombin. Following activation, platelet samples were diluted and FITC-conjugated annexin A5 was added. For experimental details, consult “Materials and methods.” Similar results were obtained for charybdotoxin- or quinine-treated platelets.
Inhibition of the procoagulant response by Gardos channel blockers is reversed by valinomycin
To verify that the inhibition by clotrimazol, charybdotoxin, and quinine was caused by blocking the efflux of K+ ions from the cell, valinomycin was used, which acts as a cage carrier selective for K+ ions. As shown in Figure 2A and 2B (hatched bars), valinomycin at a concentration of 3 μM completely reversed the inhibition caused by clotrimazol and charybdotoxin, and partially abolished the inhibitory effect of quinine. The results depicted in Figure 2 were from experiments performed in HEPES buffer. Similar findings were obtained when the experiments were performed in HEPES/choline. Moreover, also in HEPES/choline, the inhibition by clotrimazol and charybdotoxin was virtually annihilated in the presence of valinomycin (Figure 4). Valinomycin itself did not cause a procoagulant response of platelets in the absence of a stimulus, nor did it affect the procoagulant response by collagen and thrombin in the absence of inhibitors. The inhibition of the procoagulant response by high extracellular [K+] was not reversed by addition of valinomycin (data not shown). Collectively, these findings strongly indicate that an efflux of K+ ions through the Gardos channel augments the platelet procoagulant response induced by collagen plus thrombin.
The effect of Gardos channel blockers and valinomycin on collagen-plus-thrombin–induced procoagulant response in HEPES/choline buffer. Platelets at 107 mL–1 in HEPES/choline were preincubated with inhibitors for 30 minutes followed by activation with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes. Valinomycin was added 5 minutes prior to activation. Samples from these incubations were diluted 10-fold and subsequently analyzed for binding of FITC-conjugated annexin A5. Data are expressed as a percentage of the number of annexin-positive cells found upon activation in HEPES/choline in absence of the inhibitors. Data are mean values ± 1SD(n = 4).
The effect of Gardos channel blockers and valinomycin on collagen-plus-thrombin–induced procoagulant response in HEPES/choline buffer. Platelets at 107 mL–1 in HEPES/choline were preincubated with inhibitors for 30 minutes followed by activation with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes. Valinomycin was added 5 minutes prior to activation. Samples from these incubations were diluted 10-fold and subsequently analyzed for binding of FITC-conjugated annexin A5. Data are expressed as a percentage of the number of annexin-positive cells found upon activation in HEPES/choline in absence of the inhibitors. Data are mean values ± 1SD(n = 4).
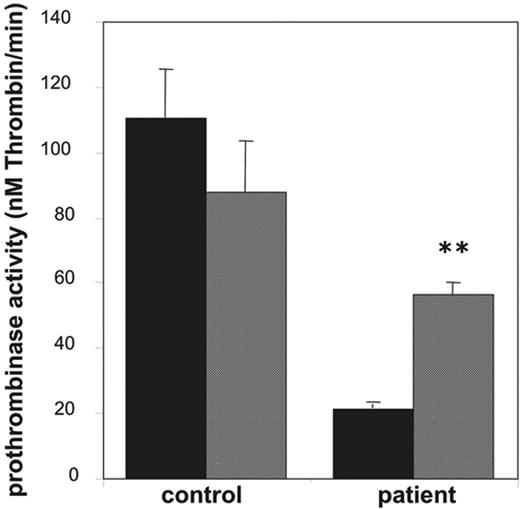
Effect of valinomycin on collagen-plus-thrombin–induced procoagulant activity of platelets from a patient with Scott syndrome. Prothrombinase activities, expressed as nanomolar thrombin formed per minute, were determined after activation with collagen (10 μg/mL) plus thrombin (4 nM) in the absence (▪) and presence (▦) of valinomycin (3 μM). Data are mean values ± 1SD(n = 6).
Effect of valinomycin on collagen-plus-thrombin–induced procoagulant activity of platelets from a patient with Scott syndrome. Prothrombinase activities, expressed as nanomolar thrombin formed per minute, were determined after activation with collagen (10 μg/mL) plus thrombin (4 nM) in the absence (▪) and presence (▦) of valinomycin (3 μM). Data are mean values ± 1SD(n = 6).
Effect of Gardos channel blockers on [Ca2+]i
It is increasingly appreciated that the procoagulant response depends on a persistent elevation of [Ca2+]i. Therefore, inhibition of the procoagulant response by Gardos channel inhibitors might be due to a reduced collagen-thrombin–induced change in [Ca2+]i. To test this hypothesis we have conducted [Ca2+]i measurements in collagen-plus-thrombin–activated platelets that were incubated with clotrimazol, charybdotoxin, or quinine. In agreement with the notion that a sustained, threshold [Ca2+]i is of importance, [Ca2+]i levels, measured 5 minutes after stimulation with collagen plus thrombin either in the absence or presence of inhibitors, were compared. In the absence of inhibitors the change in [Ca2+]i was 364 ± 18 nM. When platelets were preincubated with clotrimazol (10 μM), the increase in [Ca2+]i was significantly lower (Δ[Ca2+]i = 275 ± 10 nM; P = .01). This 24% reduction could be reversed with valinomycin (Δ[Ca2+]i = 326 ± 34 nM). With charybdotoxin (20 nM), no significant change in [Ca2+]i was found (Δ[Ca2+]i = 360 ± 23 nM). The intrinsic fluorescence properties of quinine appeared to interfere with the Fura-2 signal, impeding determination of Δ[Ca2+]i with this inhibitor.
The impaired procoagulant response of platelets from a patient with Scott syndrome can be partially corrected by valinomycin
Platelets from patients with Scott syndrome elicit a partially impaired procoagulant response when activated with collagen plus thrombin and appear to be completely defective in their response to Ca2+ ionophore. Here, we used platelets from a Welsh Scott patient, whose clinical features have been described earlier.26 Indeed, as shown in Figure 5, the prothrombinase activity after stimulation with collagen and thrombin was approximately 20% of that of an identically treated “travel” control. Stimulation with collagen plus thrombin in the presence of 3 μM valinomycin caused a significant increase in the procoagulant response of the Scott platelets, almost to the level of that observed for the control in the presence of valinomycin.
Discussion
Activation of blood platelets, particularly by the combined action of collagen and thrombin, evokes a procoagulant response, which has been attributed to surface exposure of PS in a distinct platelet population,17 thus producing a catalytic membrane surface that promotes assembly and activity of the prothrombinase and tenase complex of the blood coagulation proteins.9 In this study we wanted to investigate whether selective inhibition of K+ channels present in the plasma membrane could affect the procoagulant response of platelets. A number of studies have demonstrated the presence of both voltage-operated K+ channels and Ca2+-induced K+ channels in the plasma membrane of platelets.27-30 The latter can be subdivided in 3 different types, SK (small conductance), IK (intermediate conductance) and BK (large conductance) channels.31-33 Whether or not all 3 types are present in platelets is presently unknown. Of the Ca2+-activated K+ channels, the IK channel is identical to the Gardos channel, first described in erythrocytes.34 Distinction between different types of K+ channels can be made based on their sensitivity to specific inhibitors.18,32,33 The antifungal agent clotrimazol and charybdotoxin, a high-affinity peptide isolated from scorpion venom, specifically block the Gardos channel, although charybdotoxin can also inhibit the large conductance Ca2+-activated K+ channel. Iberiotoxin, a peptide also purified from scorpion venom and apamin, isolated from bee venom are blockers of BK and SK channels, respectively.18,32,33 The present study shows that PS exposure, microvesiculation, and prothrombinase activity of platelets stimulated with collagen plus thrombin are markedly attenuated in the presence of specific inhibitors of the Gardos K+ channel, charybdotoxin, clotrimazol, and quinine, as well as in the presence of high extracellular K+. This inhibition does not occur with inhibitors of the non-Gardos K+ channels (eg, apamin and iberiotoxin), strongly suggesting that functional Gardos channels are a requisite for the process of PS externalization. Mahaut-Smith30 has suggested that Ca2+-activated K+ channels are important in maintaining the membrane potential during stimulated cation influx. Inhibition of these channels would be expected to cause a higher degree of membrane depolarization following platelet activation and consequently reduce the driving force for Ca2+ entry. Indeed, a significant inhibition by clotrimazol was found on the [Ca2+]i measured 5 minutes after collagen-plus-thrombin activation. The inhibitory effect of the Gardos channel blockers is abolished in the presence of the K+ ionophore valinomycin, in agreement with its ability to clamp the membrane potential near to the K+ equilibrium potential.29 Although the effect of clotrimazol on the collagen-plus-thrombin–induced sustained [Ca2+]i (ie, measured after 5 minutes of stimulation) appeared quite small, it should be emphasized that these values represent the average of all platelets in suspension. As was shown recently, platelets are heterogeneous in the procoagulant response (ie, the PS-exposing platelets represent only a fraction of the total).17 This may explain why the inhibitory effect of charybdotoxin on the [Ca2+]i was not significant, since this inhibitor, on average, appeared less potent than clotrimazol.
Treatment of platelets with the Ca2+ ionophore ionomycin causes maximal PS exposure in all platelets and consequently evokes a maximal procoagulant response.17 Gardos channel blockers did not significantly affect ionomycin-induced PS exposure in platelets, which would be expected since in the presence of ionomycin the Ca2+ fluxes mainly depend on the Ca2+ gradients across the plasma membrane and the endoplasmic reticulum membrane and not the membrane potential. In contrast with our findings on platelets, Lang and coworkers7 demonstrated that clotrimazol and charybdotoxin inhibit PS exposure in erythrocytes treated with Ca2+ ionophore. Also, morphologic changes, such as cell shrinkage, microvesicle production, and formation of crenated spherocytes, were suppressed by these inhibitors. We found that these inhibitory effects in erythrocytes could be abolished by addition of valinomycin, indicating that diminished PS exposure was related to a decreased efflux of K+ ions (J.L.W., unpublished data, June 2005). We have as yet no explanation for the different effects of Gardos channel inhibitors on Ca2+ ionophore–induced responses in platelets and erythrocytes.
Interestingly, blood platelets from Scott syndrome, a rare hereditary bleeding disorder characterized by a defective scramblase mechanism, nevertheless expose PS upon activation in the presence of valinomycin. Moreover, red cells from Scott syndrome, unlike normal red cells, have been shown to maintain their biconcave structure upon treatment with Ca2+ ionophore,35 whereas normal red cells maintain their biconcave structure with Ca2+ ionophore in the presence of Gardos channel inhibitors.7 Therefore, it can be speculated that blood cells from Scott syndrome lack, or have defective, Gardos channels, preventing operation of the scramblase mechanism and thus the formation of a procoagulant platelet-membrane surface.
It is worth recalling that Scott platelets have been shown to expose PS upon treatment with local anesthetics (eg, tetracain and propranolol), which produce mitochondria-related apoptotic events.24 These local anesthetics also produce apoptosis and concurrent PS exposure in normal platelets, but this response cannot be inhibited by the Gardos channel blockers charybdotoxin and clotrimazol. This confirms that PS exposure during apoptosis differs from that during cell activation, as has been shown earlier.36
Finally, the Gardos channel inhibitors do not affect platelet aggregation and release to any appreciable extent. This may open new perspectives to explore selective inhibition of the platelet procoagulant response while maintaining the platelet functions required for the primary arrest of bleeding. Also, promoting K+ efflux in platelets from Scott syndrome could be a feasible strategy to correct this bleeding disorder.
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2006-01-009613.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Effect of high extracellular [K+] on collagen-plus-thrombin–induced platelet procoagulant response. Platelets at 107 mL–1 were activated with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes in HEPES, HEPES/choline, or HEPES/KCl, and the procoagulant response was measured as the binding of FITC-conjugated annexin A5. Data are expressed as a percentage of the response obtained in HEPES. We emphasize that the 100% value in HEPES corresponds to approximately 20% to 25% of the total cell population that becomes positive for annexin binding, depending on the donor. Data are mean values ± 1SD(n = 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/7/10.1182_blood-2006-01-009613/4/m_zh80190601770001.jpeg?Expires=1767737044&Signature=zf9DVgEPHLHpwLTr3vNAKOyuHZ3bEzyWCuGtAKTl4mQppb~F3CunWu6GWpFLhMp0f5-NcV4A0~Vy098ou2SZ6Kym0zB0srtzuTFrjJLQeAc9eYPoNDQgVFNneKWNtEwHI-dAC4Rhb2OIs3~V-j5-1jvy9VNWcnTvnj9EGfqvV3ULPmw~hLy8pVbFdZWm7gKrtwAr1F6MBC9sOSEe431ho~3jzhAsrXLjopubdEyHATjOgq1hIFw-1DYvMPlSVbhOInGcvianITpUhGMJcIcREOinj7q5Hpcnj2ImuOc2jWNgZ2E3PwuthHXRmBAJTO3QyVYslBMWJjNZpw6YNkN8mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Effect of potassium channel inhibitors on collagen-plus-thrombin–induced platelet procoagulant activity. Platelets at 107 mL–1 were preincubated with various inhibitors for 30 minutes followed by activation with collagen (10 μg/mL) plus thrombin (4 nM) for 10 minutes. Samples from these incubations were diluted 10-fold in HEPES buffer containing 3 mM CaCl2 and subsequently analyzed for prothrombinase activity (A) or percentage of annexin-positive cells (B) as described in “Materials and methods.” Valinomycin was added 5 minutes prior to activation of the platelets (to avoid long-term effects of this ionophore). ▪ indicates absence of valinomycin; and ▦, presence of valinomycin. Concentrations used: clotrimazol, 10 μM; charybdotoxin, 20 nM; quinine, 0.4 mM; apamin, 500 nM; iberiotoxin, 200 nM; and valinomycin, 3 μM. Data are expressed as percentage of control (ie, platelets in absence of inhibitor [mean ± 1 SD] panel A, n > 6; panel B, n > 3]). Student t test was used to compare data in the presence and absence of valinomycin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/7/10.1182_blood-2006-01-009613/4/m_zh80190601770002.jpeg?Expires=1767737044&Signature=rIT3q10JO4w733n6hb7vPK~221fCwuZcHcJEdzKOJrnzXrVK75~zwf5NYLAg2A52iOoLt9KlqLRqVi4VvLixYWukxu95O9hIFfLUlpKngafPts6WfEWjqMOWerCPITeeErtrIn32XmT-tfvlDcQKTXrIumVviLowpP3tOk2TjL5Trj4qABdJLixCL~S8m4Q7FjZVjurjEjqdMmebDAo1TOEfSzGvOtuYXZj9Og2u3RR1mx4lLl8yDLZPFUO8SY3I85CxJjOts~DH71oxSheTytT0c626BqOJsp4xkKXTFT7DaxtRx7edRHO8TM~K4tiRWnZBQumhu3h7408keSD~dA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal