Abstract
Hematopoietic stem and progenitor cells (HSPCs) are located in the bone marrow in close association with a highly organized 3-dimensional structure formed by stroma cells, referred to as the niche. Mobilization of HSPCs from bone marrow to peripheral blood in response to granulocyte colony-stimulating factor (G-CSF) requires de-adhesion of HSPCs from the niche. The influence of aging of HSPCs on cell-stroma interactions has not been determined in detail. Using a mouse model of G-CSF–induced mobilization, we demonstrated that the ability to mobilize hematopoietic stem cells is approximately 5-fold greater in aged mice. Competitive mobilization experiments confirmed that enhanced mobilization ability was intrinsic to the stem cell. Enhanced mobilization efficiency of primitive hematopoietic cells from aged mice correlated with reduced adhesion of hematopoietic progenitor cells to stroma and with elevated levels of GTP-bound Cdc42. These results might indicate that stroma–stem cell interactions are dynamic over a lifetime and result in physiologically relevant changes in the biology of primitive hematopoietic cells with age.
Introduction
Hematopoietic stem and progenitor cells (HSPCs) are located in the bone marrow (BM) in close association with a highly organized 3-dimensional structure formed by stroma cells, referred to as the niche.1,2 It has been demonstrated that these cell–cell interactions are vital for the biology of HSPCs. Systemic administration of cytokines and chemokines or cytotoxic agents mobilize HSPCs from the BM into peripheral blood (PB), where they are collected in clinically useful quantities for stem cell therapies.3,4 Mobilization of HSPCs in response to these factors requires the de-adhesion of HSPCs from the niche.5,6
Evidence accumulating over the past decade has proven that there is a measurable and successive functional decline in hematopoietic, intestinal, and muscle stem cell function.7,8 Given that stem cell activity is necessary to replenish lost differentiated cells, it has been hypothesized that aging of hematopoietic stem cells (HSCs) leads to reduced stem cell renewal and thus reduced tissue homeostasis in aged animals,7-11 which is emphasized by age-associated anemia and a decline in function of immune cells in elderly persons.12-19 HSC aging is intrinsic to the aged cell and cannot be reverted by exposing HSCs from aged animals to a young microenvironment.18-20 The ability of healthy, older patients to undergo stem cell mobilization in response to granulocyte colony-stimulating factor (G-CSF), the standard regimen used for clinical HSPC mobilization, has thus far not been investigated in detail because autologous hematopoietic stem cell transplantation is most often administered to adults younger than 50 years and rarely to patients older than 60 years.21,22 The limited available information is not conclusive in terms of efficiency of mobilization because it is based primarily on the analysis of mobilization of elderly patients after severe chemotherapy23,24 and possibly also because 2 different methods of determining mobilization efficiency are used (colony-forming cell [CFC] frequency vs CD34+ cell frequency in PB). Combining general clinical wisdom and these published reports, it is anticipated that the ability to mobilize HSPCs in response to G-CSF may be reduced in elderly patients,23-26 hampering the efficient collection of HSPCs for subsequent hematopoietic stem cell therapies. Whether a negative correlation exists between age and mobilization is still under debate.24,25
The mouse has been used in a wide variety of studies to model human G-CSF–induced mobilization of HSCs, with an overall good correlation between results obtained from murine studies and subsequent results obtained in humans. By investigating mobilization proficiency in aged mice, we report here that aged mice show not only decreased but increased efficiency in mobilizing hematopoietic progenitor cells (HPCs) and HSCs to PB.
Materials and methods
Animals
Young C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and were subsequently housed in the animal barrier facility at Cincinnati Children's Hospital Medical Center (CCHMC). Aged C57BL/6J animals were retired breeders derived from our own colony or were obtained from The Jackson Laboratory and subsequently aged in the animal barrier facility at CCHMC or obtained from the National Institute on Aging (NIA) aged rodent colony (Harlan). B6.SJL(BoyJ) mice were obtained either from the divisional stock (derived from animals obtained from The Jackson Laboratory) or from the National Cancer Institute (NCI; C57BL/6 Ly5.2Cr). All animals were house in a pathogen-free environment in the CCHMC barrier facility.
Mobilization
rhuG-CSF (Amgen Biologicals, Thousand Oaks, CA) was administered intraperitoneally at 12.5 μg/mL in PBS/0.1% BSA at 100 μg/kg once a day for 5 days. Animals were given injections of a volume in the range of 136 μLup to 240 μL per injection. HPC frequency in PB, BM, and spleen (SPL) was determined by a CFC assay on day 6.
CFC assay
For the CFC assay, 10 or 20 μL PB, 2 × 104 whole BM cells, 1 × 105 SPL cells, or 4 × 103 low-density BM cells in 20 μL Hanks balanced salt solution (HBSS) were admixed with 80 or 90 μL HBSS and 1 mL colony assay media (MethoCult GF M 3534; Stem Cell Technologies, Vancouver, BC, Canada) containing 50 ng/mL rm SCF, 10 ng/mL rm IL-3, and 10 ng/mL rh IL-6 (all from PeproTech, Rocky Hill, NJ) and incubated at 37°C. Each tissue sample was analyzed in triplicate. Colonies of more than 50 cells were scored in PB assays after 7 to 8 days and in BM and SPL assays after 9 to 10 days.
Flow cytometry
Immunostaining and flow cytometry analyses were performed according to standard procedures, and cells were subsequently analyzed on a FACS-Canto flow cytometer (BD Biosciences, San Jose, CA). To distinguish aged from young cells in the competitive transplantation model, anti–Ly5.2 (clone 104, FITC conjugated; BD Biosciences) and anti–Ly5.1 (clone A20, PE conjugated; BD Biosciences) monoclonal antibodies were used. For lineage (Lin) analysis in tissues, anti-CD3ϵ (clone 145-2C11, PE-Cy7 conjugated), anti-B220 (clone RA3-6B2, APC conjugated), anti-CD11b (clone M1/70, APC-Cy7 conjugated), and anti–Gr-1 (clone RB6-8C5, APC-Cy7 conjugated, all from BD Biosciences) antibodies were used. For the determination of chimerism in the HSC and HPC compartments, low-density BM cells obtained from layering whole BM cells on a density gradient (Histopaque 1083; Sigma, St Louis, MO) were incubated with a cocktail of biotinylated antibodies specific for molecules on differentiated hematopoietic cells (Lin cocktail) containing anti-CD11b (clone M1/70), anti-B220 (clone RA3-6B2), anti-CD5 (clone 53-7.3), anti–Gr-1 (clone RB6-8C5), anti-Ter119, and anti-CD3ϵ (clone 145-2C11; all from BD Biosciences) antibodies and subsequently were incubated with anti–Sca-1, clone E13-161.7, PE-Cy7–conjugated (eBiosciences, San Diego, CA), anti–c-Kit, APC-conjugated, clone 2B8, and streptavidin APC-Cy7 (all from BD Biosciences). For the determination of the expression of adhesion receptors, low-density BM cells were individually incubated with biotinylated antibodies specific against CD184 (CXCR4), CD49d (clone R1-2), or CD49e (clone 5H 10-27) and subsequently with PE-labeled Lin cocktail antibodies, anti–Sca-1 PE-Cy7, anti–c-Kit APC, and streptavidin FITC. For the determination of the genotype of CFC colonies, individual colonies were picked out of the semisolid medium at day 9 of culture, resuspended in 1 mL PBS, and stained for Ly5.1/Ly5.2 contribution to determine the contribution of aged and young cells. At least 100 live cells were acquired per colony, and only colonies showing greater than 80% contribution to either the Ly5.1 or the Ly5.2 population were scored. For live/dead discrimination in all immunophenotyping experiments, cells were resuspended in medium containing 5 μg/mL 7AAD (Molecular Probes, Eugene, OR).
Competitive transplantation
Two- to 4-month-old B6.SJL(BoyJ) (recipient) mice were irradiated with a total dosage of 11.75 Gy (7 Gy + 4.75 Gy, 4 hours apart), and cells were subsequently transplanted into the retro-orbital sinus in a volume of 200 μL in HBSS/2% FCS. For BM transplants, whole BM cells were harvested and pooled from the tibia and the femur of 2- to 3-month-old B6.SJL(BoyJ) and 25-month-old B6 mice (3 mice each age group) in HBSS supplemented with 2% FCS. Equal numbers of BM cells (2 × 106 cells of each, aged and young) were transplanted into irradiated recipients. For PB transplants, PB was harvested and pooled from 4 mobilized mice each and subjected to red blood cell lysis. Irradiated recipients underwent transplantation with equal volumes of PB cells from young and aged mice with a total of 800 μLPB per recipient to ensure engraftment. After transplantation, mice were housed in a pathogen-free environment in the CCHMC barrier facility.
Transendothelial migration
The murine cervical high-endothelial venule cell line (mHEVc) was cultivated as previously described.27 Transwell inserts (8 μm, polycarbonate membrane; Corning Glassworks, Corning, NY) were preincubated in RPMI 1640 supplemented with 20% FCS for at least 1 hour. Four thousand endothelial cells were seeded on the transwell. Confluence of the endothelial monolayer after 4 days was confirmed microscopically and by the ability of the monolayer to retain red blood cells (greater than 95% retention). Low-density BM cells obtained from layering whole BM cells from mobilized mice on a density gradient (Ficoll-Paque plus 1077; Amersham Biosiences, Freiburg, Germany) were seeded at a density of 1 × 106 cells/well on top of the transwell in RPMI 1640 supplemented with 20% FCS. CFC frequency was determined in parallel. Murine SDF-1 (PeproTech) was added to the lower chamber (100 ng/mL) where indicated. Transwell chambers were subsequently incubated at 37°C, migrated cells were harvested 18 hours later, and the cell count was determined and analyzed for CFC frequency. Lymphocyte migration across endothelial cells was linear from 0 to 24 hours; thus, the 18-hour time point chosen for our experiments was in the linear range and resulted in a reasonable percentage of cells capable of migrating.28
CAFC progenitor adhesion assay
For the cobblestone area–forming cell (CAFC) progenitor adhesion assay, FBMD-1 cells were seeded in IMDM supplemented with 15% FCS and 5% horse serum (both from Gibco, Grand Island, NY) at a density of 1000 cells per well in a 96-well plate, as previously described.29 After 1 week in culture, confluence of the FBMD-1 monolayer was verified microscopically. Whole BM cells were diluted in CAFC medium (IMDM, supplemented with 20% horse serum [Gibco] and 10–5 M hydrocortisone σ) at 3000, 1500, 750, and 375 cells per well (15 wells per cell concentration) onto the FBMD-1 stroma cell line. To determine progenitor cell adhesion, nonadherent cells were washed from the FBMD-1 stroma cells after 2 or 4 hours, and fresh CAFC medium was added to each well. The frequency of total HPCs and adherent HPCs was determined by counting the frequency of cobblestone areas at day 7 on the FBMD-1 stroma cell line. Initial experiments confirmed that the percentage of adherent plus nonadherent HPCs totaled 100% of the initially plated HPC frequency (data not shown).
Rho-GTPase effector domain pull-down assay
Relative levels of GTP-bound Rac1, Rac2, and Cdc42 were determined by an effector pull-down assay, as previously described.30,31 Briefly, whole BM cells or lineage-negative (Lin–) cells were lysed in a buffer containing 1% Triton X-100 and incubated with glutathione bead–bound GST-PAK1. Bound (activated) and unbound (nonactivated) RhoGTPases were probed by immunoblotting with antibodies specific for Rac1 (clone 102), Cdc42 (clone 44), β-actin (clone C4; all BD Biosciences), and Rac2 (polyclonal; Upstate Biotechnology, Lake Placid, NY), and the relative amount of each protein was quantified by densitometry. To obtain lineage-depleted BM cells, low-density BM cells (Ficoll Histopaque 1083; Sigma) pooled from 4 to 6 mice were incubated with biotinylated rat antimouse antibodies (Lin Abs) against surface receptors on differentiated hematopoietic cells (Lin cocktail: anti-CD11b, clone M1/70, anti-B220, clone RA3-6B2, anti-CD5, clone 53-7.3, anti–Gr-1, clone RB6-8C5, anti-Ter119, anti-CD3ϵ, clone 145-2C11; all from BD Biosciences) and subsequently with sheep anti–rat IgG antibodies coupled to magnetic beads (Dynabeads; Invitrogen, Carlsbad, CA). Cells were exposed to a magnetic field, and Lin– cells were harvested and lysed.
Statistical analyses
Paired Student t tests were used to determine the significance of the difference between means.
Results
Mobilization of progenitors is increased in aged mice
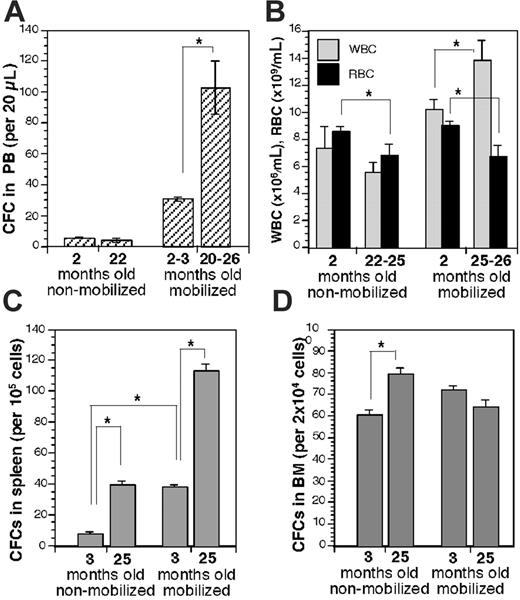
In the standard mobilization assay, which closely resembles that used in humans, young (2- to 3-month-old) and aged (20- to 26-month-old) C57BL/6 (B6) mice were given G-CSF (100 μg/kg) once a day by intraperitoneal injection for 5 days, and PB was collected on day 6. Given that the mean lifespan of these mice is approximately 800 days,32 26 months of mouse age corresponds to a human age of approximately 70 years. The frequency of HPCs in PB was determined by a standard CFC assay. The frequency of HPCs in PB of mobilized aged mice was elevated 3-fold over the frequency found in PB of young mice (Figure 1A). Interestingly, the aged animals mobilized increased numbers of HPCs despite slight, but significant, anemia (indicated by a reduced red blood cell [RBC] count; Figure 1B). Age-associated anemia has been previously reported in mice and humans.33,34 Age-associated anemia was still prevalent in mobilized aged mice (Figure 1B).
G-CSF–induced mobilization leads to a redistribution of primitive hematopoietic cells from BM to the spleen.35 Thus, the frequency of CFCs in spleen and BM was determined for young and aged mice before and after mobilization. Aged mice showed a 4-fold increase in CFC frequency in spleen over the frequency in young animals (Figure 1C). After mobilization, CFC frequency in the spleen in young animals was increased 4-fold over the premobilization frequency, whereas the frequency in mobilized aged mice was increased 3-fold (Figure 1C). The increase in the CFC content in the spleen of mobilized mice was accompanied by a moderate 19% increase in the total cell splenic number after mobilization in aged animals (2.1-2.5 × 108 cells per spleen) and a 50% increase in young mice (1.4-2.1 × 108 cells per spleen). The relative increase in CFC frequency in SPL in mobilized aged mice might have been reduced because of space restrictions given the high total splenic CFC numbers in these mice. An explanation for the increased splenic CFC content in nonmobilized aged mice might be a consequence of the slight anemia in aged mice but also warrants further investigation. Possibilities include an altered splenic microenvironment in aged mice or an altered homing ability of aged HPCs to spleen. As in spleen, a significant, but much lower, age-related increase in CFC content was detected in the BM of nonmobilized animals. A slight increase in the CFC frequency was detected in the BM of young mice as a result of the mobilization regimen (Figure 1D), in contrast to a slight decrease in BM of aged mice after mobilization. We conclude from these results that quantitative aspects of HPC distribution in response to mobilization are altered in aged animals.
Aged mice mobilize increased numbers of HPCs to PB in response to G-CSF. (A) Frequency of CFCs in 20 μL PB in young and aged mobilized (5-day G-CSF at 100 μg/kg per day intraperitoneally; PB analysis on day 6) and nonmobilized mice, n = 10 for young nonmobilized and mobilized mice, n = 3 for 22-month-old mobilized mice, and n = 7 (3 at 20 months, 1 at 23 months, 3 at 26 months) for mobilized aged mice. (B) White blood cell (WBC) and RBC counts in young and aged mobilized and nonmobilized mice, n = at least 4 per value. (C) CFC frequency in spleen in young and aged mobilized and nonmobilized mice, n = 3. (D) CFC frequency in BM in young and aged mobilized and nonmobilized mice, n = 3. Values shown are mean ± 1 SEM. *P < .05.
Aged mice mobilize increased numbers of HPCs to PB in response to G-CSF. (A) Frequency of CFCs in 20 μL PB in young and aged mobilized (5-day G-CSF at 100 μg/kg per day intraperitoneally; PB analysis on day 6) and nonmobilized mice, n = 10 for young nonmobilized and mobilized mice, n = 3 for 22-month-old mobilized mice, and n = 7 (3 at 20 months, 1 at 23 months, 3 at 26 months) for mobilized aged mice. (B) White blood cell (WBC) and RBC counts in young and aged mobilized and nonmobilized mice, n = at least 4 per value. (C) CFC frequency in spleen in young and aged mobilized and nonmobilized mice, n = 3. (D) CFC frequency in BM in young and aged mobilized and nonmobilized mice, n = 3. Values shown are mean ± 1 SEM. *P < .05.
Mobilization of hematopoietic stem cells is increased in aged mice
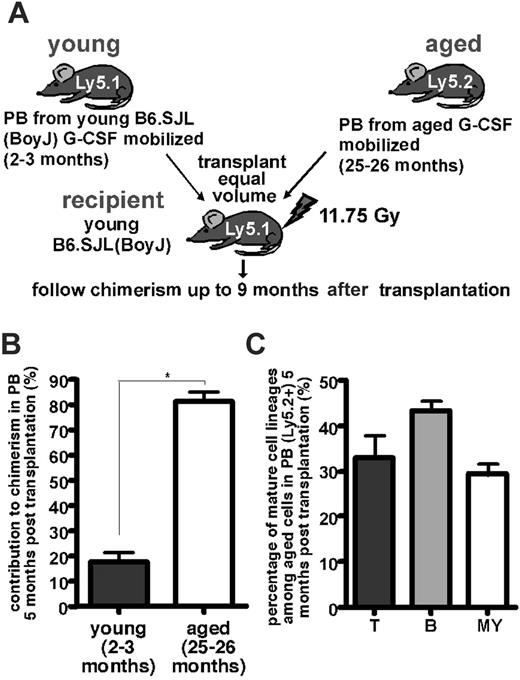
Stem cells are defined by their ability to self-renew and to support multilineage differentiation; therefore, stringent testing of stem cell activity and frequency is best accomplished by transplanting putative HSCs into lethally irradiated recipients. To unequivocally compare the frequency and differentiation capacity of HSCs mobilized to PB in young and old mice, standard competitive repopulation assays were performed. To that end, equal volumes of PB from young (2- to 3-month-old) mobilized B6.SJL(BoyJ) and aged (25- and 26-month-old) mobilized B6 mice were transplanted into lethally irradiated B6.SJL(BoyJ) animals (Figure 2A). Because hematopoietic cells from B6.SJL(BoyJ) mice expressed the Ly5.1 allele and hematopoietic cells from B6 animals expressed the Ly5.2 allele of CD45 cell surface marker, B6.SJL cells could be distinguished from B6 cells by flow cytometry using antibodies specific for the Ly5 alleles. Young B6.SJL(BoyJ) mice are identical to young B6 mice in their mobilization efficiency and characteristics.36
Analysis of the contribution of the transplanted mobilized HSCs to chimerism in PB in the recipient animals was measured up to 9 months after transplantation, when hematopoiesis in the periphery was sustained by transplanted long-term repopulating stem cells.37 HSCs mobilized to PB in aged animals contributed on average to 80% to hematopoiesis in the PB of recipients 5 months after transplantation (Figure 2B). Further analyses of PB from the recipient animals revealed that mHSCs from aged mice had multilineage differentiation ability because they contributed to the T, B, and myeloid lineages (Figure 2C).
One cohort of mice that underwent competitive transplantation was killed 9 months later, and chimerism in various hematopoietic tissue and the hematopoietic stem and progenitor cell compartments was determined by flow cytometry (Table 1). HPCs (Lin–S–K+ cells) were identified by immunophenotyping as cells negative for lineage markers (Lin–) and for the Sca-1 receptor (S–) and positive for the c-Kit receptor (K+), whereas HSCs were negative for lineage markers and positive for the Sca-1 and the c-Kit receptors (Lin–S+K+). The chimerism supported by HSCs mobilized to PB in aged mice (Ly5.2-positive cells) in the hematopoietic tissues analyzed ranged from 77% in spleen to 96% in thymus and was on average 86% in PB and BM and in the hematopoietic stem (Lin–S+K+) and progenitor cell (Lin–S–K+) compartment in BM (Table 1). This percentage of contribution is significantly increased over the 50% contribution expected in case of an equal frequency of HSCs mobilized to PB in young and aged mice. Thus, long-term repopulating HSCs were at least 5-fold enriched (85% vs 15%) in PB from aged mice after mobilization over PB from young mobilized mice. These data are in good agreement with the increase in CFC frequency in PB of aged mice after mobilization (Figure 1A).
Chimerism in hematopoietic tissue 9 months after transplantation
Tissue . | Contributions from HSCs mobilized to PB in aged mice, % . |
|---|---|
| Peripheral blood | 86.4 ± 3.0 |
| Bone marrow | 87.3 ± 4.0 |
| Bone marrow progenitor cells, Lin-S-K+ | 86.5 ± 7.1 |
| Bone marrow stem cells, Lin-S+K+ | 85.4 ± 4.3 |
| Spleen | 77.1 ± 6.4 |
| Thymus | 96.2 ± 1.9 |
Tissue . | Contributions from HSCs mobilized to PB in aged mice, % . |
|---|---|
| Peripheral blood | 86.4 ± 3.0 |
| Bone marrow | 87.3 ± 4.0 |
| Bone marrow progenitor cells, Lin-S-K+ | 86.5 ± 7.1 |
| Bone marrow stem cells, Lin-S+K+ | 85.4 ± 4.3 |
| Spleen | 77.1 ± 6.4 |
| Thymus | 96.2 ± 1.9 |
Values shown are mean ± SEM. n = 3 mice.
Aged mice mobilize a higher number of HSCs to PB in response to G-CSF. (A) Experimental setup of competitive transplantation of mobilized PB cells. (B) Flow cytometric analysis of chimerism in PB of mice that underwent competitive (50/50) transplantation with mPB from aged (Ly5.2+) and young (Ly5.1+) mice 5 months after transplantation. (C) Determination of the percentages of T, B, and myeloid (MY) cells in PB cells derived from the transplanted mobilized HSCs from aged mice in the recipient 5 months after transplantation by flow cytometry. Data are a summary of 2 independent experiments; in each, n = 3 for the number of donors per age group from which PB was collected and n = 4 for the number of recipients. One recipient animal had graft failure and was excluded from the analysis. Values shown are mean ± 1 SEM. *P < .05.
Aged mice mobilize a higher number of HSCs to PB in response to G-CSF. (A) Experimental setup of competitive transplantation of mobilized PB cells. (B) Flow cytometric analysis of chimerism in PB of mice that underwent competitive (50/50) transplantation with mPB from aged (Ly5.2+) and young (Ly5.1+) mice 5 months after transplantation. (C) Determination of the percentages of T, B, and myeloid (MY) cells in PB cells derived from the transplanted mobilized HSCs from aged mice in the recipient 5 months after transplantation by flow cytometry. Data are a summary of 2 independent experiments; in each, n = 3 for the number of donors per age group from which PB was collected and n = 4 for the number of recipients. One recipient animal had graft failure and was excluded from the analysis. Values shown are mean ± 1 SEM. *P < .05.
Enhanced mobilization proficiency with age is intrinsic to the stem cell
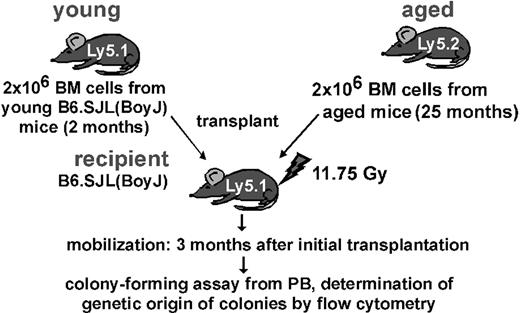
To determine whether the age-associated increased ability to mobilize was intrinsic to HSCs or was driven by extrinsic changes in the BM microenvironment, a competitive mobilization assay was performed (Figure 3).36 Lethally irradiated mice underwent competitive transplantation with equal numbers of young and aged BM cells, and premobilization chimerism in PB in the recipient animals was determined 3 months after transplantation. As previously reported and confirmed in these experiments, HSCs from aged animals contributed to only 30% chimerism in PB in the recipient animals, resulting in a 1:2 ratio in favor of young cells (Table 2).18,38 These competitively reconstituted animals were subsequently mobilized by G-CSF, and the CFC frequency in PB was determined. PB of the mobilized reconstituted animals contained on average 58 CFCs per 20 μL, which is an almost 2-fold increase over the 30 CFCs per 20 μL PB after mobilization seen in young animals (Figure 1A). The genetic origin (young/aged) of the mobilized CFCs was determined by analyzing individual colonies by flow cytometry (Table 2). On average, 85% of all CFCs mobilized to PB were derived from cells of aged mice, strongly arguing that increased mobilization proficiency is not a simple consequence of the increased CFC content in aged BM and is to a large extent intrinsic to the aged primitive hematopoietic cell.
Competitive mobilization in mice transplanted with equal number of young and old BM cells
. | Contribution to PB before mobilization, % . | Contribution to CFCs after mobilization, % . |
|---|---|---|
| Aged cells | 31 ± 2 | 85 ± 1 |
| Young cells | 69 ± 2 | 15 ± 1 |
. | Contribution to PB before mobilization, % . | Contribution to CFCs after mobilization, % . |
|---|---|---|
| Aged cells | 31 ± 2 | 85 ± 1 |
| Young cells | 69 ± 2 | 15 ± 1 |
Aged was defined as 26 months; young, as 2 months. Contribution of aged and young BM cells to PB before mobilization and of CFCs derived from aged and young cells to PB after mobilization, analyzing at least 50 CFCs per recipient. Data are derived from 3 recipient animals that underwent competitive transplantation with BM cells pooled from 3 donors each. Values shown are mean± 1 SEM.
Experimental setup of the competitive mobilization experiment. Data are presented in Table 2.
Experimental setup of the competitive mobilization experiment. Data are presented in Table 2.
HPCs from young and aged mice do not differ in their migratory behaviors
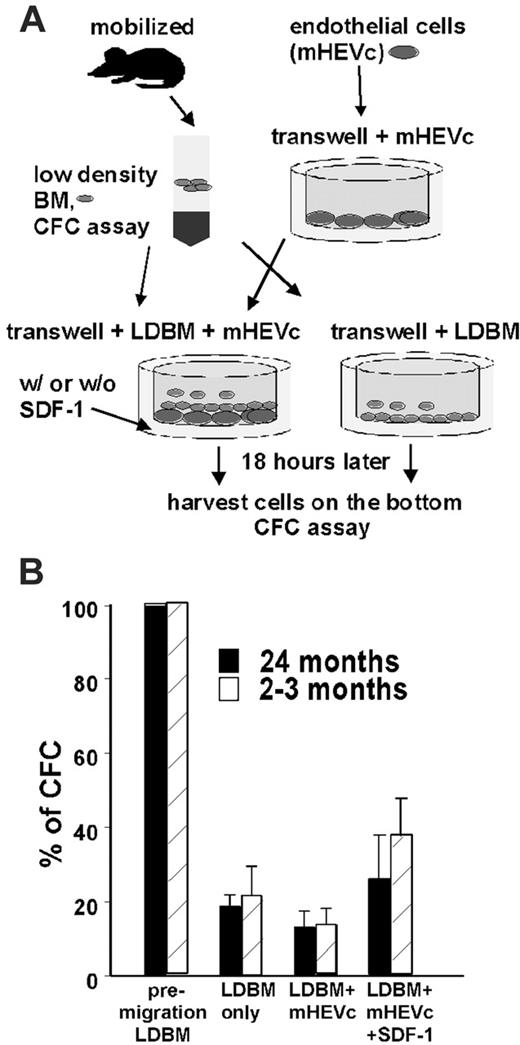
To mobilize, HSPCs must leave their stromal niche and migrate through the endothelial barrier into the circulation.3-5 It is believed that in mobilized mice, a positive gradient of stroma-derived factor-1 (SDF-1) supports the migration of primitive cells from BM across the endothelial barrier into blood vessels.39 To determine the underlying mechanism leading to increased mobilization proficiency of aged HPCs, transendothelial migration assays were performed. Low-density BM cells derived from mobilized animals were seeded on top of a membrane with an 8-μm pore size with or without a monolayer of endothelial cells.27,40 Cells seeded on an endothelial cell monolayer were further exposed to a positive SDF-1 gradient (Figure 4A). Cells from the bottom well were harvested 18 hours after seeding. The frequency of HPCs in the population seeded on top and in the migrated population was determined by a CFC assay. HPCs in BM from mobilized aged mice did not exhibit a significant difference in their ability to migrate through the membrane in the absence (aged 19% vs young 22%) or in the presence of the endothelial cell monolayer (aged 13% vs young 13%) or in the presence of the endothelial cell monolayer with an SDF-1 gradient (aged 26% vs young 38%; Figure 4B). Taken together, these data do not support differences in migratory activity between HPCs from young and aged mobilized mice as the mechanism of increased mobilization in aged mice.
Young and aged HPCs do not differ with respect to their ability to cross an endothelial cell layer. (A) Experimental setup for the transwell-migration assay. (B) Percentage of CFCs from mobilized young (2-3 months) and aged (24 months) mice migrating in 18 hours into the lower well of the transwell. n = 3. Values shown are mean ± 1 SEM. LDBM indicates low-density BM cells.
Young and aged HPCs do not differ with respect to their ability to cross an endothelial cell layer. (A) Experimental setup for the transwell-migration assay. (B) Percentage of CFCs from mobilized young (2-3 months) and aged (24 months) mice migrating in 18 hours into the lower well of the transwell. n = 3. Values shown are mean ± 1 SEM. LDBM indicates low-density BM cells.
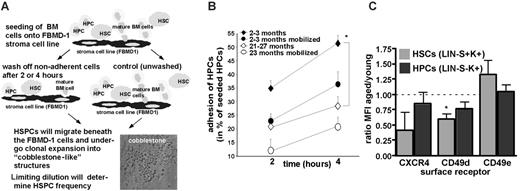
HPCs from aged mice are reduced in their ability to adhere to stroma. (A) BM cells from mobilized and nonmobilized aged (21-27 months) and young (2-3 months) mice were subjected to CAFC adhesion assay. Experimental setup. (B) Percentage of adherent HPCs after 2 or 4 hours determined as CAFC day 7 cells using the CAFC adhesion assay. n = at least 3 for each age group. Values shown are mean ± 1 SEM. (C). Ratio of the MFI of CXCR4, CD49d, CD49e on HPCs and HSCs from aged (21-22 months) and young (2-3 months) mice. No change in the MFI of expression between cells from young and aged animals resulted in ratio of 1, indicated by the dashed line. n = 3 mice each aged and young for CXCR4, and n = 4 mice each aged and young for CD49d and CD49e. *P < .05.
HPCs from aged mice are reduced in their ability to adhere to stroma. (A) BM cells from mobilized and nonmobilized aged (21-27 months) and young (2-3 months) mice were subjected to CAFC adhesion assay. Experimental setup. (B) Percentage of adherent HPCs after 2 or 4 hours determined as CAFC day 7 cells using the CAFC adhesion assay. n = at least 3 for each age group. Values shown are mean ± 1 SEM. (C). Ratio of the MFI of CXCR4, CD49d, CD49e on HPCs and HSCs from aged (21-22 months) and young (2-3 months) mice. No change in the MFI of expression between cells from young and aged animals resulted in ratio of 1, indicated by the dashed line. n = 3 mice each aged and young for CXCR4, and n = 4 mice each aged and young for CD49d and CD49e. *P < .05.
Aged HPCs are impaired in their adhesion to stroma cells
We next compared the adhesive properties of aged and young HPCs. To this end, we measured adhesion of HPCs to a bone marrow–derived stroma cell line (FBMD-1) using the CAFC adhesion assay, which is a modification of the CAFC assay.41 The CAFC assay is a well-established, limiting-dilution–type in vitro surrogate assay to measure progenitor cell activity.41 For the CAFC adhesion assay, BM cells were plated on top of a monolayer of confluent FBMD-1 cells, and the nonadhesive cells were washed off after 2 or 4 hours (Figure 5A). HPC frequency was subsequently determined at day 7, and the frequency of adhesive HPCs was compared with the initial HPC frequency.42 BM cells from nonmobilized aged mice showed a significant 2-fold reduction in the ability to adhere to FBMD-1 cells after 4 hours (Figure 5B).
To correlate changes in adhesion/mobilization efficiency with changes in the expression of adhesion receptors, the level of surface expression of the surface receptors CXCR4 (the receptor for SDF-1), CD49d (α4 integrin), and CD49e (α5 integrin) on aged and young HSCs (Lin–S+K+) and HPCs (Lin–S+K–) was compared using flow cytometry (Figure 5C). These tested surface molecules are involved in regulating HSPC adhesion/mobilization.3 The ratio of the mean fluorescence intensity (MFI) was determined for each surface receptor on aged and young cell populations. CD49d expression was significantly reduced on aged HSCs, but not on aged HPCs, whereas CXCR4 and CD49e did not show significant changes in the expression between aged and young HSPCs (Figure 5C). These results suggest that reduced adhesion of aged HPCs is independent of the expression levels of these surface receptors.
Increased level of activation of Cdc42 in aged primitive hematopoietic cells
Adhesion and de-adhesion of HSPCs from stroma requires significant changes in the cytoskeleton. These intracellular changes are regulated in part by outside-in signaling of receptors sensing the cellular environment (eg, adhesion receptors, cytokines, chemokines), which then result in inside-out reactions of the cell and finally in de-adhesion. Integrins, growth factor receptors, and chemokine receptors have been shown to signal this outside-in information through members of the family of small Rho GTPases.43,44 Small Rho GTPases, including Rac1, Rac2, and Cdc42, are involved in the regulation of adhesion in various mammalian cell types, including hematopoietic cells.40,45-47 They act as molecular switches by cycling between an active GTP-bound form and an inactive GDP-bound form. Upon activation by distinct signals originating from these “environment sensing” receptors, active GTP-bound GTPases ultimately regulate actin polymerization, integrin clustering, and adhesion receptor–mediated adhesion.48,49
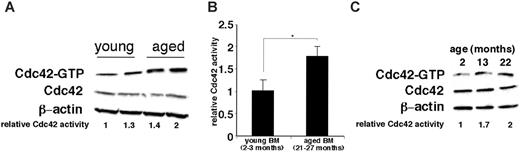
To further investigate possible mechanisms leading to the reduced adhesion of aged HPCs, the activity of the small RhoGTPases Cdc42, Ra1, and Rac2 was analyzed in BM cells and cell populations highly enriched for HPCs (Lin– cells) from 2- to 3-month, 13-month, and 21- to 27-month-old mice using an effector-domain GST fusion pull-down protocol as previously described.31 A significant increase in the GTP-bound form of Cdc42 was detected in BM cells and in Lin– cells from aged animals (Figure 6A-C), whereas the levels of activity of Rac1 and Rac2 did not change with age (data not shown). These results indicate that elevated levels of the active form of Cdc42 are associated with reduced adhesion and increased mobilization efficiency.
Discussion
Hematopoietic stem cell transplantation is most often administered to patients younger than 50 and rarely to patients older than 60 years of age21,22 because the toxicity of the chemotherapy/conditioning regimen has caused unacceptable morbidity in older patients. In contrast, the average age of patients at diagnosis of neoplasms (eg, leukemia, lymphoma) who are candidates for stem cell transplantation ranges from 65 to 70 years.50 Given that in recent years the adverse effects of the conditioning and the mobilization regimens for transplantation have been improved because of better treatment regimens and new therapeutic concepts such as the minigraft,26,51 older patients can now be expected to better tolerate transplantation regimens and thus be more likely to undergo stem cell therapy and G-CSF–induced stem cell mobilization. Because the mouse is regarded as a model for human G-CSF–induced mobilization, we compared the ability of aged and young mice to mobilize hematopoietic stem and progenitor cells to PB in response to G-CSF.
Increased activity of Cdc42 in primitive hematopoietic cells in aged mice. (A) BM cells from young (2- to 4-month-old) and aged (20- to 27-month-old) mice were subjected to an effector domain pull-down assay and subsequently probed by immunoblotting. (B) Quantification of the relative amount of the active Cdc42-GTP bound form by densitometry. n = 3 for young mice, and n = 4 for aged mice. Values shown are mean ± 1 SEM. *P < .05. (C) Lin–BM cells (enriched for HPCs) isolated from pooled BM cells from each experiment in which 4 young, middle-aged, and aged mice (2, 13, and 22 months, respectively) were subjected to an effector domain pull-down assay and subsequently probed by immunoblotting. Data are representative of 2 independent experiments.
Increased activity of Cdc42 in primitive hematopoietic cells in aged mice. (A) BM cells from young (2- to 4-month-old) and aged (20- to 27-month-old) mice were subjected to an effector domain pull-down assay and subsequently probed by immunoblotting. (B) Quantification of the relative amount of the active Cdc42-GTP bound form by densitometry. n = 3 for young mice, and n = 4 for aged mice. Values shown are mean ± 1 SEM. *P < .05. (C) Lin–BM cells (enriched for HPCs) isolated from pooled BM cells from each experiment in which 4 young, middle-aged, and aged mice (2, 13, and 22 months, respectively) were subjected to an effector domain pull-down assay and subsequently probed by immunoblotting. Data are representative of 2 independent experiments.
Aged mice showed increased G-CSF–induced mobilization efficiency for HPCs and HSCs. The data presented suggest that PB in aged, mobilized mice contains at least a 5-fold higher concentration of long-term repopulating hematopoietic stem cells than the PB of mobilized young animals and that the enhanced mobilization efficiency is intrinsic to the HSC. This intrinsic mechanism might be a direct mechanism, such as reduced adhesion of aged cells, or in an indirect one in which stem cells from aged animals are able to alter their microenvironments in a way that leads to reduced adhesion and enhanced mobilization. Further experiments will have to distinguish between such possibilities.
We and others recently described a 2- to 3-fold reduced homing ability of aged BM HSCs in comparison with young BM HSCs,18,19,38,52,53 resulting in reduced contribution of aged cells to hematopoiesis when equal numbers of BM cells from aged and young mice were transplanted into young recipients. The 77% to 96% levels of contribution of aged mobilized HSCs in recipients that underwent competitive transplantation with mobilized PB cells further suggests that mHSCs from aged mice are spared from this homing defect of BM stem cells of nonmobilized aged mice, that their homing abilities are actually enhanced, or that HSC mobilization in aged mice is even greater than that predicted from our data.
What might be the underlying molecular mechanisms for enhanced HPC mobilization in aged mice? Cell adhesion and cell migration are fundamental to the process of mobilization.5,6,40,54 Our experiments revealed that though HPCs from aged animals did not show an increased ability to migrate to or cross an endothelial cell layer, HPCs from aged animals are significantly deficient in their ability to adhere to stroma cells, thus implying that reduced adhesion of primitive hematopoietic cells to stroma might be the underlying cause of enhanced mobilization proficiency. Reduced adhesion of HSPCs from aged animals to stroma could also explain the fact that young BM cells transplanted into nonablated aged recipients are able to engraft, whereas young BM cells transplanted into young nonablated animals are not because young cells might be able to push less adhesive HSPCs in aged animals out of their niches.55 Given that aged mice do not show increased spontaneous mobilization to PB (Figure 1A), we conclude that the reduced adhesion of HPCs from aged mice is necessary, but not sufficient, for increased spontaneous mobilization and that only the combination of the action of G-CSF and reduced adhesion will result in enhanced mobilization.
Our results indicate that associated reduced adhesion and enhanced mobilization correlate with elevated levels of Cdc42 activity in primitive hematopoietic cells from aged mice. We recently reported a knock-out animal model system resulting in constitutively elevated levels of Cdc42 activity in HPCs (Cdc42 GAP–/– mice). HPCs from these mice showed reduced adhesion to fibronectin.30 Thus, we speculate that the reduced stromal adhesion of HPCs from aged animals might be a consequence of elevated activity of Cdc42 in HPCs in aged mice.
What might be the cause for the age-related increase in Cdc42 activity? Integrins, growth factor receptors, and adhesion molecules have been shown to signal outside-in information through Cdc42; thus, signaling through Cdc42 is embedded in a network and not in a linear cascade. In addition, it has been speculated that Cdc42 affects progenitor cell–cell contacts in skin by stabilizing β-catenin.56 Recently published gene expression profiles of stem cells from young and aged animals,36 together with gene expression data obtained in our own laboratories (data not shown), suggest that HSPC cell aging is accompanied by changes in the expression of signal transducers. Further experiments will be necessary to correlate age-related changes in any of these gene categories with functional consequences on Cdc42 activity and adhesion.
In summary, the data presented here demonstrate that HSC and HPC mobilization proficiency is increased in aged mice and that it is intrinsic to the stem cell. Our data support a need for further studies aimed at determining the mobilization proficiency in healthy middle-aged and aged persons. Our results also indicate that reduced adhesion to the stroma/niche might be the mechanism involved in increased HPC mobilization proficiency in aged mice and that reduced adhesion might be a consequence of increased levels of activated Cdc42 in HPCs from aged animals. The interaction of primitive hematopoietic cells with their niche might be regarded as dynamic over the lifespan of the individual and might perhaps confer other phenotypes associated with primitive hematopoietic cells in aged animals.8
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2005-12-010272.
Supported by the Mouse Core and the Flow Cytometry Core of the Division of Experimental Hematology at Cincinnati Children's Hospital Medical Center. Supported in part by National Institutes of Health grants RO1 HL076604 (H.G.) and RO1 AG022859 (G.V.Z.), and by the Ellison Medical Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Michael Jansen for providing the mHEVc cell line and for help with the transendothelial migration assay. We thank Susanne Wells, David Williams, and Jose Cancelas for critical comments on the manuscript and Matthew Hodgson for editorial assistance. H.G. is a New Scholar in Aging of The Ellison Medical Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal