Abstract
An abnormal serum immunoglobulin free light chain (FLC) ratio at diagnosis may identify risk of progression to myeloma in patients with solitary bone plasmacytoma (SBP). In the cohort of 116 patients, 43 have progressed to myeloma, with a median time to progression of 1.8 years. The FLC ratio was determined in all 116 patients on serum collected at time of diagnosis and was abnormal in 54 patients (47%). An abnormal FLC ratio was associated with a higher risk of progression to myeloma (P = .039). The risk of progression at 5 years was 44% in patients with an abnormal serum FLC ratio at diagnosis compared with 26% in those with a normal FLC ratio. One to 2 years following diagnosis, a persistent serum M protein level of 5 g/L (0.5 g/dL) or higher was an additional risk factor for progression. A risk stratification model was constructed using the 2 variables of FLC ratio and M protein level: patients with a normal FLC ratio at baseline and M protein level less than 5 g/L (0.5 g/dL) at 1 to 2 years following diagnosis (low risk, n = 31); with either risk factor abnormal (intermediate risk, n = 26); and with both an abnormal FLC ratio and M protein level of 5 g/L (0.5 g/dL) or higher (high risk, n = 18). The corresponding progression rates at 5 years were significantly different in the low, intermediate, and high groups: 13%, 26%, and 62%, respectively (P < .001).
Introduction
Solitary plasmacytoma is a condition characterized by a localized collection of monoclonal plasma cells without evidence of a systemic plasma cell proliferative disorder (eg, multiple myeloma). Plasmacytomas may be localized to bone (solitary bone plasmacytoma [SBP]) or occur in soft tissues, especially in the upper aerodigestive tract.1,2 Together, these 2 entities constitute less than 10% of all plasma cell neoplasms. SBP is usually treated with localized radiation therapy, and a dose of more than 40 Gy is associated with a low risk of local recurrence.3-5 The outcome of solitary plasmacytoma varies greatly; many patients are cured with radiation therapy or surgical resection but almost 50% develop multiple myeloma, typically after a median duration of 2 to 3 years.3,6-8
The prevalence of a monoclonal protein (M protein) in the serum or urine of patients with SBP varies from 24% to 72%, and the levels of the M protein tend to be low.3,8,9 In a previous report on 46 patients with SBP from our institution, 25 (54%) had a detectable serum or urine M protein, 4 patients had an M protein in both serum and urine, while 1 patient had only Bence Jones proteinuria.3 The M protein may decrease with successful therapy of SBP, and it has been reported that complete disappearance of the M protein 1 year after therapy is associated with prolonged disease stability.7,10
The location of disease, presence of soft tissue masses, age (> 55 years), serum M protein level, persistent M protein after therapy, microvessel density, and the presence of bulky disease have been reported to be associated with in increased risk of relapse.4,7,9-12 However, these predictors have drawbacks. For example, one of the most reliable predictors of progression, namely persistence of a serum M protein, can be assessed only a year or 2 following therapy and is therefore not available for risk assessment at the time of initial diagnosis. Thus, there is still a need for reliable predictors of outcome at the time of diagnosis in patients with solitary plasmacytoma.
Recently, an assay for serum immunoglobulin free light chains (FLCs) has become available for clinical use and is currently used to monitor patients with light chain amyloidosis, nonsecretory myeloma, and light chain myeloma.13-15 The assay allows quantitation of kappa and lambda chains that are not bound to intact immunoglobulin molecules, and allows determination of clonality based on the kappa to lambda ratio. An abnormal FLC ratio indicating presence of monoclonal free light chains has been recently shown to be an independent risk factor for progression in monoclonal gammopathy of undetermined significance (MGUS).16 Based on this, we hypothesized that an abnormal serum FLC ratio at the time of diagnosis of SBP would be an adverse prognostic factor for progression to myeloma.
Patients, materials, and methods
Patients
Following approval of this study by the Mayo Foundation institutional review board in conformity with the Declaration of Helsinki and federal regulations, we performed a search for all patients with SBP seen at Mayo Clinic Rochester between January 1, 1960, and December 31, 1995, and for whom a stored serum sample collected at the time of presentation and before the start of therapy was available. All charts were reviewed to confirm the diagnosis; patients with other plasma-cell dyscrasias were excluded; patients with recurrent solitary plasmacytoma were included if 2 or more years had passed since initial diagnosis. The diagnosis of SBP required histologic confirmation of a monoclonal population of plasma cells, the absence of bone marrow plasmacytosis (any patient with a bone marrow having more than 5% plasma cells was excluded), no additional lytic lesions on a radiographic bone survey and any other imaging modality whenever available, and absence of hypercalcemia. Patients with a serum M spike more than 30 g/L (3 g/dL) were excluded from analysis. However patients with anemia or renal insufficiency that was explained by another process other than myeloma were retained. These patients were followed forward in time until progression to multiple myeloma, death, or last follow-up.
Free light chain assays
The FLC level on all available serum samples (n = 116) was evaluated using the FLC assay (Freelite; The Binding Site, Birmingham, United Kingdom) performed on a Dade-Behring Nephelometer (Deerfield, IL). The assay consists of 2 separate measurements, one to detect free kappa (normal range, 3.3-19.4 mg/L) and the other to detect free lambda (normal range, 5.7-26.3 mg/L) light chains.13-15 Once the absolute levels of free light chains are measured, the ratio of kappa-lambda light chain levels (normal reference range, 0.26-1.65) can be used to assess clonality. If the kappa-lambda FLC ratio is less than 0.26, such a patient is considered to have a monoclonal lambda free light chain, whereas a patient with a ratio more than 1.65 is defined as having a monoclonal kappa free light chain.
Statistical analysis
Overall survival was defined as the time between diagnosis of SBP and either death or last contact with the patient. Time to progression was defined as the time between diagnosis of SBP and progression to myeloma, death, or last contact with the patient. Patients who have not progressed or died before progression were censored for purposes of time to progression. Survival and time to progression were estimated using the method of Kaplan and Meier and groups compared using log-rank tests.17 Cox proportional hazards models were used to determine the impact of FLC abnormalities and other potential risk factors on survival and time to progression. These risk factors were evaluated in both univariate and multivariable analyses.18 Comparisons between groups were performed using 2-sample t tests or rank sum tests for continuous variables and chi square tests for categorical variables. P values less than .05 were considered statistically significant.
Results
Our search identified 116 patients who met the criteria for SBP and for whom we had serum collected at the time of diagnosis and before any therapy. There were 82 males (70%), and the median age was 60 years (range, 26-93 years) (Table 1). In addition to bone surveys, 41% of patients also had magnetic resonance imaging (MRI) or computed tomography (CT) imaging done to assess disease; however, 48% of this imaging was restricted to the area of involvement. In none of these patients was there any evidence of systemic disease with this additional imaging. SBP presented as a symptomatic lytic lesion in 86 patients, with 26 patients having a pathologic fracture at diagnosis. Radiation therapy was given to 94 of the patients, while 4 patients had surgical removal of the lesion.
Clinical characteristics of 116 patients with solitary plasmacytoma of bone
Characteristic . | N . | %* . |
|---|---|---|
| Sex | ||
| Male | 82 | 70 |
| Female | 34 | 30 |
| Age, y | ||
| 0 to 49 | 33 | 28 |
| 50 to 59 | 23 | 20 |
| 60 to 69 | 29 | 25 |
| 70+ | 31 | 27 |
| Serum monoclonal protein type | ||
| No M protein | 42 | 36 |
| IgG | 57 | 49 |
| IgA | 10 | 9 |
| Light chain only | 4 | 3 |
| IgM | 1 | 1 |
| Biclonal | 2 | 2 |
| Size of serum monoclonal protein, g/dL | ||
| 0.5 or more | 63 | 54 |
| 0.51 to 1.0 | 11 | 10 |
| 1.1 to 1.99 | 29 | 25 |
| 2.0 or more | 13 | 11 |
| Urine monoclonal protein type | ||
| Not done | 11 | 10 |
| Kappa | 32 | 27 |
| Lambda | 10 | 9 |
| No monoclonal protein | 63 | 54 |
| Site of plasmacytoma | ||
| Skull | 5 | 4 |
| Spine | 49 | 45 |
| Pelvis | 17 | 16 |
| Long bones | 14 | 13 |
| Ribs | 14 | 13 |
| Other | 0 | 9 |
| Dose of radiation | ||
| No radiation | 4 | 3 |
| Less than 3000 | 1 | 1 |
| 3000 to 3999 | 13 | 12 |
| 4000 to 4999 | 42 | 39 |
| 5000 to 5999 | 32 | 30 |
| 6000 or more | 4 | 3 |
| Unknown | 13 | 12 |
Characteristic . | N . | %* . |
|---|---|---|
| Sex | ||
| Male | 82 | 70 |
| Female | 34 | 30 |
| Age, y | ||
| 0 to 49 | 33 | 28 |
| 50 to 59 | 23 | 20 |
| 60 to 69 | 29 | 25 |
| 70+ | 31 | 27 |
| Serum monoclonal protein type | ||
| No M protein | 42 | 36 |
| IgG | 57 | 49 |
| IgA | 10 | 9 |
| Light chain only | 4 | 3 |
| IgM | 1 | 1 |
| Biclonal | 2 | 2 |
| Size of serum monoclonal protein, g/dL | ||
| 0.5 or more | 63 | 54 |
| 0.51 to 1.0 | 11 | 10 |
| 1.1 to 1.99 | 29 | 25 |
| 2.0 or more | 13 | 11 |
| Urine monoclonal protein type | ||
| Not done | 11 | 10 |
| Kappa | 32 | 27 |
| Lambda | 10 | 9 |
| No monoclonal protein | 63 | 54 |
| Site of plasmacytoma | ||
| Skull | 5 | 4 |
| Spine | 49 | 45 |
| Pelvis | 17 | 16 |
| Long bones | 14 | 13 |
| Ribs | 14 | 13 |
| Other | 0 | 9 |
| Dose of radiation | ||
| No radiation | 4 | 3 |
| Less than 3000 | 1 | 1 |
| 3000 to 3999 | 13 | 12 |
| 4000 to 4999 | 42 | 39 |
| 5000 to 5999 | 32 | 30 |
| 6000 or more | 4 | 3 |
| Unknown | 13 | 12 |
Percentages may not total 100 because of rounding.
An M protein was detected in the serum of 74 patients (64%), and this could be accurately quantitated (> 3 g/L [0.3 g/dL]) in 63 patients; in 11 patients the serum was positive only by immunofixation. The remaining 42 patients had no M spike (0 g/L [0 g/dL]). The median size of the serum M spike was 5 g/L (0.5 g/dL; range, 0-30 g/L [0-3.0 g/dL]). Urine protein electrophoresis was performed on 105 patients. Of these, an M protein in the urine was detectable in 42 (40%) patients, with 16 patients having only Bence Jones proteinuria. The median urine M spike was 0 g/24 hours (range, 0-0.2 g/24 hours). Overall, an M protein could be detected in the serum or urine at baseline in 83 patients (72%).
Results of the FLC assay
The FLC ratio was normal in 62 (53%) patients and abnormal in 54 (47%). Relevant demographic, clinical, and laboratory characteristics of the patients, based on the presence or absence of an abnormal FLC ratio, are presented in Table 2. Patients with an abnormal FLC had a higher incidence of monoclonal protein in the urine (P < .001) and a larger serum M spike (P = .04).
Demographic, clinical, and laboratory characteristics of patients with solitary plasmacytoma of bone for whom an FLC ratio was available at the time of diagnosis (N = 116)
. | FLC ratio . | . | . | |
|---|---|---|---|---|
| Characteristic . | Abnormal . | Normal . | P . | |
| Sex, no. (%) | .63 | |||
| Male | 37 (68.5) | 45 (72.6) | ||
| Female | 17 (31.5) | 17 (27.4) | ||
| Age at diagnosis, y | .74 | |||
| Median | 61.5 | 59.5 | ||
| Range | 26.0-93.0 | 30.0-86.0 | ||
| Fracture, no. (%) | .96 | |||
| Yes | 11 (20.5) | 12 (20.0) | ||
| No | 43 (79.6) | 48 (80.0) | ||
| Hemoglobin, g/L | .53 | |||
| Median | 133 | 138 | ||
| Range | (95-170) | (87-170) | ||
| Serum monoclonal protein, no. (%) | .32 | |||
| Positive | 37 (68.5) | 37 (59.7) | ||
| Negative | 17 (31.5) | 25 (40.3) | ||
| Serum M spike, g/dL | .04 | |||
| Median | 1.0 | 0.3 | ||
| Range | 0.0-3.0 | 0.0-2.6 | ||
| Urine monoclonal protein, no. (%) | < .001 | |||
| Positive | 31 (60.8) | 11 (20.4) | ||
| Negative | 20 (39.2) | 43 (79.6) | ||
| Urine M spike, g/24 h | .58 | |||
| Median | 0.0 | 0.0 | ||
| Range | 0.0-0.2 | 0.0-0.2 | ||
| Reduction in uninvolved Igs, no. (%) | .19 | |||
| 0 | 15 (51.7) | 21 (72.4) | ||
| 1 | 10 (34.5) | 7 (24.1) | ||
| 2 | 1 (3.4) | 4 (13.8) | ||
. | FLC ratio . | . | . | |
|---|---|---|---|---|
| Characteristic . | Abnormal . | Normal . | P . | |
| Sex, no. (%) | .63 | |||
| Male | 37 (68.5) | 45 (72.6) | ||
| Female | 17 (31.5) | 17 (27.4) | ||
| Age at diagnosis, y | .74 | |||
| Median | 61.5 | 59.5 | ||
| Range | 26.0-93.0 | 30.0-86.0 | ||
| Fracture, no. (%) | .96 | |||
| Yes | 11 (20.5) | 12 (20.0) | ||
| No | 43 (79.6) | 48 (80.0) | ||
| Hemoglobin, g/L | .53 | |||
| Median | 133 | 138 | ||
| Range | (95-170) | (87-170) | ||
| Serum monoclonal protein, no. (%) | .32 | |||
| Positive | 37 (68.5) | 37 (59.7) | ||
| Negative | 17 (31.5) | 25 (40.3) | ||
| Serum M spike, g/dL | .04 | |||
| Median | 1.0 | 0.3 | ||
| Range | 0.0-3.0 | 0.0-2.6 | ||
| Urine monoclonal protein, no. (%) | < .001 | |||
| Positive | 31 (60.8) | 11 (20.4) | ||
| Negative | 20 (39.2) | 43 (79.6) | ||
| Urine M spike, g/24 h | .58 | |||
| Median | 0.0 | 0.0 | ||
| Range | 0.0-0.2 | 0.0-0.2 | ||
| Reduction in uninvolved Igs, no. (%) | .19 | |||
| 0 | 15 (51.7) | 21 (72.4) | ||
| 1 | 10 (34.5) | 7 (24.1) | ||
| 2 | 1 (3.4) | 4 (13.8) | ||
Univariate analysis for progression and survival using risk factors at the time of diagnosis
With a median follow-up of 9.1 years (range, 25 days–27 years), 78 patients have died and 38 are alive. In this cohort of patients, 43 (37%) have progressed to multiple myeloma, including one patient who had an IgM monoclonal protein. For those patients who progressed to myeloma, the median time to progression was 1.8 years (range, 0.1-12.7 years).
Results of univariate analysis of risk factors at the time of first diagnosis for time to progression are listed on Table 3. Age, sex, baseline hemoglobin concentration, the presence or size of a serum monoclonal protein, and size of urine monoclonal protein were not significant adverse predictors of progression. The presence of an abnormal FLC ratio (defined as being outside the conventional reference range of 0.26-1.65; ie, < 0.26 or > 1.65) and the presence of a urinary M protein at baseline were significant predictors of progression. Patients with an abnormal FLC ratio (< 0.26 or > 1.65) had a higher risk of progression to multiple myeloma compared with those with a normal ratio (P = .039) (Table 3), with the risk being 44% at 5 years, 51% at 10 years, and 51% at 15 years (Figure 1A). For patients with a normal FLC ratio, the corresponding risk of progression over the same time intervals was 26%, 32%, and 36%, respectively. The adverse effect of FLC ratio was also noted when it was defined as being outside the revised normal range of 0.25 to 4.0 that has been proposed for MGUS (P = .014) (Table 3).
Univariate analysis at diagnosis for time to progression to multiple myeloma in patients with solitary plasmacytoma of bone
Parameter . | N . | Hazard ratio . | Confidence interval . | P . |
|---|---|---|---|---|
| Age | 116 | 1.02 | 1.00-1.04 | .078 |
| Male sex | 116 | 0.62 | 0.33-1.18 | .144 |
| Hemoglobin | 116 | 0.93 | 0.80-1.10 | .431 |
| Serum M spike | ||||
| Positive | 116 | 1.50 | 0.77-2.92 | .235 |
| Size | 116 | 1.17 | 0.84-1.62 | .360 |
| Urine M spike | ||||
| Positive | 105 | 2.55 | 1.33-4.89 | .005 |
| Size | 105 | 0.70 | 0.31-1.62 | .409 |
| Free light chain assay | ||||
| Ratio less than 0.26 or more than 1.65 | 116 | 1.89 | 1.03-3.45 | .039 |
| Ratio less than 0.25 or more than 4.0 | 116 | 2.14 | 1.17-3.95 | .014 |
| Elevated light chain | 107 | 1.68 | 0.89-3.16 | .107 |
| Reduced uninvolved Igs | 60 | 0.80 | 0.41-1.57 | .513 |
| Type of treatment | 116 | 1.67 | 0.91-3.05 | .095 |
| Site of plasmacytoma | 115 | 1.37 | 0.33-5.68 | .662 |
Parameter . | N . | Hazard ratio . | Confidence interval . | P . |
|---|---|---|---|---|
| Age | 116 | 1.02 | 1.00-1.04 | .078 |
| Male sex | 116 | 0.62 | 0.33-1.18 | .144 |
| Hemoglobin | 116 | 0.93 | 0.80-1.10 | .431 |
| Serum M spike | ||||
| Positive | 116 | 1.50 | 0.77-2.92 | .235 |
| Size | 116 | 1.17 | 0.84-1.62 | .360 |
| Urine M spike | ||||
| Positive | 105 | 2.55 | 1.33-4.89 | .005 |
| Size | 105 | 0.70 | 0.31-1.62 | .409 |
| Free light chain assay | ||||
| Ratio less than 0.26 or more than 1.65 | 116 | 1.89 | 1.03-3.45 | .039 |
| Ratio less than 0.25 or more than 4.0 | 116 | 2.14 | 1.17-3.95 | .014 |
| Elevated light chain | 107 | 1.68 | 0.89-3.16 | .107 |
| Reduced uninvolved Igs | 60 | 0.80 | 0.41-1.57 | .513 |
| Type of treatment | 116 | 1.67 | 0.91-3.05 | .095 |
| Site of plasmacytoma | 115 | 1.37 | 0.33-5.68 | .662 |
In addition to effects on time to progression, there was an adverse effect of an abnormal (< 0.25 or > 4.0) baseline FLC ratio on overall survival as well (P = .033) (Figure 1B). Other risk factors for overall survival on univariate analysis are listed on Table 4.
Univariate analysis for overall survival in patients with solitary plasmacytoma of bone
Parameter . | N . | Hazard ratio . | Confidence interval . | P . |
|---|---|---|---|---|
| Age | 116 | 1.07 | 1.04-1.09 | < .001 |
| Male sex | 116 | 0.65 | 0.40-1.06 | .083 |
| Hemoglobin | 116 | 0.91 | 0.80-1.03 | .013 |
| Serum M spike | ||||
| Positive | 116 | 0.99 | 0.62-1.59 | .980 |
| Size | 116 | 0.99 | 0.77-1.27 | .927 |
| Urine M spike | ||||
| Positive | 105 | 1.58 | 0.97-2.56 | .066 |
| Size | 105 | 0.74 | 0.43-1.25 | .262 |
| Free light chain assay | ||||
| Ratio less than 0.26 or more than 1.65 | 116 | 1.51 | 0.97-2.36 | .070 |
| Ratio less than 0.25 or more than 4.0 | 116 | 1.68 | 1.04-2.69 | .033 |
| Elevated light chain | 107 | 1.28 | 0.81-2.02 | .300 |
Parameter . | N . | Hazard ratio . | Confidence interval . | P . |
|---|---|---|---|---|
| Age | 116 | 1.07 | 1.04-1.09 | < .001 |
| Male sex | 116 | 0.65 | 0.40-1.06 | .083 |
| Hemoglobin | 116 | 0.91 | 0.80-1.03 | .013 |
| Serum M spike | ||||
| Positive | 116 | 0.99 | 0.62-1.59 | .980 |
| Size | 116 | 0.99 | 0.77-1.27 | .927 |
| Urine M spike | ||||
| Positive | 105 | 1.58 | 0.97-2.56 | .066 |
| Size | 105 | 0.74 | 0.43-1.25 | .262 |
| Free light chain assay | ||||
| Ratio less than 0.26 or more than 1.65 | 116 | 1.51 | 0.97-2.36 | .070 |
| Ratio less than 0.25 or more than 4.0 | 116 | 1.68 | 1.04-2.69 | .033 |
| Elevated light chain | 107 | 1.28 | 0.81-2.02 | .300 |
Effect of positive monoclonal protein and abnormal serum free light chain ratio 1 to 2 years following therapy
Unlike the risk factors studied in the last section, the prognostic effect of a persistent M protein is available as a risk factor only after completion of therapy. It is typically assessed as a risk factor 1 to 2 years following initial diagnosis. The prognostic effect of a positive serum M protein by electrophoresis or immunofixation 1 to 2 years following diagnosis of solitary plasmacytoma was assessed in 75 patients who had repeat estimations done in that time period and had not already progressed to myeloma. On univariate analysis, the presence of a serum M protein 1 to 2 years following diagnosis was a significant predictor of adverse prognosis, with a hazard ratio of 3.0 for time to progression (95% confidence interval [CI], 1.2-7.4; P = .02). The corresponding hazard ratio for the presence of a urine M protein 1 to 2 years following initial diagnosis was 3.6 (95% CI, 1.6-8.1; P = .002). The size of the serum M protein (when studied as a continuous variable) was associated with an adverse outcome, with a hazard ratio of 2.0 for time to progression (95% CI, 1.4-2.9; P < .001). Patients with a serum M protein level of 5 g/L (0.5 g/dL) or more (n = 32) 1 to 2 years following diagnosis had a higher risk of progression compared with patients with serum M protein level of 0.0 to 5 g/L (0.5 g/dL; n = 43), 50% versus 13% at 5 years, respectively (P < .001).
Similarly, we assessed the prognostic value of an abnormal serum free light chain ratio 1 to 2 years following diagnosis in 44 patients in whom serum samples during that time period were available for analysis. On univariate analysis, abnormal free light chain ratio (< 0.26 or > 1.65) 1 to 2 years following diagnosis was noted in 17 patients, and was a significant predictor of adverse prognosis, with a hazard ratio of 3.5 for time to progression (95% confidence interval [CI], 1.3-8.9; P = .01).
Kaplan-Meier plots for time to progression and overall survival in our cohort of patients with solitary bone plasmacytoma. For the analysis, 116 patients with available free light chain (FLC) ratios were evaluated. An abnormal FLC ratio at diagnosis had an adverse effect on both time to progression to multiple myeloma (A) and on overall survival (B).
Kaplan-Meier plots for time to progression and overall survival in our cohort of patients with solitary bone plasmacytoma. For the analysis, 116 patients with available free light chain (FLC) ratios were evaluated. An abnormal FLC ratio at diagnosis had an adverse effect on both time to progression to multiple myeloma (A) and on overall survival (B).
Multivariate analysis
Among risk factors tested at baseline, since serum FLC assay and urine M protein assessment at baseline essentially measure the same risk factor (ie, monoclonal light chains), they cannot be tested for independence in a multivariate model.
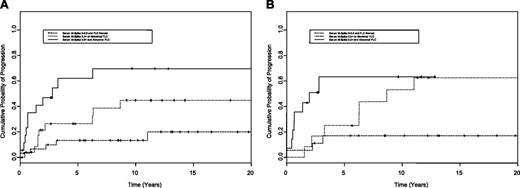
At 1 to 2 years following diagnosis, 2 variables were determined on univariate analysis to have prognostic value for time to progression: abnormal free light chain ratio at diagnosis and serum M protein level of 5 g/L (0.5 g/dL) or more. A risk stratification model was constructed using 2 variables of FLC ratio and M protein level: patients with a normal FLC ratio and serum M protein level less than 5 g/L (0.5 g/dL) 1 to 2 years following diagnosis (low risk, n = 31); with either risk factor abnormal (intermediate risk, n = 26); and with both an abnormal FLC ratio and M protein level of 5 g/L (0.5 g/dL) or more (high risk, n = 18). The corresponding progression rates at 5 years were significantly different in the low, intermediate, and high groups: 13%, 26%, and 62%, respectively (P < .001; Figure 2A).
A risk stratification model was also constructed using repeat FLC measurements 1 to 2 years following diagnosis and serum M protein at 1 to 2 years following diagnosis: patients with a normal FLC ratio and serum M protein level less than 5 g/L (0.5 g/dL) 1 to 2 years following diagnosis (low risk; n = 18), either risk factor abnormal (intermediate risk, n = 12), and both an abnormal FLC ratio and M protein level of 5 g/L (0.5 g/dL) or more (high risk, n = 14). The corresponding progression rates at 5 years were significantly different in the low, intermediate, and high groups, 17%, 25%, and 63%, respectively (P = .01) (Figure 2B).
Discussion
Solitary plasmacytoma of bone is a heterogenous condition. Some patients have a single bone lesion due to local monoclonal plasma-cell proliferation, while in others SBP may be precursor to multiple myeloma. With the availability of magnetic resonance imaging, the risk of misdiagnosing SBP for early multiple myeloma seems to be less.7,19 However, the potential risk of eventual progression to myeloma persists, and therefore the clinical management of these patients would be significantly enhanced if factors that reliably identify patients at high risk of progression were available.
An abnormal FLC ratio has been recently shown to be a powerful prognostic factor in determining the risk of progression of MGUS to multiple myeloma.16 Thus, we hypothesized that the same assay may be useful in patients with SBP. In this study, we demonstrate that the immunoglobulin FLC ratio obtained at the time of diagnosis is abnormal in nearly half of patients and is a powerful predictor of the risk of progression to multiple myeloma in patients with SBP. Patients with a normal ratio have a small risk of progression despite extended follow-up. In this study, the median time to progression in those who progressed to myeloma was 1.8 years (range, 0.1-12.7 years), which is very similar to what has been observed by others.3,6-8 This suggests that our results are not due to bias but represent a typical population with SBP. Our results indicate that patients with an abnormal FLC ratio are at highest risk of progression early after diagnosis; after 10 years, the risk seems to stabilize although it does not disappear. Thus, patients with SBP and an abnormal FLC ratio at diagnosis require frequent re-evaluation after therapy to minimize complications of active myeloma should it occur.
Complete disappearance of the monoclonal protein following therapy has also been reported to be associated with a low risk of progression to myeloma.10 While our observations do support this conclusion, by necessity, assessment of this risk factor requires at least a year of observation and therefore is not a useful prognostic tool at the time of diagnosis. In this respect, the serum FLC ratio at diagnosis is unique since this simple assay can provide powerful prognostic information that can guide management of these patients. The persistence of a serum M protein and the serum FLC assay can complement each other in estimating risk. In this paper, we have developed a risk-stratification model using the serum FLC ratio and the persistence of a serum M protein one year or more following diagnosis. Despite the small sample size, the model identifies 3 cohorts of patients with markedly different risk of progression to myeloma.
Progression of solitary bone plasmacytoma. Risk of progression of solitary bone plasmacytoma to myeloma using a risk stratification model that incorporates the serum free light chain (FLC) ratio measured at baseline (A) or 1 to 2 years following diagnosis (B) and the persistence of serum monoclonal protein 1 to 2 years following diagnosis. The top curve illustrates risk of progression with time in patients with both risk factors, namely an abnormal serum free light chain ratio (< 0.26 or > 1.65) and M protein level of 5 g/L (0.5 g/dL) or more (high risk); the second gives the risk of progression in patients with any one of the 2 risk factors (intermediate risk); the third curve illustrates the risk of progression with neither risk factor present (low risk). The date at which the test for presence of serum M protein 1 to 2 years following diagnosis was determined was used as time 0 for this analysis.
Progression of solitary bone plasmacytoma. Risk of progression of solitary bone plasmacytoma to myeloma using a risk stratification model that incorporates the serum free light chain (FLC) ratio measured at baseline (A) or 1 to 2 years following diagnosis (B) and the persistence of serum monoclonal protein 1 to 2 years following diagnosis. The top curve illustrates risk of progression with time in patients with both risk factors, namely an abnormal serum free light chain ratio (< 0.26 or > 1.65) and M protein level of 5 g/L (0.5 g/dL) or more (high risk); the second gives the risk of progression in patients with any one of the 2 risk factors (intermediate risk); the third curve illustrates the risk of progression with neither risk factor present (low risk). The date at which the test for presence of serum M protein 1 to 2 years following diagnosis was determined was used as time 0 for this analysis.
Although it has been reported that the presence or size of the serum M protein at the time of initial diagnosis can be a predictor of risk, our studies do not support such a conclusion. On the other hand, presence of a urinary M protein was a risk factor in this study. This is an expected finding since both the urine protein electrophoresis and immunofixation studies and the serum FLC assay identify the presence of the same risk factor (ie, free monoclonal kappa or lambda light chains) albeit with different levels of sensitivity. The serum assay has the obvious advantage of not requiring a 24-hour urine protein collection, which is cumbersome. But our study provides evidence for the first time that the presence of monoclonal free light chains at the time of initial diagnosis in patients with solitary plasmacytoma regardless of the method of detection (serum free light chain assay or urine monoclonal protein studies) is an important risk factor for progression. The choice of how to detect clonal free light chains—urine studies versus serum free light chain ratio—is something clinicians can decide based on which test is felt to be more economical and convenient.
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2006-04-015784.
Supported in part by grants CA62242, CA85818, CA93842, and CA100080 from the National Cancer Institute, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal