Abstract
Autoimmune polyendocrine syndrome type I (APS I) is an inherited recessive disorder with a progressive immunological destruction of many tissues including the adrenal cortex, the parathyroid glands, and the gonads. APS I is caused by mutations in the AIRE gene (autoimmune regulator), expressed in cells of the thymus and spleen, suggesting a role in central and peripheral tolerance. Aire–/– mice replicate the autoimmune features of APS I patients with the presence of multiple autoantibodies and lymphocytic infiltrates in various tissues, but young mice appear clinically healthy. We here report the investigation of 15- to 24-month-old Aire–/– mice. We did not observe any endocrinological abnormalities, nor did sera from these mice recognize known APS I autoantigens. Interestingly, however, there was a high frequency of marginal zone B-cell lymphoma in Aire–/– mice and liver infiltrates of B cells, suggesting chronic antigen exposure and exaggerated activation. Furthermore, increased numbers of monocytes in blood were identified as well as augmented numbers of metallophilic macrophages in the spleen. We propose that Aire, in addition to its function in the thymus, also has a peripheral regulatory role by controlling the development of antigen-presenting cells (APCs) and marginal zone B-cell activation.
Introduction
In autoimmune polyendocrine syndrome type I1 (APS I, or autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy [APECED]; Online Mendelian Inheritance in Man [OMIM] no. 240300), an inherited autoimmune disorder without major histocompatibility complex (MHC) linkage,2,3 patients progressively lose tolerance to various tissue-specific antigens, with ensuing endocrine and nonendocrine disorders. APS I patients suffer from multiple organ failures due to immunologic destruction of the adrenal cortex, the parathyroid glands, the liver, β-cells of the islets of Langerhans, and enterochromaffin cells of the small intestine. In addition, probably as a sign of immune dysfunction, the patients suffer from a chronic mucocutaneous candidiasis.1 The prevalence of APS I is increased in Finland, Sardinia, and among Iranian Jews.4-6 The disease-causing gene was localized to the long arm of chromosome 217 and has later been identified and named the autoimmune regulator (AIRE).8,9 AIRE has been proposed to function as a transcription factor.10-14
Aire is expressed in thymic medullary epithelial cells, in dendritic cells,15-18 and in blood monocytes,19 suggesting a role of Aire in both central and peripheral tolerance. To define the role of Aire in immunologic tolerance, we previously reported an Aire–/– mouse line engineered to reproduce the most common Finnish APS I mutation.20 In analogy with APS I, Aire–/– mice display multiple organ-specific autoantibodies and lymphocytic infiltrates.20,21 Since Aire is expressed in medullary epithelial cells of the thymus (mTEC), studies have so far focused on central tolerance and have addressed the role of Aire in negative selection.21,22 Aire has been proposed to activate transcription of organ-specific antigens in thymic medullary epithelial cells and by this way induce deletion of autoreactive T cells,21 but since autoreactivity against antigens normally expressed in Aire–/– mTECs has been observed,23 and additional genes than organ-specific antigens have instead been found to be differentially regulated in Aire–/– mTECs,24 it has become clear that the mechanism through which Aire controls negative selection is more complicated than initially thought. We have recently described the role of Aire in peripheral antigen-presenting cells (APCs), demonstrating a regulatory function of APCs dependent on costimulatory molecules.25 Maturation, distribution, and activation of T cells appear normal in young Aire–/– mice, and no tissue destruction has been observed under normal- or low-pathogen environmental conditions.20,21 Furthermore, the number and function of CD4+CD25+ regulatory T cells have also been found to be normal.23,26
The antigens involved in mouse autoimmunity have not been defined, whereas many of the organ-specific autoantigens in APS I have been identified.27 The presence of autoantibodies among APS I patients is of high predictive value28 since they frequently precede the onset of clinical disease and signal ongoing organ destruction.29 Most studies have hitherto been performed on mice younger than 12 weeks old, without apparent disease. As autoimmunity in human patients with APS I develops over time, typically over many years or even decades, we here report a study on 15- to 24-month-old Aire–/– mice. Our data suggest that the defect produced in Aire–/– mice has consequences early in the hematologic development of the monocyte lineage and causes an increased risk to develop marginal zone B-cell lymphoma (MZL) in aged Aire–/– mice.
Materials and methods
Animals
The generation and breeding of Aire (B6.129S4-Airetm1Pltn)–/– mice has been described previously.20 The mice used herein were back-crossed for 6 generations onto the C57Bl/6 background. A cohort of age- and sex-matched 15- to 24-month-old Aire–/– and Aire+/+ littermates was used for the experiments. The work was approved by the local ethics committee (Uppsala Djurförsöksetiska Nämnd).
Immunofluorescent stainings
For immunofluorescent stainings, we used cryostat sections of frozen livers and adrenal glands. The sections were washed in phosphate-buffered saline (PBS) and blocked for 30 minutes with 1% bovine serum albumin (BSA), 2% goat or donkey serum (Sigma, Saint Louis, MO) in PBS. The slides were incubated with primary antibody or mouse serum overnight at 4°C. Slides were washed in PBS, incubated with fluorochrome conjugated secondary antibodies for 45 minutes, washed, and mounted in Vectashield (Vector Laboratories, Burlingame, CA). The primary antibodies used were rat IgG2b anti-IAb (M5/114; American Type Culture Collection, Manassas, VA), rat IgG2a anti-CD3 (Santa Cruz Biotechnology, Santa Cruz, CA), rat IgG2a anti-B220 (BD Pharmingen, San Diego, CA), and rat IgG2a anti-CD9 (BD Pharmingen). The secondary antibody was goat anti–rat Ig-FITC (BD Pharmingen) or donkey anti–mouse Ig-FITC (Jackson Immunoresearch, West Grove, PA) for detection of autoantibodies in mouse sera. Pictures of the stained slides were taken with a LEICA DMRB microscope and LEICA DC 200 camera (Leica, Heidelberg, Germany), and they were acquired with LEICA QWin software. A longer exposure time was used for negative control slides in order to visualize the otherwise too dark tissue.
Flow cytometry
Spleens, thymi, and livers were homogenized to single-cell suspensions, red blood cells from spleens were lysed with ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM EDTA at pH 7.2), and the cells were resuspended in fluorescence-activated cell-sorting (FACS) buffer (PBS, 2% fetal calf serum [FCS], 0.05% sodium azide). Bone marrow cells were prepared as described in “Hematologic evaluation.” The cells were stained for flow cytometry using the following antibodies: FITC-conjugated antibodies, anti-B220, anti-IgD, anti-CD3, and anti-Gr1; PE-conjugated antibodies, anti-CD21, and anti-IAb; APC-conjugated antibodies, anti-CD11b, and anti-CD19; and biotin-conjugated antibodies, anti-CD1d (BD Pharmingen). Biotinylated antibodies were detected using streptavidin-PE or streptavidin-PerCP (BD Pharmingen). The data were collected with a FACS-Calibur flow cytometer and analyzed with the Cell Quest pro software (Becton Dickinson, San Jose, CA).
Immunohistochemical stainings
Metallophilic macrophages were detected using FITC-conjugated MOMA-1 (Serotec, Raleigh, NC) and marginal zone B cells (MZBs) using biotinylated anti-CD1d antibody (BD Pharmingen). Secondary antibodies for immunohistochemistry, anti-FITC F(ab′) horseradish peroxidase (HRP), or antibiotin F(ab′) alkaline phosphatase (AP) were from DAKO (Carpinteria, CA). Vector Blue Alkaline Phosphatase Substrate (Vector Laboratories) and DAB peroxidase substrate (DAKO) were used for development of immunohistochemistry stains. Stainings were done on cryosections of spleens that were fixated in acetone for 3.5 minutes and blocked with 2% goat serum in PBS. Thereafter the slides were incubated with primary antibodies for 1 hour at room temp. Slides were washed in PBS and incubated with HRP and AP-conjugated secondary antibodies for 1 hour. After washing with PBS, substrates for HRP and then AP were added. Pictures were taken with the LEICA microscope and subject to color adjustment with Adobe Photoshop 7.0 (Adobe, San Jose, CA).
Histology
Tissues from mice were fixed by immersion in 10% formalin overnight, rinsed in PBS, dehydrated through increasing concentrations of ethanol, cleared and embedded in paraffin, cut into 6-μm sections, and mounted on slides. The sections were stained with hematoxylin and eosin (H&E) and examined in a blind fashion by a mouse pathologist.
Hematologic evaluation
Aire–/– and Aire+/+ mice were killed and whole blood was collected immediately from the heart using EDTA-coated syringes to prevent coagulation and was placed into EDTA-coated tubes (Becton Dickinson). Differential counts of leukocyte subsets were performed on May-Grünwald-Giemsa–stained blood smears by identifying at least 100 cells. The femurs were surgically removed from the animals and the femoral head was cut. Bone marrow was flushed out 5 times with 2 mL PBS containing 50% FCS (Invitrogen, Paisley, United Kingdom) by using a 19-gauge needle and a 2 mL syringe. The bone marrow cell suspension was underlayered with 1 mL FCS and centrifuged at 300g for 5 minutes at 4°C. The pellet was resuspended in 2 mL of ice-cold PBS containing 0.5% BSA, and the total number of cells was calculated using a hemacytometer. Cells were diluted to 2 × 106/mL and 3 cytospin glasses were prepared using a cytofunnel sample chamber (Shandon, Pittsburgh, PA). Cytospins were stained with May-Grünwald-Giemsa and microscopical evaluation and classification of 300 cells into erythroid, myeloid, and lymphoid cells was performed in a blind fashion by an experienced hematopathologist.
BrdU incorporation
Mice were given intraperitoneal injections of 2 mg bromodeoxiuridine (BrdU). After 1 hour they were killed, and spleen and femurs were collected. Cells from spleen and bone marrow were prepared and stained for cell-surface markers as described for flow cytometry and then they were fixed and stained for BrdU using the FITC BrdU Flow kit (BD Pharmingen) according to manufacturer's instructions.
Clonality PCR
Genomic DNA was extracted from spleens using the EZNA Tissue DNA Kit (Omega Bio-Tek, Doraville, GA). The heavy chain of the immunoglobulin gene was amplified at the D-J rearrangement junction using 2 previously published primers:30 forward DSF, 5′AGGGATCCTTGTGAAGGGATCTACTACTGTG 3′ and reverse JH4-FAM 5′AAAGACCTGCAGAGGCCATTCTTACC 3′; the reverse primer was fluorescently labeled with blue-6-FAM. The polymerase chain reaction (PCR) amplification of splenic DNA was performed using 94°C for denaturation, 59°C for annealing, and 72°C for extension with 1 minute each for 39 cycles. The PCR products were mixed with HiDi formamide (Applied Biosystems, Foster City, CA) and GeneScan 400HD Rox dye standard (Applied Biosystems), denaturated for 2 minutes at 95°C and loaded on an ABI Prism 310 Genetic Analyzer (Applied Biosystems) for capillary electrophoresis. Results were analyzed using the GeneScan 3.7 software (Applied Biosystems).
Statistical methods
The Mann Whitney U test and the Student t test were used in order to test the hypothesis of no difference between the 2 groups, Aire+/+ and Aire–/–, in a set of outcome variables. The used test is indicated in the figure legends. The Mann Whitney U test was used when the data were not of interval type, or when the data were not approximately normally distributed, or when the variances in the 2 groups were not approximately homogeneous. The Mann Whitney U test is a technique for testing the hypothesis of a general difference in distributions between Aire+/+ and Aire–/–, which can be interpreted as a general difference in median values, under favorable circumstances. When these assumptions are satisfied the variables are analyzed with the Student t test. The Student t test is used to test the hypothesis of a general difference in mean values between the Aire+/+ and the Aire–/– individuals. The splenic extramedullary hematopoiesis was estimated in a 3-grade scale and the frequency was compared between Aire+/+ and Aire–/– mice with Kruskal-Wallis test. The frequency of lymphoma was compared with one sided Fisher exact test using the assumption that the frequency of lymphoma is higher in Aire–/– mice since MZL has not been observed in aged Sv129 and C57Bl/6 strains,31 and it is absent in the Aire+/+ group in this study.
In order to test the hypothesis of a general association between development of lymphoma, lymphocytic infiltrates in liver and B-cell infiltrates in thymus Spearman rank order correlation was used. P values equaling .05 were considered statistically significant.
Results
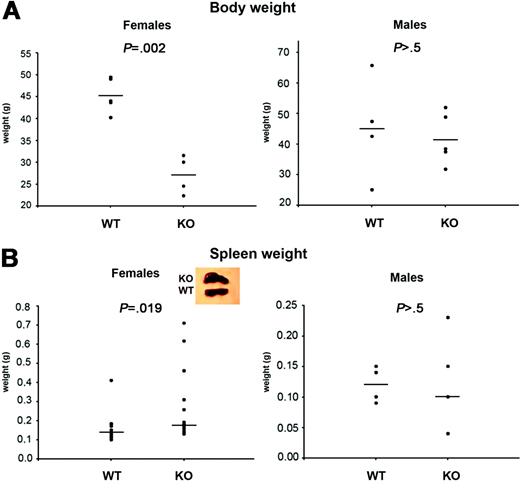
Decreased body weight and splenomegaly among female Aire–/– mice
Young female and male Aire–/– mice appear healthy and have normal weight compared with Aire+/+ littermates (not shown). However, at the age of 15 to 24 months, when Aire–/– females were investigated, they weighed significantly less than Aire+/+ littermate females (Figure 1A, left panel) (P = .002). The body weights of male Aire–/– mice and Aire+/+ mice did not statistically differ (Figure 1A, right panel) (P > .5). Spleens from aged Aire–/– females appeared abnormal on visual inspection (Figure 1B, left panel insert), and their weights were significantly increased (P = .019). Spleens from male Aire–/– mice appeared normal and were of similar size as those from their Aire+/+ counterparts (Figure 1B, right panel) (P > .5).
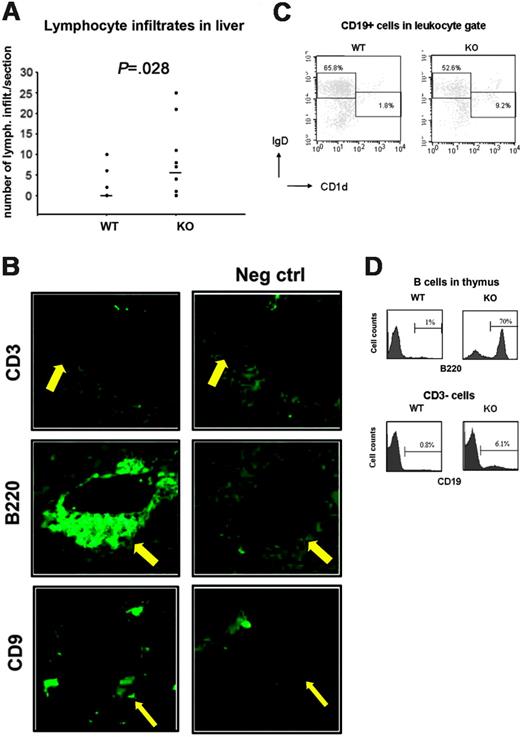
Liver infiltrates in Aire–/– mice are composed of B cells
Previously we reported lymphocytic infiltrates in the livers of Aire–/– mice.20 The livers of older Aire–/– mice displayed a significantly higher frequency (P = .028) (Figure 2A) and larger inflammatory cell infiltrates in comparison with livers from Aire+/+ mice. In order to further characterize these infiltrates, Aire–/– liver sections were analyzed and stained negatively for the T-cell marker CD3 (Figure 2B), but positively for the B-cell marker B220 (Figure 2B), class II H2-IAb (not shown), and the marginal zone B-cell and plasma cell marker CD932 (Figure 2B). A further characterization of the B-cell infiltrates in the liver by flow cytometry showed an enrichment of CD19+ B cells with an IgDloCD1dhi marginal zone phenotype and a depletion of B cells with a follicular IgDhiCD1dlo/– phenotype33 in Aire–/– mice (Figure 2C). Furthermore, 3 of 8 Aire–/– mice displayed a higher frequency (more than 10%) of B220+ cells in the thymus, ranging from 14% to 70%. In one Aire–/– mouse the thymus was totally overtaken by B220+ cells (Figure 2D). Since the B220 marker can be expressed by subsets of T cells in addition to B cells, we performed double stainings for CD19 and CD3, and we could confirm that the B220+ infiltrates that we had observed were composed of CD19+CD3– B cells (Figure 2E). This demonstrates that the majority of infiltrating lymphocytes in Aire–/– mice are of B-cell origin.
Body and spleen weight of aged Aire-deficient mice. (A) Body and (B) spleen weight were measured in 15-month-old Aire wild-type (WT) or knock-out (KO) mice kept in a conventional animal facility. Each symbol corresponds to a different animal. P values were calculated with t test for panel A and Mann-Whitney test for panel B. Mean values (A) or median (B) are indicated by horizontal lines. n = 4 WT males, 5 KO males (body and spleen weight); n = 5 WT females, 5 KO females (body weight); n = 13 WT females, 14 KO females (spleen weight).
Body and spleen weight of aged Aire-deficient mice. (A) Body and (B) spleen weight were measured in 15-month-old Aire wild-type (WT) or knock-out (KO) mice kept in a conventional animal facility. Each symbol corresponds to a different animal. P values were calculated with t test for panel A and Mann-Whitney test for panel B. Mean values (A) or median (B) are indicated by horizontal lines. n = 4 WT males, 5 KO males (body and spleen weight); n = 5 WT females, 5 KO females (body weight); n = 13 WT females, 14 KO females (spleen weight).
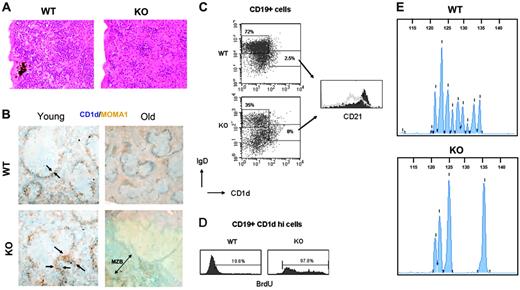
Aire-deficient mice develop MZLs and fail to expand extramedullary hematopoiesis in the spleen
The histologic examination of the following organs—thyroid, thymus, pancreas, kidney, adrenal gland, ovary, testis, and lung—showed changes consistent with aging (data not shown). As reported previously,20,21,34 lymphocytic infiltrates were found in the pancreas, lung, adrenal, thyroid, and ovary of Aire–/– mice (not shown), with the highest frequency observed in the liver (Figure 2A) and lung (P = .045, not shown). The spleens of Aire+/+ mice had a normal age-dependent expansion of the extramedullary hematopoiesis (EH),35 while surprisingly, in Aire–/– mice EH was very low or absent (P = .05) (Figure 3A). Another interesting finding was a hyperplasia of the marginal zone in 4 of 11 Aire–/– mice (P = .04). Importantly, 3 of 4 cases had developed into an early-stage MZL with irregular nucleus, clumped chromatin, and increased cytoplasm with villous appearance (Figure 3B, bottom right panel). Interestingly, the MZL correlated with the highest number of B-lymphocytic infiltrates in liver (P < .05) and in the thymus (P < .05), and all the lymphoma mice displayed antibodies against liver and adrenal sections (not shown). MZL was not present in any of the Aire+/+ mice (Figure 3B, top right panel) nor in young (6-week-old) Aire–/– mice (Figure 3B, bottom left panel). In addition, judged by histologic evaluation the marginal zone of young Aire–/– mice displayed a higher number of metallophilic macrophages (Figure 3B, bottom left panel) but normal numbers of marginal zone macrophages (not shown) and red pulp macrophages (not shown). We further characterized the phenotype of the splenic B cells by flow cytometric stainings of Aire–/– spleens with and without MZL and could observe an expansion of IgDloCD1dhi MZBs even in elderly Aire–/– mice without MZL with a corresponding decrease of IgDhiCD1dlo/– follicular B cell numbers (Figure 3C, left panel). Interestingly, the MZB of Aire–/– mice displayed a strong down-regulation of the complement receptor 2 CD21, which is normally expressed at high levels in this B-cell subset36 (Figure 3C, right panel). To confirm an increased activation and proliferation of MZB in Aire–/– mice, we studied in vivo incorporation of BrdU in the spleen after a short 1-hour pulsing. A higher proliferation of B cells was confirmed in Aire–/– mice, and in particular, one mouse with MZL displayed 98% BrdU+ MZB (Figure 3D). To further confirm that the proliferation and expansion of the MZBs in Aire-deficient mice give rise to a clonal lymphoma, we analyzed the rearrangement of the B-cell receptor (BCR) heavy-chain immunoglobulin gene at the D-J rearrangement junction on genomic DNA extracted from Aire–/– and Aire+/+ spleens using PCR. DNA extracted from Aire+/+ spleens showed a high degree of complexity with many different lengths of the heavy-chain immunoglobulin gene PCR products as a sign of an intact BCR polyclonality (Figure 3E, top panel). In contrast, DNA from 4 of 9 Aire–/– spleens displayed an oligoclonal BCR pattern with only a few PCR product lengths, suggesting the development of a clonal B-cell expansion (Figure 3E, bottom panel) at the expense of the normal diverse BCR repertoire seen in Aire+/+ mice. Thus, histology, BrdU incorporation and BCR clonality demonstrate the development of MZB lymphomas in aged Aire–/– mice.
B cells infiltrate liver and thymus of aged Aire-deficient mice. (A) Number of lymphocytic infiltrates in liver sections. n = 11 WT, 11 KO. Each symbol corresponds to a different animal, and median is indicated by horizontal lines. P value was calculated with Mann-Whitney test. (B) Immunofluorescent stainings for CD3, B220 (×20/1.0 NA objective magnification) and CD9 (×10/1.0 NA objective magnification) were performed on cryostat sections of livers from 3 aged Aire KO mice. Negative control stainings were performed on adjacent sections by adding secondary antibody in the absence of primary antibody. In addition, the negatively staining anti-CD3 antibody is an isotype control of the anti-B220 and anti-CD9 antibodies. Arrows point at the site of lymphocytic infiltration. (C) The follicular versus marginal zone phenotype of the liver B-cell infiltrates was measured by FACS. Leukocytes were gated on forward scatter/side scatter (FSC/SSC) by comparison with a spleen, and then CD19+ cells were further gated. n = 6 WT, 5 KO. Two representative mice are shown. (D) Aire WT or KO thymi were FACSed for B220 expression (1 KO and 1 WT shown). Histograms shown are gated on lymphocytes from FSC/SSC. In an additional experiment, Aire WT or KO thymi were investigated by FACS for CD19 expression. Histograms shown are gated on CD3– lymphocytes.
B cells infiltrate liver and thymus of aged Aire-deficient mice. (A) Number of lymphocytic infiltrates in liver sections. n = 11 WT, 11 KO. Each symbol corresponds to a different animal, and median is indicated by horizontal lines. P value was calculated with Mann-Whitney test. (B) Immunofluorescent stainings for CD3, B220 (×20/1.0 NA objective magnification) and CD9 (×10/1.0 NA objective magnification) were performed on cryostat sections of livers from 3 aged Aire KO mice. Negative control stainings were performed on adjacent sections by adding secondary antibody in the absence of primary antibody. In addition, the negatively staining anti-CD3 antibody is an isotype control of the anti-B220 and anti-CD9 antibodies. Arrows point at the site of lymphocytic infiltration. (C) The follicular versus marginal zone phenotype of the liver B-cell infiltrates was measured by FACS. Leukocytes were gated on forward scatter/side scatter (FSC/SSC) by comparison with a spleen, and then CD19+ cells were further gated. n = 6 WT, 5 KO. Two representative mice are shown. (D) Aire WT or KO thymi were FACSed for B220 expression (1 KO and 1 WT shown). Histograms shown are gated on lymphocytes from FSC/SSC. In an additional experiment, Aire WT or KO thymi were investigated by FACS for CD19 expression. Histograms shown are gated on CD3– lymphocytes.
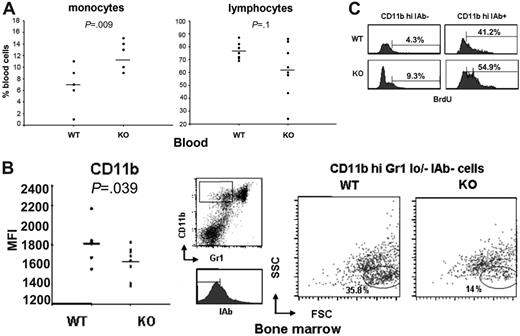
Aire–/– mice have an increased turnover of monocytic myeloid precursors in bone marrow
To further characterize hematopoiesis in Aire-deficient mice, differential cell counts in blood and bone marrow were performed. We observed a significant increase in the percentage of monocytes (P = .009) and a trend to decreased percentage of lymphocytes (P = .1) (Figure 4A) in the blood of Aire–/– mice, while no difference was found in the neutrophil and eosinophil counts (data not shown). The monocytelymphocyte ratio was significantly increased in Aire–/– mice (P = .009). In bone marrow there was no difference in the lymphocytic counts (not shown), in the myeloid-erythroid ratio (Figure S1, left panel, available on the Blood website; see the Supplemental Material link at the top of the online article), or in the megakaryocyte counts (Figure S1, right panel). To investigate the monocyte precursor lineage, we analyzed bone marrow with regards to the marker CD11b. The number of CD11bhi bone marrow cells, which include monocyte precursors,37 appeared decreased in Aire–/– mice but not significantly (data not shown). Furthermore, a lower expression level of CD11b was observed in the CD11bhi population of Aire–/– bone marrow cells (P = .039) (Figure 4B, left panel). To further dissect this issue, we stained bone marrow cells with CD11b, the neutrophil marker Gr-1,38,39 and class II IAb and studied the CD11bhiGr1lo/– IAb– immature myeloid precursors for size and granularity. We could clearly observe a decreased number of myeloid precursors with typical size and granularity of monocytes in Aire–/– mice, whereas the granulocyte precursors were not altered (Figure 4B, right panel). The lower number of myeloid monocytic precursors might be due to a lower proliferation or to a premature differentiation and bone marrow exit of these cells. To distinguish between these 2 possibilities, we studied in vivo incorporation of BrdU in bone marrow after 1 hour of pulsing. The total BrdU incorporation in bone marrow was not altered in Aire–/– mice but differences could be found in the myeloid monocytic precursors, where the more immature CD11bhiIAb– population displayed an increased percentage of highly BrdU-incorporating cells, whereas the more mature CD11bhiIAb+ population displayed an increased number of low BrdU-incorporating cells (Figure 4C). This pattern with a combination of an increased proliferation at early and late stages of myelogenesis (Figure 4C) and accelerated differentiation of monocytic precursors (Figure 4B) suggest a disturbed maturation and a premature exit from the bone marrow, which might account for the increased number of blood monocytes (Figure 4A) and metallophilic macrophages (Figure 3B, bottom left panel) in Aire–/– mice.
Discussion
In previous reports of the Aire–/– mice, a mild phenotype has been described, with decreased fertility, development of autoantibodies, and lymphocytic infiltrates in several organs,20,21 but no apparent clinical disease. This might in part be due to the genetic background of C57Bl/6, a strain that has been reported to be resistant to several autoimmune disease models,40-44 suggesting that Aire interacts with other autoimmunity susceptibility genes to initiate autoimmune disease or that specific environmental triggers are necessary. The influence of the genetic background on the impact of Aire on the phenotype has been recently highlighted in crosses with the nonobese diabetic (NOD) strain, where Aire deficiency causes death by pneumonitis.34 In this report, we extended the phenotypic characterization by analyzing 15- to 24-month-old mice. In APS I, the number of affected organs increases with age27 ; however, aged mice express a phenotype with only minor aberrations compared with the wild-type mice. Aged Aire–/– female mice weigh significantly less than Aire+/+ controls (Figure 1A) and display splenomegaly (Figure 1B), which are both signs of an ongoing clinical disease. The males, however, had normal body and spleen weights. This sex difference is interesting, since it may indicate an autoimmune destruction of some tissue that we have not yet been able to identify. In APS I, males are partially protected from the development of autoimmune hypoparathyroidism.45 Also, gonadal insufficiency is recognized in 60% of the female patients1 but is rarely seen among the male patients.
Hematologic abnormalities of Aire–/– spleens. (A) Extramedullary hematopoiesis of spleens from aged Aire KO and WT mice (×20/1.0 NA objective magnification); sections were stained with H&E. (B) Representative immunohistochemical analysis of 6-week-old (left panel) and 15-month-old (right panel) Aire WT and KO mice. At least 4 serial sections from each mouse were stained for MOMA-1+ (brown) metallophilic macrophages and CD1dhi marginal zone B cells (blue). Objective magnification: ×10/1.0 NA for young mice, ×4/1.0 NA for old mice. The arrows in the left panels outline the thickness of the metallophilic macrophages, and the double arrow in the bottom right panel outlines the extent of the CD1d-positive marginal zone. (C) The follicular versus marginal zone phenotype of the splenic B cells was measured by FACS. The dot plots are gated on CD19+ lymphocytes; the histogram on the right shows the expression of CD21 on IgDloCD1dhi marginal zone B cells in WT (▪) vs KO (gray line). n = 6 WT, 5 KO. Two representative mice are shown. (D) One-hour incorporation of BrdU in CD19+CD1dhi splenic marginal zone B cells of a WT versus a KO mouse with MZL. (E) PCR of splenic DNA at the immunoglobulin heavy-chain gene D-J rearrangement junction with fluorescent primer, analyzed with capillary electrophoresis. Length of the PCR product in base pairs is indicated on the top scale. n = 3 WT, 9 KO. Two representative mice are shown.
Hematologic abnormalities of Aire–/– spleens. (A) Extramedullary hematopoiesis of spleens from aged Aire KO and WT mice (×20/1.0 NA objective magnification); sections were stained with H&E. (B) Representative immunohistochemical analysis of 6-week-old (left panel) and 15-month-old (right panel) Aire WT and KO mice. At least 4 serial sections from each mouse were stained for MOMA-1+ (brown) metallophilic macrophages and CD1dhi marginal zone B cells (blue). Objective magnification: ×10/1.0 NA for young mice, ×4/1.0 NA for old mice. The arrows in the left panels outline the thickness of the metallophilic macrophages, and the double arrow in the bottom right panel outlines the extent of the CD1d-positive marginal zone. (C) The follicular versus marginal zone phenotype of the splenic B cells was measured by FACS. The dot plots are gated on CD19+ lymphocytes; the histogram on the right shows the expression of CD21 on IgDloCD1dhi marginal zone B cells in WT (▪) vs KO (gray line). n = 6 WT, 5 KO. Two representative mice are shown. (D) One-hour incorporation of BrdU in CD19+CD1dhi splenic marginal zone B cells of a WT versus a KO mouse with MZL. (E) PCR of splenic DNA at the immunoglobulin heavy-chain gene D-J rearrangement junction with fluorescent primer, analyzed with capillary electrophoresis. Length of the PCR product in base pairs is indicated on the top scale. n = 3 WT, 9 KO. Two representative mice are shown.
Normal bone marrow hematopoiesis but increased turnover of myeloid monocytic precursors in aged Aire–/– mice. (A) Differential count of monocytes and lymphocytes on blood smears. n = 7 WT, 7 KO. (B) Mean fluorescence intensity of CD11b in CD11bhi cells in bone marrow measured by flow cytometry (left panel); n = 8 WT, 8 KO. Each symbol corresponds to a different animal. P value was calculated with t test. Mean values are indicated by horizontal lines. Size and granularity of CD11bhiGr1lo/–IAb– bone marrow myeloid cells (right panel); gating strategy shown in the middle panel. n = 6 WT, 5 KO. Two representative mice are shown. (C) One-hour BrdU incorporation in CD11bhi IAb– and CD11bhi IAb+ bone marrow myeloid cells gated on monocyte precursors from FSC/SSC. n = 6 WT, 5 KO. Two representative mice are shown.
Normal bone marrow hematopoiesis but increased turnover of myeloid monocytic precursors in aged Aire–/– mice. (A) Differential count of monocytes and lymphocytes on blood smears. n = 7 WT, 7 KO. (B) Mean fluorescence intensity of CD11b in CD11bhi cells in bone marrow measured by flow cytometry (left panel); n = 8 WT, 8 KO. Each symbol corresponds to a different animal. P value was calculated with t test. Mean values are indicated by horizontal lines. Size and granularity of CD11bhiGr1lo/–IAb– bone marrow myeloid cells (right panel); gating strategy shown in the middle panel. n = 6 WT, 5 KO. Two representative mice are shown. (C) One-hour BrdU incorporation in CD11bhi IAb– and CD11bhi IAb+ bone marrow myeloid cells gated on monocyte precursors from FSC/SSC. n = 6 WT, 5 KO. Two representative mice are shown.
In Aire–/– mice, the typical endocrine and ectodermal manifestations present in patients with APS I are absent. We did not find any signs of mucocutaneous Candida infection, hypoparathyroidism (Figure S2A), or Addison disease (Figure S2B) in Aire–/– mice. Thus the classical tissues targeted in APS I seem to be unaffected in Aire–/– mice when investigated endocrinologically, with the sole exception of the liver where a few cases of Aire–/– mice developed elevated serum transaminases as an indirect sign of ongoing hepatocyte damage (Figure S2C).
The organ-specific autoantigens of APS I are well characterized.27 Usually, they are key enzymes in the synthesis of hormones or neurotransmitters in endocrine cells, but the autoantigens recognized by the autoantibodies of Aire–/– mice have not been studied thoroughly. Autoantibodies from APS I patients recognize conserved sites of organ-specific autoantigens from different species, and they inhibit the enzymatic in vitro activity.46 Sera from Aire–/– mice did not recognize APS I autoantigens (Figure S4), implying that the organ-specific autoantigens of Aire–/– mice are distinct from those of APS I. When we compare the self-antigens differentially regulated in Aire–/– mTECs21 to previously identified autoantigens in APS I patients,28 the only antigen in common is the liver enzyme CYP1A2, which was not recognized by sera from Aire–/– mice (Figure S4). Furthermore, liver stainings with Aire–/– sera20 do not correspond to the distribution of the CYP1A2 APS I autoantigen,47 suggesting another identity of the liver antigen recognized by Aire–/– sera. The only autoantigen that has been identified in Aire–/– mice is α-fodrin,23 but the α-fodrin transcript was normally regulated in Aire–/– mTECs. At the present state, there seems to be no correlation between the self-antigens differentially regulated in mTECs by Aire and the autoantigens targeted by autoantibodies in Aire-deficient mice, but this issue needs to be investigated further by identification of autoantigens in Aire-deficient mice and study of their expression in Aire-deficient mTECs.
The differential leukocyte counts in blood showed an increased percentage of monocytes and a decreased percentage of lymphocytes (Figure 4A) in Aire–/– mice. AIRE has been shown to be expressed in blood monocytes,19 and its expression increases during in vitro monocyte differentiation into dendritic cells (DCs).48 AIRE might therefore have a direct function in monocyte and DC development. Myeloid and lymphocyte counts were normal in the bone marrow, but the CD11bhiGr1lo/–IAb– subpopulation of immature monocytic myeloid precursors was decreased (Figure 4B, right panel) in spite of a higher proliferation in Aire–/– mice (Figure 4C). More mature stages of monocytic myeloid precursors displayed also a higher proliferation (Figure 4C), suggesting that Aire–/– monocytes proliferate, differentiate, and migrate out from the bone marrow at a faster rate, causing increased numbers of monocytes in the circulation (Figure 4A).
Aged female Aire–/– mice developed splenomegaly (Figure 1B). In addition, independent of sex and spleen size, 36% of Aire–/– mice displayed a splenic MZL, which was absent in young mice (Figure 3B,D). A high frequency of MZL has previously been described in the New Zealand Black (NZB) strain, in p53-haploinsufficient mice, and in murine leukemia virus–infected NFS.V+ mice,49-51 but there are no reports on C57Bl/6 nor the Sv129 strains,31,50 the backgrounds of the Aire–/– mice. Since none of the Aire+/+ mice developed MZL, it is likely that the development of MZL in Aire–/– mice is a consequence of the missing transcriptional regulator. MZBs usually recognize T-independent (TI) type 2 antigens and are rapidly activated during bacterial infections in the blood.33,36,52 In addition to particulate bacterial antigens, they are often reactive to self-antigens.53,54 It is believed that the initial step in the development of MZL is the chronic exposure of the MZBs to either a pathogen or a self-antigen.55-57 It is in fact well established that in certain autoimmune diseases, especially in Sjögren syndrome, there is a strong association with lymphoma, and in particular with MZL.58-60 This is thought to occur due to constant activation of B cells, which in turn increases the probability of lymphoma development. If this would be the case in Aire–/– mice, it must be an indirect effect, since Aire is not expressed in B cells.25 Indeed, the abnormalities found in the Aire-expressing monocyte population (Figure 4) could point toward such a possibility. Macrophages have previously been shown to be involved in the activation of MZBs.61,62 In one report, marginal metallophilic macrophages have been described to be necessary for the serologic TI type 2 response,63 which is mediated by MZBs, and might therefore be important for the activation of MZBs. Aire–/– mice displayed an increased number of metallophilic macrophages in the spleen (Figure 3B), suggesting that an increased activation mediated by these cells might contribute to the lymphoma development of MZBs. The CD19+IgDloCD1dhi MZBs of Aire–/– mice displayed a low CD21 expression (Figure 3C). Such a down-regulation has been shown to be caused by antigen-dependent activation in both human and mouse B cells,64-67 and is typical in the MZBs of human systemic lupus erythematosus (SLE) and of the mouse MRL/lpr SLE model.67-69 The down-regulation of CD21 is therefore a sign of high activation of MZBs in Aire–/– mice. It is interesting to observe that Aire–/– mice have been found to develop Sjögren syndrome.23
The presence of lymphocytic infiltrates in Aire–/– livers has been used as an argument for a thymus function in bone marrow chimera experiments, but the infiltrates have not been further characterized.21 To our surprise, these infiltrates were not composed of CD3+ T cells (Figure 2B, top panel), but were found to be mainly B cells and plasma cells (Figure 2B, middle and bottom panel), pointing out the B cells as important actors in the pathology of the liver. Interestingly, 3 of 8 Aire–/– mice displayed a high number of B cells in the thymus, and the thymus was totally overtaken by B cells in one of these mice (Figure 2D). B cells in the thymus are present in certain forms of myasthenia gravis (MG) and in Grave Disease (GD) in humans.70 In MG, the infiltrating B cells are typically organized in a follicular histology similar to that of the lymph nodes,71 whereas in GD they constitute a part of the infiltrating cells and are thought to be of MZB origin.70 The high correlation of B-cell infiltrates in the thymus of old Aire–/– mice with MZL suggested that the infiltrates of the thymus are composed of MZL cells. Part of the liver infiltrates were found to be CD9 positive 32 (Figure 2B, bottom panel) and enriched for CD19+IgDloCD1dhi MZBs (Figure 2C) even in Aire–/– mice without MZL, but most of them were composed of follicular B cells in both Aire+/+ and Aire–/– mice.
In previous reports, evidence for autoantibody production has been claimed in Aire-deficient mice.20,21 The exaggerated marginal zone, increased MOMA-1, CD1d staining, and BrdU incorporation observed in Aire–/– mice suggest an altered activation of cells in the marginal zone, an area thought to be involved in the presentation of bloodborne antigens. Splenic B cells enriched for MZBs from antibody-positive Aire–/– mice were able to transfer antibody production in recipient Aire+/+ mice by day 6 (Figure S5). It is therefore tempting to speculate that at least part of the antibodies present in sera from Aire-deficient mice are a product of plasma cells of MZB origin. Furthermore, since MZBs are independent of T-cell help for maturation, and the low-affinity antibodies produced are often recognizing carbohydrate antigens, it is likely that aged B-cell lymphoma–prone Aire–/– mice produce some antibodies against antigens independent of an autoimmune process. However, the presence of antibodies and follicular B-cell infiltrates in livers of Aire–/– mice suggests that MZBs are not the exclusive producers of these antibodies.
In aged Aire–/– mice there was a very low grade of splenic hematopoiesis but normal bone marrow hematopoiesis with no difference in the myeloid-erythroid ratio, in the lymphocyte and plasma cell counts, and in the total number of cells; therefore, we would have expected anemia. A more extensive analysis of red blood cell (RBC) and platelet parameters in blood only revealed a trend toward decreased red blood cell counts and decreased hematocrit (Table S1). Thus, Aire–/– mice have a normal hematopoiesis in bone marrow but do not expand the extramedullary hematopoiesis in the spleen as commonly seen in aging mice. The MZL observed in Aire–/– mice was at a too early stage to be able to displace hematopoiesis in the red pulp or cause an autoimmune hemolytic anemia, and it cannot explain the low splenic hematopoiesis. Therefore, it is more likely that there is either a defect in the migration of stem cells to the Aire–/– spleens or that their spleens have an unfavorable cytokine milieu for extramedullary hematopoeisis, probably because of the altered activation in the marginal zone.
Taken together, these results suggest that Aire may have a role in autoimmunity at several different levels such as hematopoiesis, thymic development, and selection and in the peripheral immune system. In this paper, we show that Aire may play a role in the development of APCs of the myeloid lineage, namely blood monocytes and metallophilic macrophages, and therefore modify their phenotype in the periphery. Increased antigen uptake in the marginal zone mediated by metallophilic macrophages might be a contributing cause to the frequent lymphoma of the MZBs in aged Aire–/– mice. In summary, our data suggest that Aire plays a role in modulating peripheral tolerance mechanisms of the marginal zone. We also suggest that Aire–/– mice may serve as a spontaneous model to study MZL development.
Prepublished online as Blood First Edition Paper, May 18, 2006; DOI 10.1182/blood-2006-04-019679.
Supported by the Swedish Medical Council, the Lundberg foundation, Ronald McDonald foundation, the Grönwalls foundation, the Magnus Bergvall foundation, the Sven Jerring Foundation, the Swedish Medical Society, the Swedish Juvenile Diabetes foundation, Agnes and Mac Rudbergs foundation and the Queen Silvia Jubilee foundation (S.H.).
S.H. performed research, analyzed data, wrote the paper; C.R. performed research; M.C.K. performed research, analyzed data; D.L. performed research; B.H. performed research; B.R. performed pathological analysis; M.B. performed statistical analysis; L.P. contributed vital new reagents and analytical tools; O.K. contributed vital new reagents and analytical tools; O.W. designed research, wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Bernt Jones and Dr Inger Lilliehöök, Department of Clinical Chemistry, Division of Diagnostic Imaging and Clinical Pathology, Faculty of Veterinary Medicine and Animal Science, Swedish University of Agricultural Sciences; and to Dr Ala Saad, AstraZeneca R&D, Södertälje for useful advice, Åsa Hallgren and Katrin Österlund for excellent technical assistance, and to Peter Janson for help in setting up the GeneScan clonality assay.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal