Abstract
Vascular endothelial growth factor (VEGF) and VEGF receptor-1 (VEGFR-1/Flt-1) were shown to be involved in pathological angiogenesis, particularly rheumatoid arthritis (RA). However, the molecular basis of their actions is not fully understood. Here we report that in a murine model of RA, deletion of the tyrosine kinase (TK) domain of VEGFR-1 decreased the incidence and clinical symptoms of RA. Pathological symptoms, such as synovial hyperplasia, inflammatory infiltrates, pannus formation, and cartilage/bone destruction, became milder in Vegfr-1 tk–/– mice compared with wild-type (Wt) mice in the human T-cell leukemia virus-1 (HTLV-1) pX–induced chronic models. VEGFR-1 TK-deficient bone marrow cells showed a suppression of multilineage colony formation. Furthermore, macrophages induced to differentiate in vitro showed a decrease in immunologic reactions such as phagocytosis and the secretion of interleukin-6 (IL-6) and VEGF-A. Treatment of this RA model with a small molecule inhibitor for VEGFR TK, KRN951, also attenuated the arthritis. These results indicate that the VEGFR-1 TK signaling modulates the proliferation of bone marrow hematopoietic cells and immunity of monocytes/macrophages and promotes chronic inflammation, which may be a new target in the treatment of RA.
Introduction
Vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs), including VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4), form a regulatory system crucial for normal development and pathological angiogenesis.1-4 VEGFRs are structurally related to the Fms/Kit/PDGFR family and contain an extracellular domain carrying 7 immunoglobulin (Ig)–like repeats and a cytoplasmic tyrosine kinase (TK) domain.5,6 VEGFR-1 and VEGFR-2 are highly expressed on vascular endothelial cells.7-10 We and others have recently shown that VEGFR-1 is expressed not only in vascular endothelial cells but also in monocytes/macrophages,11,12 and its signaling is involved in the migration of macrophages toward VEGF-A.13 In addition to VEGF-A, VEGF-B and PlGF are also ligands for VEGFR-1; thus, they could play a role in this signaling under both physiological and pathological conditions. Other nonendothelial cells, including smooth muscle cells, trophoblasts, and osteoblasts, were reported to express VEGFR-1.14
Mice lacking Vegfr-2 die in the embryonic stage due to a severe deficiency of vascular development.15 In contrast, mice lacking Vegfr-1 die due to overgrowth and disorganization of the vascular system.16 Interestingly, however, mouse embryos lacking the TK domain of Vegfr-1 survive without significant defects,17 suggesting that VEGFR-1 functions as a negative regulator of vascular development by trapping VEGF-A via its ligand-binding domain.14 Recently, various studies including ours indicated that the expression of VEGFs and VEGFRs is up-regulated in various diseases.2,4,18-20 VEGFR-1–mediated signaling was shown to play a significant role in a variety of pathological conditions such as carcinogenesis and inflammatory diseases.19,21,22 VEGFR-1 signaling facilitates tumor angiogenesis and spontaneous lung metastases by inducing the expression of matrix metalloproteinase 9 (MMP-9).22-24 A recent study also showed that VEGFR-1 is important for the reconstitution of hematopoiesis in bone marrow (BM) after irradiation.25
Rheumatoid arthritis (RA)26 is a chronic systemic disease characterized by an inflammatory erosive synovitis, which shows marked neovascularization, inflammatory cell infiltration, and synovial hyperplasia. These pathological reactions gradually induce a pannus, with inflammatory vascular tissue leading to an irreversible loss of cartilage and bone.27,28 VEGF-A is highly expressed in synovial fluid in RA.29 Immunohistochemical and in situ hybridization studies on the synovial tissues have shown that VEGF-A is strongly expressed in synovial macrophages, fibroblasts surrounding microvessels, and vascular smooth muscle cells.29,30 In inflamed joints, many cytokines, including VEGF and the proinflammatory interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α), play important roles in the pathogenesis of RA.31
Iwakura et al have recently reported that the human T-cell leukemia virus-1 (HTLV-1) pX transgenic mouse is a useful model for studying RA32 in that it is more similar to human RA than other models, such as collagen-antibody–induced arthritis, in terms of chronic progression, the production of rheumatoid factor, and pathological findings.33 Interesting characteristics of pX transgenic mice include a high incidence of arthritis in the BALB/c genetic background34 and up-regulation of the production of cytokines such as IL-6 and TNF-α.35 However, it is not clear yet how the signaling of VEGFR-1 is involved in pX-induced or anticollagen-antibody–induced RA.
In this study, we examined these points using wild-type and Vegfr-1 tk–deficient mice. We have found that in Vegfr-1 tk–/– mice, the arthritis was significantly suppressed through a decrease in the inflammatory response of monocytes/macrophages and hematopoietic proliferation.
Materials and methods
Mice and the marker-assisted selection protocol (MASP)
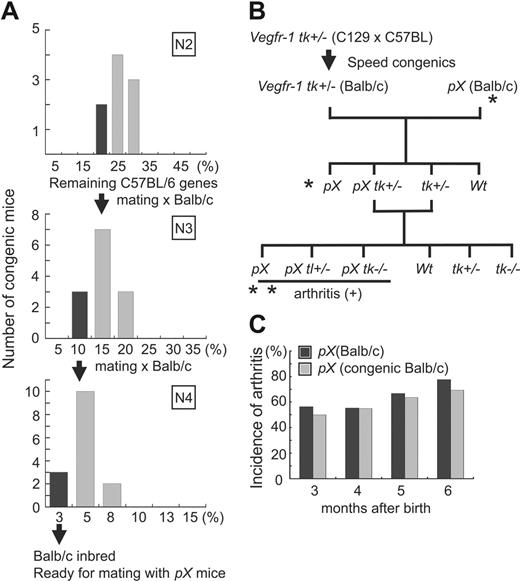
All the experiments using animal models were carried out according to the guidelines set by the Animal Center of The Institute of Medical Science, The University of Tokyo. To obtain Vegfr-1 tk–/– mice with the BALB/c background, we backcrossed Vegfr-1 tk+/– heterozygous males (original genetic background is 50% 129SV and 50% C57BL/6) with BALB/c females using marker-assisted selection protocol (MASP) (speed congenics). First, a total of 60 polymorphic polymerase chain reaction (PCR)–based microsatellite loci were randomly obtained from Mouse Genome Informatics of The Jackson Laboratory36 (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Two to 6 markers were set for each chromosome with an average spacing of 20 cM. At every generation, the mouse carrying the fewest C57BL/6 background loci was used for backcrossing. After 4 generations, less than 3% of C57BL/6 background loci were left, and then crosses were made with pX transgenic mice to compare the incidence of arthritis in newly prepared congenic mice with that in the control pX mice. Finally, pX Vegfr-1 tk+/– mice were crossed with Vegfr-1 tk+/– mice for the experiments (Figure 1B).
For the pX-induced arthritis, animals were observed every month from age 2 to 6 months and then killed for assessment. For the collagen-antibody–induced arthritis, 8-week-old BALB/c female Vegfr-1 tk–/– and wild-type mice were used. Acute arthritis was induced by the intraperitoneal injection of 1 mg of an antitype II collagen–monoclonal antibody cocktail (Chondrex, Redmond, WA) on day 0 followed by the intraperitoneal injection of 50 μg lipopolysaccharide (LPS) on day 3. Animals were killed for assessment on day 7.
Arthritis and histologic score
The incidence and severity of arthritis were examined monthly for pX mice and daily for mice with collagen-antibody–induced arthritis by qualitative clinical scoring as follows: 0, normal; 1, mild redness and swelling of the ankle or wrist; 2, moderate redness and swelling; 3, severe redness and swelling of the entire paw; 4, maximally inflamed limb within multiple joints. The number of joints observed was 33 to 122 for each genotype and each month. Mice were killed by neck dislocation, and joints were fixed in paraformaldehyde (PFA) and decalcified in EDTA. Then sagittal paw paraffin sections were examined by hematoxylin and eosin (HE) staining and immunohistochemical staining with von Willebrand factor (VWF) antibody (Dakopatts, Glostrup, Denmark). Histologic examination including morphologic analysis was carried out by semiquantitive grade scoring. In brief, for each paw joint, synovial hyperplasia, inflammatory cell infiltration, pannus formation, and bone and cartilage destruction were scored as follows: 0, none; 1, mild; 2, moderate; 3, severe. The sum of the scores for each paw was used as a histologic score index of the 64 paws for pX mice and 50 paws for pX Vegfr-1 tk–/– mice. The density of VWF-positive vessels in the hyperplasic synovia was determined by measuring the immunoreactive area in 3 chosen paw joints, which had an average grade. Vascular density was analyzed using a Kurabo Angiogenesis Image Analyzer (Kurabo, Osaka, Japan).
Macrophage activities for cytokine secretion and phagocytosis
For the cytokine secretion assay, mouse peritoneal macrophages were collected 3 days after the intraperitoneal injection of 4% thioglycolate. Peritoneal lavage was collected with 0.5% BSA, 2 mM EDTA (pH 7.2), and PBS, washed twice, and purified using anti–mouse CD11b magnetic-activated cell sorting (MACS) (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of macrophages was more than 90%. About 4 × 105 macrophages in 0.2 mL of 0.1% BSA–RPMI 1640 medium were seeded into a 96-well plate and incubated with recombinant human VEGF-A (hVEGF-A) (100 ng/mL) or control PBS, with or without a VEGFR inhibitor, SU541637 or KRN633.38 After 48 hours of incubation, the supernatants were collected after centrifugation and cytokine concentrations were measured using a mouse IL-6 and VEGF-A enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN). Total RNA from hVEGF-A–stimulated macrophages after 24 hours of incubation was isolated using an RNeasy Quick spin column (QIAGEN, Hilden, Germany). The total RNA was analyzed using a real-time reverse transcriptase (RT)–PCR according to the manufacturer's instructions (Takara Bio, Shiga, Japan). All the primers used for the amplification are listed in Table S2. For the phagocytosis assay, BM mononuclear cells (BMMNCs) of both Vegfr-1 tk–/– and wild-type mice were collected from the femur, and then 3 × 106 cells in 3.0 mL were cultured in 6-well plates with 100 ng/mL M-CSF (R&D Systems) in 10% FCS–RPMI 1640 medium. On days 2 and 5 of culture, nonadherent cells were discarded. On day 7, the confluent monolayers of adherent macrophages were used for the phagocytosis assay. Confluent macrophages were washed twice with cold PBS, resuspended in 1 mL RPMI 1640 medium containing FITC-dextran (Sigma, St Louis, MO) (100 ng/mL) or Alexa LPS (20 ng/mL) (Molecular Probes, Eugene, OR), and incubated for 2 hours at 37°C. After the incubation, plates were immediately transferred onto ice. This was followed by a quick aspiration of the medium and the washing of cells to remove unphagocytosed particles. Adding PFA stopped the phagocytotic reaction. Total fluorescence was measured with a fluorescence-activated cell sorting (FACS) machine Epics-XL (Beckman Coulter, Fullerton CA). The instrument was set to collect 2 × 104 cells, and profiles of phagocytosis were analyzed using FlowJo software (Tree Star, Ashland, OR).
Treatment of arthritis-model mice with a kinase inhibitor
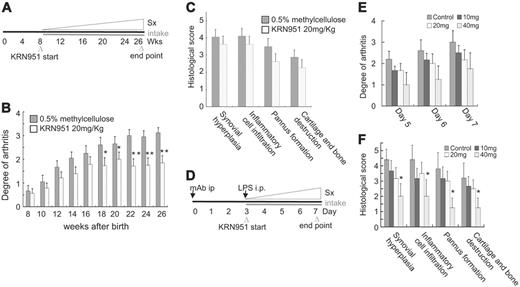
A low-molecular-weight chemical, KRN951, was obtained from the Kirin Brewery (Gunma, Japan). KRN951 is a VEGFR inhibitor suppressing the TK of VEGFR-1, VEGFR-2, and VEGFR-3. Inhibition of the phosphorylation of VEGFR-1 and -2 with KRN951 is shown in Figure S1. KRN951 was dissolved in 0.5% methylcellulose, Metolose SM15 (Shin-Etsu Chemical, Tokyo, Japan). The KRN951 solution was orally administered to mice with pX-induced arthritis at 20 mg/kg/d for 5 days a week, with a 2-day rest period, between the age of 8 and 26 weeks. The mice with collagen-antibody–induced arthritis were given the same inhibitor from day 3 to day 7 at 10, 20, and 40 mg/kg/d, respectively. The control solution was 0.5% methylcellulose without the inhibitor.
Colony formation assay
About 1 × 105 BMMNCs per 35-mm plate (triplicate) were cultured in complete methylcellulose containing 50 ng/mL recombinant murine SCF (rmSCF), 10 ng/mL rmIL-3, 10 ng/mL rhIL-6, and 3 U/mL recombinant human erythropoietin (rhEPO) (Stem Cell Technologies, Vancouver, BC, Canada). Colonies were scored and phenotyped on an inverted phase microscope 10 days after seeding.
Flow cytometric analysis of BM cells
Mouse BM cells were isolated by flushing the femur BM tissues with 1% FBS–PBS, and a single-cell suspension was obtained. Remaining red blood cells were removed with lysis buffer (10 mM NH4Cl). BMMNCs were stained with monoclonal antibodies for Sca-1 (BD Pharmingen, San Diego, CA) and CD34 (BD Pharmingen) and analyzed using a flow cytometer (Epics-XL, Beckman Coulter). The instrument was set to collect 2 × 104 cells, and these cells were analyzed using FlowJo software (Tree Star). All experiments were performed in triplicate.
Statistical analysis
An unpaired Student t test was used for all analyses. Differences were considered to be statistically significant for P values below .05 and .01.
Results
Speed congenics significantly shorten the period of backcrossing for establishing Vegfr-1 tk–/– BALB/c mice
The incidence of arthritis in pX transgenic mice is significantly higher in the genetic background of BALB/c than C57BL/6. Because the original genetic background of Vegfr-1 tk–/– mice is 50% 129SV and 50% C57BL/6, we prepared BALB/c-background congenic mice carrying the Vegfr-1 tk+/– gene. To shorten the crossing time, we used a MASP or speed congenics to establish BALB/c congenic mice (see “Materials and methods”). The mouse showing the least number of C57BL/6-background loci was selected for backcrossing. After 4 generations, less than 3% of the C57BL/6 background was left (Figure 1A). After the speed congenics, Vegfr-1 tk+/– BALB/c congenic mice were mated with pX BALB/c mice, which generated 4 genotypes (pX, pX Vegfr-1+/–, Vegfr-1+/–, and wild-type) (Figure 1B).
To confirm that the congenic strain efficiently changed to the BALB/c background, we compared the incidence of arthritis between the original pX mice and the newly prepared congenic pX mice. The original pX mice and the speed congenic BALB/c pX mice showed severe arthritis with no difference in the incidence of disease (Figure 1C). After verification, we mated pX Vegfr-1 tk+/– and Vegfr-1 tk+/– mice to generate the transgenic mice and other mice for the experiments (Figure 1B).
Signals from VEGFR-1 TK contribute to the onset and the progression of arthritis
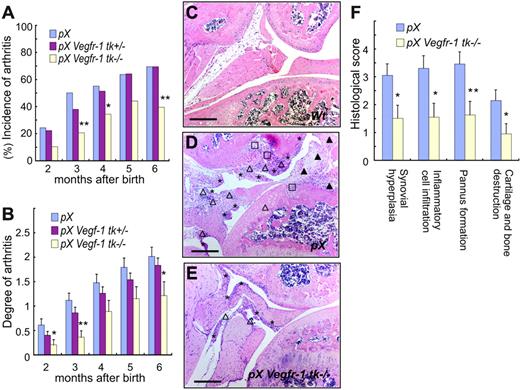
We measured the incidence and clinical grade of arthritis in the presence or absence of VEGFR-1 TK signals using pX, pX Vegfr-1 tk+/–, and pX Vegfr-1 tk–/– mice (Figure 1B). The incidence of arthritis, detected as paw swelling, erythema, and ankylosis, was significantly lower in pX Vegfr-1 tk–/– mice than Vegfr-1 wild-type pX transgenic mice at all the stages examined (P < .072 at 2 to 6 months) (Figure 2A). In addition, the incidence of arthritis was lower in the heterozygous Vegfr-1 tk+/– mice than pX transgenic wild-type mice before 4 months of age, although the difference was not statistically significant (Figure 2A). Clinical scores measured based on redness and swelling of the ankle or wrist were also significantly lower in pX Vegfr-1 tk–/– mice than pX wild-type mice (P < .058 at 2 to 6 months). Furthermore, pX Vegfr-1 tk+/– mice showed mild clinical scores between those of pX mice and pX Vegfr-1 tk–/– mice (P = .025 to .454 at 2 to 6 months) (Figure 2B).
Next we examined the histologic difference between pX mice and pX Vegfr-1 tk–/– mice. Synovial hyperplasia, inflammatory infiltrates, pannus formation, and loss of cartilage/bone were reduced by about half in pX Vegfr-1 tk–/– mice compared with pX wild-type mice (P = .015, .011, .007, and .026, respectively) (Figure 2F). Typical histologic findings are shown in Figure 2C-E.
Experimental design and speed congenics used to shorten the period of backcrossing. (A) Percentage of the C57BL/6 polymorphism loci remaining in the backcrossed generation of mice. After 4 generations, a few mice had less than 3% of C57BL/6 background loci. (B) Experimental design for pX-induced RA in the Vegfr-1 tk–/– background. *Confirmation of the incidence of arthritis in newly prepared BALB/c speed congenic pX and original BALB/c pX transgenic mice. **The incidence and degree of arthritis in these mice were examined. (C) Comparison of the incidence of arthritis between newly prepared BALB/c speed congenic pX transgenic mice and original BALB/c pX transgenic mice. No difference was observed during the first 3 to 6 months after birth.
Experimental design and speed congenics used to shorten the period of backcrossing. (A) Percentage of the C57BL/6 polymorphism loci remaining in the backcrossed generation of mice. After 4 generations, a few mice had less than 3% of C57BL/6 background loci. (B) Experimental design for pX-induced RA in the Vegfr-1 tk–/– background. *Confirmation of the incidence of arthritis in newly prepared BALB/c speed congenic pX and original BALB/c pX transgenic mice. **The incidence and degree of arthritis in these mice were examined. (C) Comparison of the incidence of arthritis between newly prepared BALB/c speed congenic pX transgenic mice and original BALB/c pX transgenic mice. No difference was observed during the first 3 to 6 months after birth.
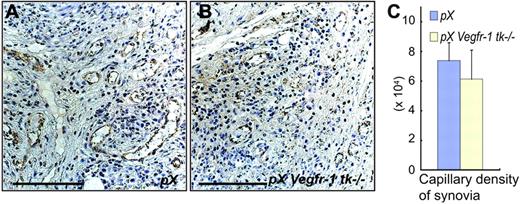
Because VEGFR-1 is considered to be associated with angiogenesis, we examined capillary formation in the pannus of synovial tissue. Capillary densities were about 17% lower in pX Vegfr-1 tk–/– mice than pX wild-type mice; however, the difference was not significant (P = .623) (Figure 3A-C). These results suggest that VEGFR-1 TK–dependent signals contribute to the symptoms of arthritis, including pathological findings, in a gene-dosage–dependent manner.
Production of cytokines by monocytes/macrophages and the function of these cells are important for arthritis
We and others already showed that VEGFR-1 is expressed on monocytes/macrophages12,13 and that the VEGF-dependent migration of macrophages is suppressed in Vegfr-1 tk–/– mice.17 Therefore, we examined local infiltration and the functions of monocytes/macrophages in these mice. The infiltration of inflammatory cells into arthritic joints was significantly less extensive in pX Vegfr-1 tk–/– mice than in pX wild-type mice (Figure 2D-F). We also observed using real-time RT-PCR that angiogenic factors and proinflammatory cytokines are up-regulated in their expression in inflammatory joints of pX transgenic mice compared with wild-type mice (Figure S2).
Signals from VEGFR-1 TK contribute to the onset and progression of arthritis. (A) The incidence of arthritis was significantly lower in pX Vegfr-1 tk–/– mice than pX mice. In addition, the incidence of arthritis in pX Vegfr-1 tk+/– heterozygotes was slightly lower in the early stages. (B) Clinical grades of arthritis were reduced depending on the deficiency of the VEGFR-1 TK domain from 2 to 6 months after birth. pX Vegfr-1 tk+/– mice showed mild clinical scores between those of pX and pX Vegfr-1 tk–/– mice. The data (A-B) represent the mean ± SEM obtained from 33 to 122 mice. *P < .05 versus pX; **P < .01 versus pX. (C-E) Cross-sections of ankle joints in control and RA mice. Joints of wild-type mice (C) show no remarkable change. Joints of pX mice (D) show synovial hyperplasia (*), inflammatory cell infiltration (▵), pannus formation (▴), and loss of cartilage and bone (□). These findings are milder in pX Vegfr-1 tk–/– mice (E). Sections are taken from average cases in these mice. Scale bars, 200 μm. Images in panels C-E were taken with a Nikon Eclipse TE600 microscope (Nikon, Tokyo, Japan) using AxioVision 3.0 software (Carl Zeiss, Jena, Germany) and a 10×/0.30 NA objective lens, then processed with Photoshop CS (Adobe Systems, San Jose, CA). (F) Histologic scores of the degree of pathology in paws and ankles. Scores of pX Vegfr-1 tk–/– mice were about half those of the pX mice. The data represent the mean ± SEM. *P < .05 versus pX; **P <.01 versus pX.
Signals from VEGFR-1 TK contribute to the onset and progression of arthritis. (A) The incidence of arthritis was significantly lower in pX Vegfr-1 tk–/– mice than pX mice. In addition, the incidence of arthritis in pX Vegfr-1 tk+/– heterozygotes was slightly lower in the early stages. (B) Clinical grades of arthritis were reduced depending on the deficiency of the VEGFR-1 TK domain from 2 to 6 months after birth. pX Vegfr-1 tk+/– mice showed mild clinical scores between those of pX and pX Vegfr-1 tk–/– mice. The data (A-B) represent the mean ± SEM obtained from 33 to 122 mice. *P < .05 versus pX; **P < .01 versus pX. (C-E) Cross-sections of ankle joints in control and RA mice. Joints of wild-type mice (C) show no remarkable change. Joints of pX mice (D) show synovial hyperplasia (*), inflammatory cell infiltration (▵), pannus formation (▴), and loss of cartilage and bone (□). These findings are milder in pX Vegfr-1 tk–/– mice (E). Sections are taken from average cases in these mice. Scale bars, 200 μm. Images in panels C-E were taken with a Nikon Eclipse TE600 microscope (Nikon, Tokyo, Japan) using AxioVision 3.0 software (Carl Zeiss, Jena, Germany) and a 10×/0.30 NA objective lens, then processed with Photoshop CS (Adobe Systems, San Jose, CA). (F) Histologic scores of the degree of pathology in paws and ankles. Scores of pX Vegfr-1 tk–/– mice were about half those of the pX mice. The data represent the mean ± SEM. *P < .05 versus pX; **P <.01 versus pX.
Secretion of cytokines and phagocytosis are attenuated in Vegfr-1 tk–/– macrophages
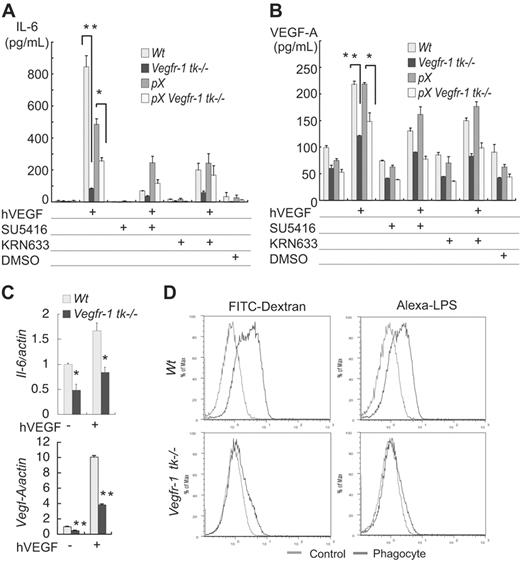
Saijo et al39 recently reported that pX Il-1–/– mice show a dramatically decreased incidence of arthritis. Because macrophages are an important source of these cytokines, we next focused on the function of macrophages derived from wild-type and Vegfr-1 tk–deficient mice. Secretion of IL-6 and VEGF-A was measured in the presence or absence of hVEGF-A. IL-6 was secreted in response to hVEGF-A both in the wild-type and in the Vegfr-1 tk–/– mice (Figure 4A). However, much less IL-6 was secreted from Vegfr-1 tk–/– macrophages than Vegfr-1 wild-type cells. In addition, the secretion of IL-6 was partially suppressed with VEGFR inhibitors, SU5416 and KRN633, in the presence of hVEGF-A (Figure 4A).
VEGF-A was secreted from macrophages, and the secretion increased in response to exogenous hVEGF-A (Figure 4B). However, the secretion of VEGF-A from Vegfr-1 tk–/– macrophages was about half that in the wild-type mice (Figure 4B).
We examined the mRNA levels of Il-6 and Vegf-A in macrophages in the presence or absence of hVEGF-A by real-time RT-PCR. The expression of both Il-6 and Vegf-A was significantly suppressed in Vegfr-1 tk–/– macrophages (P = .021 and < .001, respectively) (Figure 4C). These results are consistent with the levels of protein determined by ELISA (Figure 4A-B).
Macrophages are multifunctional cells involved in immunologic reactions and phagocytosis. Therefore, we next examined the extent of phagocytosis using macrophages that were induced to differentiate in culture. Wild-type BM-derived and M-CSF–stimulated macrophages efficiently phagocytized both fluorescent dextran and LPS. Surprisingly, however, macrophages from Vegfr-1 tk–deficient mice showed significantly less phagocytotic activity (Figure 4D).
No significant difference was observed in newly formed vessel densities in the hyperplastic synovia and pannus between pX mice and pX Vegfr-1 tk–/– mice. (A-B) Staining for VWF in the newly formed capillary vessels in the hyperplastic synovia and pannus. Scale bars, 50 μm. Images were taken with a Nikon Eclipse TE600 microscope, using AxioVision 3.0 software and a 40×/0.75 NA objective lens, then processed with Photoshop CS. (C) Vascular densities in the hyperplastic synovia are slightly lower in pX Vegfr-1 tk–/– mice than in pX mice (P = .623). The data represent the mean ± SEM.
No significant difference was observed in newly formed vessel densities in the hyperplastic synovia and pannus between pX mice and pX Vegfr-1 tk–/– mice. (A-B) Staining for VWF in the newly formed capillary vessels in the hyperplastic synovia and pannus. Scale bars, 50 μm. Images were taken with a Nikon Eclipse TE600 microscope, using AxioVision 3.0 software and a 40×/0.75 NA objective lens, then processed with Photoshop CS. (C) Vascular densities in the hyperplastic synovia are slightly lower in pX Vegfr-1 tk–/– mice than in pX mice (P = .623). The data represent the mean ± SEM.
Therefore, in addition to the suppression of VEGF-A–dependent migration, Vegfr-1 tk-deficient macrophages were dysfunctional in the secretion of IL-6 and VEGF-A as well as phagocytosis under these experimental conditions.
VEGFR TK inhibitor, KRN951, suppressed the progression of arthritis
To confirm the therapeutic effect of VEGFR kinase inhibitors on arthritis, we administered such an inhibitor, KRN951, to mice with pX-induced chronic arthritis and collagen-antibody–induced acute arthritis. We treated the mice with KRN951 for 5 straight days (oral, 20 mg/kg/d) a week from 8 to 26 weeks of age (Figure 5A). Administration of KRN951 reduced the progression of arthritis compared with the control (P < .041 from 18 weeks to 26 weeks) (Figure 5B). Histologic abnormalities in the treated group decreased 11% to 25% compared with the control (statistically not significant) (Figure 5C). In our preliminary experiments, a dose of KRN951 (1.0 mg/kg body weight) lower than those used in this experiment strongly suppressed the growth of several solid tumors in vivo in mice, suggesting that the dose of KRN951 used here could block the TK of VEGFR1 and VEGFR2 in vivo (K.N. and M.S., unpublished data, April 2006).
Cytokine secretion and phagocytosis are attenuated in the macrophages in Vegfr-1 tk–/– mice. (A-B) Mouse peritoneal macrophages were stimulated with hVEGF-A (100 ng/mL), and then levels of cytokines (IL-6 and VEGF-A) secreted into the medium were measured by ELISA after a 48-hour incubation. (A) IL-6 was secreted in response to hVEGF-A, and this secretion was partially suppressed by VEGFR inhibitors, SU5416 and KRN633. Secretion of IL-6 from Vegfr-1 tk–/– macrophages was low compared with that from the wild-type macrophages. (B) Mouse VEGF-A (mVEGF-A) was secreted from macrophages in the absence of hVEGF-A, and the secretion increased on stimulation with exogenous hVEGF-A. The secretion of mVEGF-A was partially suppressed by VEGFR inhibitors. The secretion from Vegfr-1 tk–/– macrophages was about half that from wild-type macrophages. Results represent the mean ± SEM from 2 to 3 experiments. (C) The mRNA expression of Il-6 and Vegf-A on treatment with hVEGF-A was examined by real-time RT-PCR analysis. Il-6 is weakly expressed in Vegfr-1 tk–/– macrophages. Vegf-A expression in Vegfr-1 tk–/– macrophages is about half that in the wild-type. (D) Macrophages derived from wild-type BM cells in cultures phagocytized dextran and LPS (upper row). On the other hand, macrophages from Vegfr-1 tk–/– BM did not show strong phagocytosis (lower row). Results are representative of at least 3 independent experiments. The data represent the mean ± SEM. *P < .05, **P < .01; wild-type versus Vegfr-1 tk–/– macrophages.
Cytokine secretion and phagocytosis are attenuated in the macrophages in Vegfr-1 tk–/– mice. (A-B) Mouse peritoneal macrophages were stimulated with hVEGF-A (100 ng/mL), and then levels of cytokines (IL-6 and VEGF-A) secreted into the medium were measured by ELISA after a 48-hour incubation. (A) IL-6 was secreted in response to hVEGF-A, and this secretion was partially suppressed by VEGFR inhibitors, SU5416 and KRN633. Secretion of IL-6 from Vegfr-1 tk–/– macrophages was low compared with that from the wild-type macrophages. (B) Mouse VEGF-A (mVEGF-A) was secreted from macrophages in the absence of hVEGF-A, and the secretion increased on stimulation with exogenous hVEGF-A. The secretion of mVEGF-A was partially suppressed by VEGFR inhibitors. The secretion from Vegfr-1 tk–/– macrophages was about half that from wild-type macrophages. Results represent the mean ± SEM from 2 to 3 experiments. (C) The mRNA expression of Il-6 and Vegf-A on treatment with hVEGF-A was examined by real-time RT-PCR analysis. Il-6 is weakly expressed in Vegfr-1 tk–/– macrophages. Vegf-A expression in Vegfr-1 tk–/– macrophages is about half that in the wild-type. (D) Macrophages derived from wild-type BM cells in cultures phagocytized dextran and LPS (upper row). On the other hand, macrophages from Vegfr-1 tk–/– BM did not show strong phagocytosis (lower row). Results are representative of at least 3 independent experiments. The data represent the mean ± SEM. *P < .05, **P < .01; wild-type versus Vegfr-1 tk–/– macrophages.
A VEGFR TK inhibitor, KRN951, suppressed the progression of pX-induced chronic and collagen-antibody–induced acute arthritis. (A) Experimental design for the treatment of mice with pX-induced chronic arthritis with a VEGFR inhibitor, KRN951. The VEGFR inhibitor was orally administered from 8 to 26 weeks of age (5 d/wk). (B) Clinical grades of arthritis gradually increased with age. The KRN951-administered group showed a reduction in the progression of arthritis compared with the untreated group. The data represent the mean ± SEM. *P < .05, **P < .01; KRN951-treated group versus control. (C) The scores of histologic findings in the treated group were also decreased (statistically not significant). (D) Experimental design for the treatment of mice with collagen-antibody–induced acute arthritis. Anticollagen-antibody was injected into the peritoneum of mice, followed by a peritoneal injection of LPS after 3 days. The administration of KRN951 started at day 3 and ended at day 7. (E-F) Clinical grade and histologic score of acute arthritis decreased in a KRN951 dose–dependent manner. The data represent the mean ± SEM. *P < .05; 40 mg/kg/d versus control. Sx indicates symptom.
A VEGFR TK inhibitor, KRN951, suppressed the progression of pX-induced chronic and collagen-antibody–induced acute arthritis. (A) Experimental design for the treatment of mice with pX-induced chronic arthritis with a VEGFR inhibitor, KRN951. The VEGFR inhibitor was orally administered from 8 to 26 weeks of age (5 d/wk). (B) Clinical grades of arthritis gradually increased with age. The KRN951-administered group showed a reduction in the progression of arthritis compared with the untreated group. The data represent the mean ± SEM. *P < .05, **P < .01; KRN951-treated group versus control. (C) The scores of histologic findings in the treated group were also decreased (statistically not significant). (D) Experimental design for the treatment of mice with collagen-antibody–induced acute arthritis. Anticollagen-antibody was injected into the peritoneum of mice, followed by a peritoneal injection of LPS after 3 days. The administration of KRN951 started at day 3 and ended at day 7. (E-F) Clinical grade and histologic score of acute arthritis decreased in a KRN951 dose–dependent manner. The data represent the mean ± SEM. *P < .05; 40 mg/kg/d versus control. Sx indicates symptom.
The administration of KRN951 in the acute model also reduced the progression of the symptoms of arthritis in a dose-dependent manner (P = .142 to .227 on day 5 to day 7. All values are 40 mg/kg/d versus control) (Figure 5E). KRN951 is a pan-VEGFR inhibitor; however, the arthritic symptoms and histologic abnormalities with KRN951 were as mild as those of pX Vegfr-1 tk–deficient mice. Taken together, these results suggest that VEGFR TKs, particularly VEGFR-1, contribute to the progression of arthritis.
VEGFR-1 is closely associated with the proliferation/differentiation of hematopoietic cells but not the number of BM hematopoietic stem cells
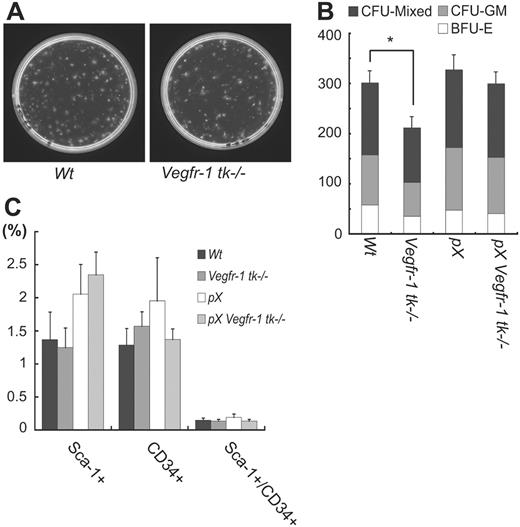
Another hallmark of hematopoietic activity is the capacity for colonies to form BMMNCs in vitro.40 The colony-forming ability of Vegfr-1 tk–/– BMMNCs was reduced to about 70% of that of wild-type BMMNCs (P = .013), and all the progenitors including erythroid colonies, myeloid colonies, and more immature mixed colonies were equally affected (P = .002, .091, and .021, respectively) (Figure 6A-B).
These results suggest at least 2 possibilities: a decrease in the activity of BM hematopoietic stem cells (HSCs) or a decrease in the number of HSCs in Vegfr-1 tk–/– BM. To distinguish between these possibilities, we examined the number of HSCs by FACS analysis. Numbers of Sca-1+ and CD34+ cells corresponding to HSCs among BMMNCs were almost the same between wild-type and Vegfr-1 tk–/– mice (Figure 6C). Therefore, a deficiency of VEGFR-1 signaling may reduce the proliferation of HSCs but not the number of these cells in BM.
VEGFR-1 is associated with the proliferation of hematopoietic cells but not the number of BM HSCs. (A-B) The number of colony-forming units (CFU) in Vegfr-1 tk–/– was decreased in all progenitor cells (BFU-E, CFU-GM, CFU-mixed) compared with that in wild-type mice (P = .013) (A). Each progenitor cell also showed a decrease in the number of colonies (P = .002 in BFU-E, .091 in CFU-GM, and .021 in CFU-mixed). Data represent the mean ± SEM for 3 mice. *P < .05; wild-type BM versus Vegfr-1 tk–/– BM (B). (C) The number of HSCs (Sca-1+ and CD34+ cells) in BM was not influenced by Vegfr-1 tk deficiency. Percentages of HSCs among BMMNCs were analyzed by flow cytometer. Results represent the mean ± SEM for 3 mice.
VEGFR-1 is associated with the proliferation of hematopoietic cells but not the number of BM HSCs. (A-B) The number of colony-forming units (CFU) in Vegfr-1 tk–/– was decreased in all progenitor cells (BFU-E, CFU-GM, CFU-mixed) compared with that in wild-type mice (P = .013) (A). Each progenitor cell also showed a decrease in the number of colonies (P = .002 in BFU-E, .091 in CFU-GM, and .021 in CFU-mixed). Data represent the mean ± SEM for 3 mice. *P < .05; wild-type BM versus Vegfr-1 tk–/– BM (B). (C) The number of HSCs (Sca-1+ and CD34+ cells) in BM was not influenced by Vegfr-1 tk deficiency. Percentages of HSCs among BMMNCs were analyzed by flow cytometer. Results represent the mean ± SEM for 3 mice.
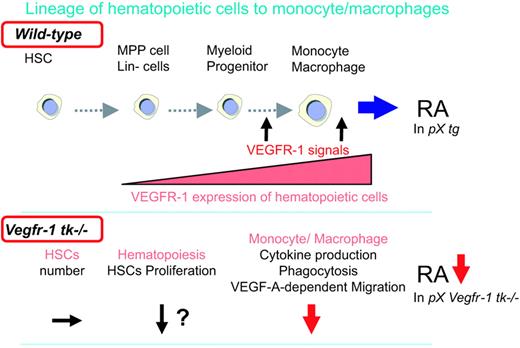
VEGFR-1 TK signaling is involved in arthritis by modulating hematopoiesis and promoting the differentiation of monocytes/macrophages. A schematic model of the VEGFR-1 TK signals associated with arthritis. (Top) Immature monocytes/macrophages derived from BM hematopoietic cells differentiate and migrate into the circulation. VEGFR-1 is expressed in monocyte/macrophage lineages. VEGFR-1 signals mobilize inflammatory cells to the peripheral tissues and RA joints and stimulate secretion of inflammatory cytokines to promote RA. (Bottom) VEGFR-1 signal–deficient macrophages show suppressed cytokine secretion, phagocytosis, and VEGF-dependent migration, resulting in a decrease in RA.
VEGFR-1 TK signaling is involved in arthritis by modulating hematopoiesis and promoting the differentiation of monocytes/macrophages. A schematic model of the VEGFR-1 TK signals associated with arthritis. (Top) Immature monocytes/macrophages derived from BM hematopoietic cells differentiate and migrate into the circulation. VEGFR-1 is expressed in monocyte/macrophage lineages. VEGFR-1 signals mobilize inflammatory cells to the peripheral tissues and RA joints and stimulate secretion of inflammatory cytokines to promote RA. (Bottom) VEGFR-1 signal–deficient macrophages show suppressed cytokine secretion, phagocytosis, and VEGF-dependent migration, resulting in a decrease in RA.
Discussion
In this study, we have shown that VEGFR-1 TK signals play a significant role in the progression of RA in murine models of both chronic and acute arthritis. Furthermore, the involvement of VEGFR-1 signals is considered to be gene-dosage dependent because the clinical and histologic scores of RA in Vegfr-1 tk+/– heterozygous pX mice were between those of wild-type pX and Vegfr-1 tk–/– mice. Several functions of macrophages such as the secretion of IL-6 and VEGF-A, phagocytosis, and VEGF-A–dependent migration were clearly suppressed in Vegfr-1 tk–/– mice. In addition, the proliferation of HSCs decreased about 30% in the in vitro assay in these mice. These results suggest that the kinase activity of VEGFR-1 is important in a variety of steps during the progression of pathological inflammatory diseases such as RA (Figure 7).
Our results raise the question of which receptor for VEGFs, VEGFR-1 or VEGFR-2, is tightly linked to RA. The TK of VEGFR-2 appears to be essential for the maintenance of blood vessel networks in adulthood4,15,20,41-44 ; thus, Vegfr-2 tk– mice, similar to the Vegfr-1 tk–/– mice in this study, are not available. However, the suppressive effect of Vegfr-1 tk deficiency on the RA model and that of the treatment with the pan-VEGFR kinase inhibitor KRN951 were similar (Figures 2 and 5A), suggesting that VEGFR-1 signaling is more strongly related to the progression of RA than VEGFR-2 signaling. In addition, the amount of newly formed capillary vessels in the pannus did not differ significantly between pX Vegfr-1 tk+/+ and pX Vegfr-1 tk–/– mice. These results imply that inflammatory responses are more closely associated with VEGFR-1 than VEGFR-2. Consistent with these findings, Luttun et al22 found that, using blocking antibodies against mouse VEGFR-1 or VEGFR-2, treatment with anti–VEGFR-1 Ab more efficiently suppressed an adjuvant-induced inflammatory arthritis than did treatment with anti–VEGFR-2 Ab. Furthermore, De Bandt et al45 recently reported that VEGFR-1 is involved in a model of chronic arthritis in which the KRN/NOD (αvβ6 T-cell receptor [TCR] transgene)/nonobese diabetic) mice were used for the induction of inflammation. Their model system is different from ours; therefore, results obtained with several independent animal models of arthritis clearly support the importance of VEGFR-1 in this disease.
It is of interest that the suppression of chronic RA in the pX mice is dependent on the dosage of the Vegfr-1 tk gene to some extent. We and others previously showed that the kinase activity of VEGFR-1 is one order of magnitude lower than that of VEGFR-2.46,47 These results suggest that the limiting step in the VEGFR-1 signaling pathway to promote RA is not the amount of ligand such as VEGF-A but the amount of VEGFR-1 itself. They may also support the idea that VEGFR-1 TK activity is a good pharmaceutical target for control of chronic RA.
Several groups including ours have shown that VEGFR-1 is well expressed in monocytes/macrophages at both the mRNA and protein levels12,13 and that VEGFR-1 is important for the VEGF-A–dependent migration of these cells.11,17 Therefore, it is reasonable that the infiltration of inflammatory cells was significantly less extensive in Vegfr-1 tk–deficient pX mice than wild-type pX mice (Figure 2D-F). Surprisingly, the blocking of the VEGFR-1 signals via deletion of its TK domain clearly suppressed the additional functions of these macrophages such as the secretion of IL-6 and VEGF-A stimulated by hVEGF and the phagocytotic reaction to dextran and LPS. The latter phenomenon is particularly interesting because the process of phagocytosis was thought to be a broad/nonspecific reaction against exogenous materials entering the body. On the other hand, macrophages obtained directly from the abdominal cavity of Vegfr-1 tk–/– mice exhibit phagocytotic activity similar to that of their wild-type counterparts (data not shown). It should be clarified whether the process of phagocytosis is directly dependent on the signaling of VEGFR-1 or whether the maturation of macrophages to obtain phagocytotic activity is delayed under VEGFR-1 signal–deficient conditions.
A VEGFR TK inhibitor, KRN951, also had suppressive effects on the pX-induced RA mouse model (Figure 5). Although the histologic scores indicated in Figure 5C are not statistically significant, the differences in the degree of arthritis more than 18 weeks after birth are significant (Figure 5B). Interestingly, the changes in RA observed between pX and pX/Vegfr-1 tk–/– mice (Figure 2F) were more significant than those in Figure 5C. To explain these results, we suggest that Vegfr-1 tk–deficient mice lost all signaling from VEGFR-1, whereas the treatment of mice with a VEGFR kinase inhibitor such as KRN951 is usually incomplete in blocking the kinase activity.
Our results suggest that VEGFR-1 has an effect on the proliferation of HSCs in CFU-B. All of the multilineage colonies (ie, BFU-E, CFU-GM, and CFU-mixed) are suppressed in Vegfr-1 tk–deficient BM HSCs. However, the number of HSCs positive for Sca-1 and CD34 was not so different from that in wild-type mice (Figure 6C). There are several potential explanations for these results: (1) Vegfr-1 tk deficiency causes a functional defect in macrophages in BM, which, in turn, indirectly causes an abnormality among HSCs; (2) VEGFR-1 expressed on the HSCs has a direct effect on cell signaling, and the lack of this signaling results in an abnormal phenotype; or (3) both mechanisms synergistically affect the function of HSCs. Although the number of these cells is small, a quantitative analysis appears to be necessary to clarify these possibilities.
RA is a chronic disease of late onset, and multiple pathways of inflammation and immune systems appear to be involved. A recent study indicated that the artificial blocking of TNF-α and IL-6 receptor by neutralizing antibodies significantly (but not completely) suppressed clinical as well as histologic scores in patients.48 VEGFR-1 signaling is involved in multiple functions of macrophages as a part of the functions of HSCs. Because these functions are at least partly independent of the TNF-α–IL-6 pathway, it appears reasonable that, in addition to inhibition of the TNF-α–IL-6 pathway, suppression of VEGFR-1 signaling by either a specific antibody or a small inhibitor molecule may significantly improve the condition of RA patients.
Prepublished online as Blood First Edition Paper, May 18, 2006; DOI 10.1182/blood-2006-04-016030.
Supported by Grant-in-Aid Special Project Research on Cancer-Bioscience 12215024 from the Ministry of Education, Culture, Sports, Science and Technology in Japan; a grant for the program “Research for the Future” from the Japan Society for Promotion of Science; and the program “Promotion of Fundamental Research in Health Science” from the Organization for Pharmaceutical Safety and Research (OPSR).
M.M., Y.I., and M.S. participated in designing the research; M.M., S.I., and M.Y. performed the research; M.M. centralized the pathological review and analyzed data; S.H. and Y.I provided gene-targeting mice; K.N. provided new reagents; M.M. and M.S. wrote the manuscript; and all authors checked the final version of the manuscript.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Saijo (Division of Cell Biology) and Dr K. Hattori and Dr Y Morita (Division of Stem Cell Therapy, Center for Experimental Medicine, Institute of Medical Science, University of Tokyo) for technical assistance and helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal