Comment on Amrolia et al, page 1797
Hematopoietic stem cell grafts engineered to eliminate graft-versus-host alloreactivity rapidly restore cell-mediated immunity in human leukocyte antigen–mismatched pediatric transplant patients.
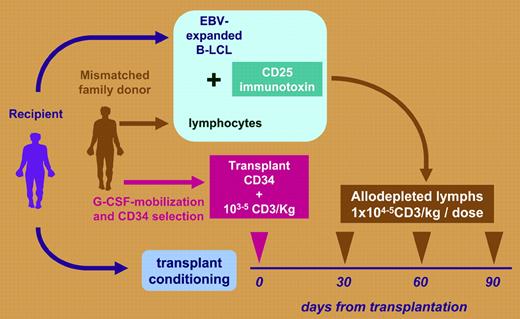
There is a high mortality rate from treatment failure in patients receiving stem cell transplants from human leukocyte antigen–haploidentical family donors. With immunosuppression and profound T-cell depletion, acute graft-versus-host disease (GVHD) can be avoided, but the price paid is severely delayed immune recovery, high mortality from infection, and reduction in any T-cell–mediated graft-versus-leukemia (GVL) effect. The paper by Amrolia and colleagues in this issue of Blood describes a way to break this impasse between GVHD control and compromised immune recovery. Following T-cell–depleted stem cell transplantation (SCT), patients were infused with up to 105/kg donor T cells depleted of alloimmune responsiveness at monthly intervals after transplantation. The process of allodepletion involved a 72-hour in vitro exposure of donor lymphocytes to the recipient's irradiated antigen-presenting cells (APCs). Activated donor T cells up-regulating the CD25 interleukin-2 receptor were eliminated by adding a ricin-conjugated anti-CD25. The allodepleted washed product was then transfused (see figure). In a clinical trial, 16 pediatric recipients of T-cell–depleted mismatched stem cell transplants received allodepleted T cells. Only 2 developed grade II or higher acute GVHD. Doses of 105/kg, but not 104/kg, allodepleted T cells resulted in impressive early numerical and functional lymphocyte recovery derived from the peripheral expansion of memory T cells. The strength of this article is the authors' meticulous description of immune recovery, using Vβ spectratyping T-cell repertoire analysis, naive effector–memory phenotyping, T-cell–receptor excision circle (TREC) analysis, and viral-specific T-cell responses to cytomegalovirus, Epstein-Barr virus (EBV), and adenovirus. This study is an important step toward the goal of engineering stem cell transplants to rapidly reconstitute immunity without causing GVHD. Results extend observations from other trials using the CD25 immunotoxin, confirming that allodepletion can reduce GVHD while supplying postthymic T cells for immunity reconstitution.1-3 However, there is still a long way to go with this approach. Not surprisingly, only 5 of these high-risk refractory patients survived disease free; 9 died from relapsed leukemia or infection.FIG1
Graft engineering to prevent graft-versus-host disease. Mismatched T-cell–depleted stem cell transplantation followed by transfusion of allodepleted donor lymphocytes.
Graft engineering to prevent graft-versus-host disease. Mismatched T-cell–depleted stem cell transplantation followed by transfusion of allodepleted donor lymphocytes.
Much remains to be understood about the mechanism of allodepletion and ways to optimize it: residual unmanipulated T cells in the graft may have contributed to both GVHD and immune recovery, and we can only guess at the identity of the antigenic targets stimulating the donor alloresponse. Furthermore, CD25 depletion might even risk enhancing GVHD, by removing protective regulatory CD4 T cells constituitively expressing CD25. The fact that GVHD appears reduced and not provoked after allodepleted SCT suggests that provided alloresponding T cells are silenced, any reduction in regulatory T cells is safe. The best choice of host APCs is yet to be defined: Amrolia and colleagues used EBV-transformed recipient B cells to convey the GVHD message to the donor; others have used blood monuclear cells or T cells.3 With B cells, there is a concern that protective T-cell immunity to EBV and graft-versus-malignancy alloresponses to B-cell malignancies could be lost. There are also practical obstacles to overcome: doses of 105 T cells/kg probably represent a lower threshold for immune recovery, and the enhancement of GVL may require many more cells. Scaling up the technique will be difficult and not always reproducible, and alternative allodepletion techniques (for example, photodepletion) deserve exploration.3 However, if significant doses of allodepleted T cells could be given without immunosuppression and without causing GVHD, the applicability and success of haploidentical transplantations for refractory malignancy would increase: well worth the extra cost of manufacturing the “perfect transplant.” ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal