Imatinib (IM) is currently the first line treatment for patients with chronic myelogenous leukemia (CML).1,2 However, in a substantial proportion of advanced-phase patients, mutant forms of the Bcr-Abl oncoprotein occur, conferring resistance to the treatment.3 New drugs, such as dasatinib, have been shown to overcome IM resistance due to Bcr-Abl mutants, with the exception of T315I.4 To date, the kinetics of the appearance of ABL kinase domain mutations is not well understood.
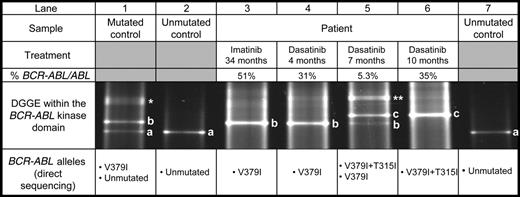
We report the case of a 68-year-old patient with CML treated with IM after failure of interferon-alpha therapy and admitted to our institution in accelerated phase after 34 months of IM treatment. To determine the mechanism of IM resistance, we screened the BCR-ABL kinase domain for mutations by denaturing gradient gel electrophoresis (DGGE) as previously described.5 At that time, the V379I mutation was detected by DGGE and characterized by direct sequencing. As shown in Figure 1, lane 3, the unmutated BCR-ABL allele was replaced by the mutated allele (band b). IM was then stopped and the patient was started on dasatinib therapy. After 7 months of treatment, a minor cytogenetic response and a 1-log reduction of the BCR-ABL/ABL ratio were observed. However, the mutation follow-up revealed the progressive disappearance of the V379I BCR-ABL allele and its replacement by a novel BCR-ABL allele (Figure 1, lanes 4-6, band c). Direct sequencing further characterized a double mutation V379I and T315I (Figure S1, available at the Blood website; see the Supplemental Figure link at the top of the online letter). It is noteworthy that the 2 mutations were present on the same allele, since only 1 band was seen in DGGE after 10 months of treatment (Figure 1, lane 6) and the V379I and T315I mutations were observed on the same electropherogram without detection of the nonmutated sequence. As the BCR-ABL/ABL ratio increased to 35% after 10 months of dasatinib therapy and only hematopoietic cells carrying the 2 mutations were detected, the treatment was interrupted.
DGGE follow-up of a CML patient treated successively by imatinib and dasatinib. A DGGE restricted to BCR-ABL RT-PCR fragments was performed for the patient (lanes 3-6), an unmutated control (lanes 2 and 7) and a V379I heterozygous mutated control (lane 1). Concerning the patient, the treatment, its duration, and the BCR-ABL/ABL ratio are shown. For each experiment, direct sequencing characterizes the BCR-ABL alleles. Letters in the DGGE profile indicate the homoduplex bands corresponding respectively to the BCR-ABL unmutated allele (a), the V379I BCR-ABL allele (b), and the V379I + T315I BCR-ABL allele (c). The asterisks (* and **) indicate the heteroduplexes, characteristics of the presence of 2 homoduplexes.
DGGE follow-up of a CML patient treated successively by imatinib and dasatinib. A DGGE restricted to BCR-ABL RT-PCR fragments was performed for the patient (lanes 3-6), an unmutated control (lanes 2 and 7) and a V379I heterozygous mutated control (lane 1). Concerning the patient, the treatment, its duration, and the BCR-ABL/ABL ratio are shown. For each experiment, direct sequencing characterizes the BCR-ABL alleles. Letters in the DGGE profile indicate the homoduplex bands corresponding respectively to the BCR-ABL unmutated allele (a), the V379I BCR-ABL allele (b), and the V379I + T315I BCR-ABL allele (c). The asterisks (* and **) indicate the heteroduplexes, characteristics of the presence of 2 homoduplexes.
Our observation confirms that targeted therapy of CML can lead to a clonal selection of BCR-ABL mutants. In our patient, IM therapy induced the selection of V379I mutants, then dasatinib selected among these IM-resistant cells those carrying the T315I mutation. These data highlight a problem inherent to targeted therapy that could directly induce a resistance phenomenon by means of selection of pre-existing mutants.6 The utility and feasibility of a sensitive and accurate mutation analysis before IM therapy has recently been studied, but even for the T315I mutant such an approach remains controversial.7 Moreover, whatever the methodology used, it seems improbable to detect any rare pre-existing mutant stem cells, especially the quiescent ones. Komarova and Wodarz8 suggested that the combination of 3 targeting drugs could overcome the problem of resistance in CML. Current protocols allow only the use of tyrosine kinase inhibitors as single agents. Consequently, when a resistance is observed, mutation screening within the BCR-ABL kinase domain is mandatory. In conclusion, our study demonstrates a sequential emergence of ABL kinase domain mutations in a patient treated with anti-tyrosine kinase agents and highlights the importance of a mutation follow-up for therapeutic decisions.
Supported by grants from the Ligue Contre le Cancer (comité de la Vienne).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal