Chronic inflammation is accompanied by impaired T-cell immunity. In the mouse, myeloid cell-associated arginase accounts for the suppression of immune reactivity in various models of tumor growth and chronic infections. Here we show that arginase I is liberated from human granulocytes, and very high activities accumulate extracellularly during purulent inflammatory reactions. Human granulocyte arginase induces a profound suppression of T-cell proliferation and cytokine synthesis. This T-cell phenotype is due to arginase-mediated depletion of arginine in the T-cell environment, which leads to CD3ζ chain down-regulation but does not alter T-cell viability. Our study therefore demonstrates that human granulocytes possess a previously unanticipated immunosuppressive effector function. Human granulocyte arginase is a promising pharmacologic target to reverse unwanted immunosuppression.
Introduction
A disturbed balance between proinflammatory and anti-inflammatory effector systems accounts for a wide variety of chronic inflammatory and autoimmune diseases, cancer, and immunopathology associated with infectious diseases.1,2 One of the main features of inflammation-triggered immune dysfunction is a profound impairment of T-cell-mediated immune responses.3,4
In the murine immune system, various myeloid cell types have been shown to actively down-regulate T-cell functions in the context of cancer or chronic inflammation via arginase-mediated arginine depletion.5 Two arginase isozymes (arginase I and II) exist in mammals, which both hydrolyze arginine to ornithine and urea but differ in subcellular localization, regulation, and function.6 T helper 2 (Th2) cytokine-stimulated macrophages and dendritic cells express the enzyme arginase,7,8 deplete arginine, and down-regulate the CD3ζ chain of interacting T cells.9 In tumor-bearing mice, GR-1-positive immature myeloid cells (myeloid suppressor cells [MSCs]) expand and inhibit the function of activated T cells by a mechanism that is partially or completely due to metabolism of arginine via arginase.10-13 If murine myeloid cell-associated arginase activity is inhibited, tumor-associated immune dysfunction is abolished and tumor cytotoxicity is restored.12-15
The expression and regulation of arginase within human leukocytes differ significantly from the murine immune system.16 In human leukocytes, arginase activity is only present at high levels in circulating polymorphonuclear leukocytes (PMNs), and no resting or inducible activity is detectable in human monocytes, macrophages, and dendritic cells.16 During inflammatory reactions, PMNs die by apoptosis or by necrosis with the liberation of tissue-destructive and immunomodulatory intracellular mediators.17
In this study we demonstrate that arginase I is liberated from human PMNs and that the enzyme specifically depletes extracellular arginine. This amino acid deprivation induces profound suppression of various T-cell functions. The suppressed T cells remain viable but down-regulate their CD3ζ chain. Finally, very high arginase activities are detected in vivo in the context of prototypical PMN-dominated inflammation within human pus, and arginase fully accounts for its profound T-cell suppressive potential.
Materials and methods
Human subjects
Human studies were approved by the ethics committee of the University of Heidelberg, and informed consent was obtained from all subjects.
Reagents
If not otherwise stated, chemicals were purchased from Sigma-Aldrich (St Louis, MO). n-ω-hydroxy-nor-l-arginine (nor-NOHA) was from Bachem (Weil am Rhein, Germany). A polyclonal rabbit anti-human arginase I antiserum was generated by immunization of rabbits with human recombinant arginase I. RPMI 1640 medium without arginine was purchased from PromoCell (Heidelberg, Germany) and supplemented with MnCl2 to a physiologic concentration (4 μM). Human recombinant arginase I was purified after overexpression in a bacterial expression system according to standard techniques.
Isolation of human PBMCs, PMNs, and T cells
Human peripheral blood mononuclear cells (PBMCs) and PMNs were purified from EDTA-anticoagulated peripheral blood of healthy human donors exactly as described.16 Human T cells were purified from PBMCs by E-rosette formation with sheep red blood cells (ICN Biomedicals, Costa Mesa, CA)18 and were 90% to 95% positive for CD3.
Arginase enzymatic assay
Arginase activity was measured in cell lysates as previously described.16 One unit of enzyme activity is defined as the amount of enzyme that catalyzes the formation of 1 μmol of urea per minute.
Generation of PMN sonicates
Human PMNs were purified from peripheral blood,16 resuspended in PBS (40 × 106 cells per milliliter), sonicated for 3 minutes (amplitude 80) in a Vibracell Sonicator (Sonics & Materials, Newtown, CT), and centrifuged for 30 minutes at 20 000g and 4°C. The supernatant was filtered (0.2 μm), protein concentration and arginase activity were determined, and aliquots were frozen at -80°C until further use.
Amino acid measurements
Suspensions were sonicated, and sulfosalicylic acid was added. The samples were allowed to stand for 10 minutes at 4°C and then centrifuged. A total of 300 μL of the supernatant was diluted with 100 μL physiologic sample dilution buffer (Onken, Gründau, Germany). Amino acid analysis was performed in the physiologic mode of an automatic amino acid analyzer (Biotronic LC 3000; Biotronik, Maintal, Germany). Ion exchange chromatography is used to separate amino acids by increasing pH, ion strength, and temperature of the elution buffers (Onken). After postcolumn derivatization with ninhydrin, amino acids (except tryptophan) were detected at 440 nm and 570 nm. For data collection and processing, ChromStar (SCPA, Weyme-Leeste, Germany) was used. For the quantification of tryptophan, 400 μL trichloroacetic acid (10%) was added to 100 μL suspension, and the mixture was allowed to stand at 0°C for 10 minutes. After centrifugation, 30 μL of the supernatant was used for high-performance liquid chromatography (HPLC) analysis with an Ultrasphere column on a Beckmann System Gold 127 coupled to a fluorescence detector RF-551 (Shimadzu, Duisburg, Germany) with λex 278 nm und λem 363 nm.
Immunoblot analysis
Cells were lysed for 30 minutes on ice in lysis buffer,16 cell debris was spun down at 18 000g for 5 minutes at 4°C, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was done as previously described.8 The proteins were transferred to a Hybond-P polyvinylidene difluoride membrane (Amersham Biosciences, Freiburg, Germany). After blocking with 5% nonfat dry milk in TBST buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% Tween 20) for 2 hours, the membranes were incubated with a polyclonal rabbit antihuman arginase I antiserum (see “Reagents,” 1:5000 in TBST, 5% bovine serum albumin). Antibody reactivity was monitored with horseradish peroxidase-conjugated antirabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA), followed by visualization with the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences).
In vitro proliferation assays and cytokine determinations
All cell culture experiments were performed with arginine-free medium (Arg(-) medium) that was supplemented with 150 μM arginine as indicated (Arg(+) medium). The medium was further supplemented with 10% FCS, penicillin/streptomycin, and 2% glutamine. Purified T cells (2 × 105 per well) were cultured in triplicate in flat-bottomed 96-well plates at 37°C and 5% CO2. For cell stimulation, wells were either precoated with goat antimouse antibody (5 μg/mL in PBS for crosslinking) and subsequently with OKT-3 (concentrations as indicated), or T cells were activated with paramagnetic microbeads that are coupled with anti-CD3 and anti-CD28 (T-cell expander; Dynal Biotech, Oslo, Norway). After 48 hours, the cells were pulsed with [3H]thymidine (Amersham Biosciences) (1 μCi [37 kBq] per well) for 16 hours. Cells were harvested on glass fiber filters using an automatic cell harvester (Packard Instruments, Downers Grove, IL). The incorporation of [3H]thymidine was measured in a microplate scintillation counter (Packard Instruments). Alternatively, T cells were labeled with 25 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Karlsruhe, Germany) for 15 minutes according to the manufacturer's instructions and subsequently used for cell activation assays. Cells were analyzed by flow cytometry (FL-1) at the indicated time points. For cytokine determinations, supernatants of stimulation cultures were harvested after 40 hours and diluted 1:2 with RPMI 1640 medium, and cytokine concentrations were measured by specific capture enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BD Biosciences, Heidelberg, Germany).
Real-time RT-PCR quantification
A total of 3 × 106 T cells were collected in 300 μL lysis buffer from the MagnaPure mRNA Isolation Kit I (Roche Diagnostics, Mannheim, Germany), and mRNA was isolated with the MagnaPure-LC device using the mRNA-I standard protocol. An aliquot of 8.2 μL RNA was reverse transcribed using AMV-reverse transcriptase (RT) and oligo(dT) as primer (First Strand cDNA synthesis kit; Roche Diagnostics) according to the manufacturer's protocol. After termination of the cDNA synthesis, the reaction mix was diluted to a final volume of 500 μL and stored at -20°C until polymerase chain reaction (PCR) analysis. Primer sets specific for the sequences of human IFN-γ, TNF-α, and IL-2 were optimized for the LightCycler (Roche Diagnostics, Heidelberg, Germany) and provided by SEARCH-LC. The PCR was performed with the LightCycler FastStart DNA Sybr GreenI kit (Roche Diagnostics) according to the protocol provided in the parameter-specific kits. The calculated copy numbers were normalized according to the average expression of 2 housekeeping genes, cyclophilin B and β-actin. Values were thus given as input adjusted copy number per microliter of cDNA.
Flow cytometry and apoptosis measurement
T cells were incubated with phycoerythrin (PE)-labeled anti-CD3. For surface marker staining, T cells were incubated subsequently with FITC-labeled anti-CD25, anti-HLA-DR, or anti-CD69 (all antibodies from BD Biosciences) according to the manufacturer's instructions. For intracellular detection of CD3ζ, cells were first stained with FITC-labeled anti-CD3 and subsequently fixed with 0.25% paraformaldehyde (in PBS) for 10 minutes at room temperature. After washing, cells were resuspended in 200 μL PBS (2.5% FCS) containing 100 μg/mL digitonin plus 1 μg anti-CD3ζ (Beckman Coulter, Fullerton, CA) or 1 μg murine IgG1-PE isotype control antibody.9 After 10 minutes of incubation at 4°C, cells were washed and analyzed. Flow cytometry analysis was performed with a FacScan flow cytometer (BD Biosciences). To analyze apoptosis and cell death, 105 human T cells were resuspended in saline containing 0.05% annexin V (Roche Diagnostics) and 100 ng/mL propidium iodide (PI). After incubation at 4°C for 30 minutes, the cells were analyzed by flow cytometry.
Results
Suppression of T-cell proliferation by human PMN arginase
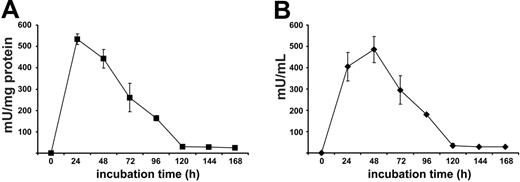
Despite the expression of high arginase activity, live human PMNs do not significantly metabolize arginine in the micromilieu.16 This is likely due to the localization and confinement of the enzyme in the azurophil granular compartment, the toxic contents of which are strictly separated from the surroundings.16 Because this strict separation may be lost after cell death, we first monitored arginase liberation after spontaneous PMN cell death. We found that arginase activity increased during the first 24 to 48 hours in the cellfree supernatant of purified human PMNs (Figure 1). Subsequently, arginase activity decreased to a baseline level that was still detectable after 14 days in this static system of dying PMNs with no influx of living cells. To standardize PMN necrosis with consecutive arginase liberation into the surroundings, we disrupted human PMNs by sonication and determined arginase activity in the PMN sonicate (PMN-S) solution. In 14 independent experiments using PMNs from different blood donors, mean arginase activity of the PMN-S was 1550 ± 459 mU/mg protein, confirming our previously published data on human PMN arginase activity after detergent-mediated solubilization of PMNs (mean, 1644 ± 423 mU/mg protein).16 Early inflammation is normally dominated by PMNs, and T cells begin to appear only after 1 to 2 days.1 We therefore preincubated RPMI 1640 cell culture medium, containing a physiologic concentration of arginine (150 μM = Arg(+) medium), with PMN-S for 12 to 16 hours to allow for possible PMN arginase-mediated arginine depletion (phase 1). This cell culture medium (Arg(+) medium + PMN-S) was then used for T-cell stimulation assays with platebound antibody-crosslinked anti-CD3 (OKT3) antibody (phase 2). We first verified that human T-cell proliferation is severely blunted in the absence of arginine (Arg(-) medium: RPMI 1640 cell culture medium without l-arginine) (Figure 2A). When T cells were stimulated in Arg(+) medium + PMN-S, proliferation was always completely inhibited in a total of 38 independent experiments. To investigate the contribution of arginase-mediated arginine depletion in this suppressive function, we used the specific arginase inhibitor nor-NOHA during phase 1. This inhibition of arginase by nor-NOHA completely rescued proliferation of T cells (Figure 2A) by preventing the degradation of arginine. The same results were seen when recombinant human arginase I (300 mU/mL) was used instead of human PMN-S (data not shown). The arginine that is present in FCS likely accounts for the residual proliferative activity of human T cells in Arg(-) medium (Figure 2A). When FCS was repetitively dialyzed to remove arginine and then used for medium supplementation, the suppression of T-cell activation in Arg(-) medium was complete (data not shown).
Arginase is liberated from dying human PMNs. Purified human PMNs (5 × 106 cells per 200 μL in PBS) were cultured for the indicated time. Cellfree supernatant was harvested after centrifugation, and protein concentration as well as arginase activity were determined in the supernatant. Arginase activity is shown as milliunits per milligram of protein (A) and milliunits per milliliter of supernatant (B). Data are representative of 3 different experiments (mean of triplicates ± SD).
Arginase is liberated from dying human PMNs. Purified human PMNs (5 × 106 cells per 200 μL in PBS) were cultured for the indicated time. Cellfree supernatant was harvested after centrifugation, and protein concentration as well as arginase activity were determined in the supernatant. Arginase activity is shown as milliunits per milligram of protein (A) and milliunits per milliliter of supernatant (B). Data are representative of 3 different experiments (mean of triplicates ± SD).
Human T-cell proliferation is suppressed by human PMN arginase. (A) Purified human T cells were stimulated with crosslinked anti-CD3 antibody at various concentrations, and proliferation was assessed by [3H]thymidine incorporation after 48 hours in triplicate wells. The cells were either stimulated in the presence (Arg(+) medium) or absence (Arg(-) medium) of 150 μM l-arginine. Alternatively, Arg(+) medium was preincubated for 12 hours with an aliquot of human PMN sonicate (PMN-S), corresponding to a final arginase activity of 300 mU/mL, in the presence or absence of the specific arginase inhibitor nor-NOHA, and T cells were then stimulated in these different media. Data are representative of 38 different experiments. (B) T-cell proliferation was analyzed as in panel A, but nor-NOHA and/or l-arginine (1 mM each) were added to arginine-depleted medium (Arg(+) medium + PMN-S) at the same time that the T cells were added. Results are expressed as mean cpm of triplicate cultures ± SD. (C) Human T-cell proliferation was assessed by cell division-mediated loss of intracellular CFSE fluorescence. T cells were labeled with CFSE and stimulated for 96 hours with antihuman CD3- and antihuman CD28-coupled microbeads in different cell culture media as described for panel A. Additionally, arginine was depleted in Arg(+) medium by 12 hours of preincubation with recombinant human arginase I (activity 300 mU/mL). Cell division (ie, loss of FL-1 fluorescence) was analyzed by flow cytometry. Data are representative of 4 different experiments.
Human T-cell proliferation is suppressed by human PMN arginase. (A) Purified human T cells were stimulated with crosslinked anti-CD3 antibody at various concentrations, and proliferation was assessed by [3H]thymidine incorporation after 48 hours in triplicate wells. The cells were either stimulated in the presence (Arg(+) medium) or absence (Arg(-) medium) of 150 μM l-arginine. Alternatively, Arg(+) medium was preincubated for 12 hours with an aliquot of human PMN sonicate (PMN-S), corresponding to a final arginase activity of 300 mU/mL, in the presence or absence of the specific arginase inhibitor nor-NOHA, and T cells were then stimulated in these different media. Data are representative of 38 different experiments. (B) T-cell proliferation was analyzed as in panel A, but nor-NOHA and/or l-arginine (1 mM each) were added to arginine-depleted medium (Arg(+) medium + PMN-S) at the same time that the T cells were added. Results are expressed as mean cpm of triplicate cultures ± SD. (C) Human T-cell proliferation was assessed by cell division-mediated loss of intracellular CFSE fluorescence. T cells were labeled with CFSE and stimulated for 96 hours with antihuman CD3- and antihuman CD28-coupled microbeads in different cell culture media as described for panel A. Additionally, arginine was depleted in Arg(+) medium by 12 hours of preincubation with recombinant human arginase I (activity 300 mU/mL). Cell division (ie, loss of FL-1 fluorescence) was analyzed by flow cytometry. Data are representative of 4 different experiments.
In titration experiments we observed that T-cell proliferation could be completely prevented when cell culture medium was preincubated with PMN-S for only 1 hour (data not shown) and when the final arginase activity exceeded 100 to 200 mU/mL cell culture medium (data not shown). In all further experiments we therefore diluted the PMN-S to a final arginase activity of 300 mU/mL from the start of phase 1. This activity corresponds to a mean of 0.213 ± 0.071 mg PMN protein per milliliter or the cellular contents of 4.5 ± 1.7 × 106 PMNs per milliliter.
The inhibition of arginase with nor-NOHA from the beginning of phase 1 likely prevents the enzymatic depletion of arginine from the cell culture medium and enables T cells to proliferate during the activation assays. We also tested if T-cell function can be rescued if fresh substrate (arginine) is supplemented at the start of phase 2 (ie, when the T cells are added to arginase-preincubated medium). As shown in Figure 2B, supraphysiologic arginine substitution (1000 μM) alone was only able to partially rescue T-cell proliferation. This is due to arginase that is still present in the cell culture medium as demonstrated by the complete rescue of cell proliferation when the arginase inhibitor nor-NOHA was added at the same time as arginine (at the beginning of phase 2) This experiment also demonstrates that downstream metabolites of the arginase enzymatic reaction (eg, ornithine, polyamines) generated during phase 1 are likely not involved in the observed T-cell suppression in phase 2.
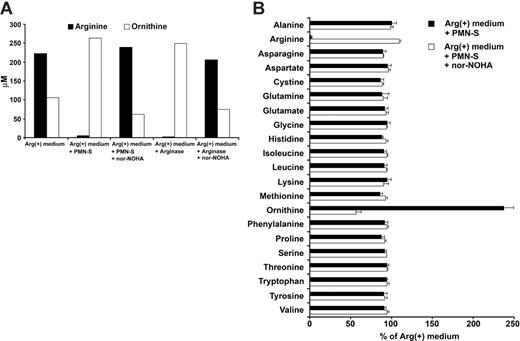
Extracellular human PMN arginase specifically depletes arginine under physiologic conditions. (A) RPMI 1640 cell culture medium containing 150 μM l-arginine (Arg(+) medium) was incubated for 12 hours at 37°C either alone or with an aliquot of a sonicate of human PMNs (PMN-S) of a defined arginase activity (300 mU/mL). Alternatively, human recombinant arginase I (final activity also 300 mU/mL) was used instead of PMN-S. When indicated, arginase activity was blocked with nor-NOHA (1 mM) during the incubation. The concentrations of arginine and ornithine were determined by ion exchange chromatography at the end of the incubation period. Representative data from 1 of 3 independent experiments are shown. (B) Experimental set-up was as in panel A. To allow for better comparison between experiments, the concentration of each amino acid in Arg(+) medium was used as control and set as 100%. The relative concentration of each amino acid (in percentage of control) after incubation with PMN-S (with or without nor-NOHA inhibition) is shown. Data are demonstrated as mean ± SD from 3 independent experiments.
Extracellular human PMN arginase specifically depletes arginine under physiologic conditions. (A) RPMI 1640 cell culture medium containing 150 μM l-arginine (Arg(+) medium) was incubated for 12 hours at 37°C either alone or with an aliquot of a sonicate of human PMNs (PMN-S) of a defined arginase activity (300 mU/mL). Alternatively, human recombinant arginase I (final activity also 300 mU/mL) was used instead of PMN-S. When indicated, arginase activity was blocked with nor-NOHA (1 mM) during the incubation. The concentrations of arginine and ornithine were determined by ion exchange chromatography at the end of the incubation period. Representative data from 1 of 3 independent experiments are shown. (B) Experimental set-up was as in panel A. To allow for better comparison between experiments, the concentration of each amino acid in Arg(+) medium was used as control and set as 100%. The relative concentration of each amino acid (in percentage of control) after incubation with PMN-S (with or without nor-NOHA inhibition) is shown. Data are demonstrated as mean ± SD from 3 independent experiments.
We also monitored T-cell proliferation by labeling the cells with CFSE and stimulating them with anti-CD3-anti-CD28-coupled microbeads. This costimulatory mode of activation was used because it provided maximal stimulation to the T cells and led to the highest number of cell divisions in Arg(+) medium. In contrast, T cells did not divide in Arg(-) medium or in Arg(+) medium that had been preincubated with PMN-S. Again, when arginase was inhibited with nor-NOHA in the latter condition, T-cell division could be rescued comparably to control cultures in Arg(+) medium (Figure 2C). The same results were seen when recombinant human arginase I was used instead of human PMN-S (Figure 2C).
Arginine is specifically depleted by liberated human PMN arginase
After having demonstrated the profound influence of liberated PMN arginase on T-cell proliferation, we studied the arginase-mediated arginine depletion directly. We measured substrate (arginine) and product (ornithine) by ion exchange chromatography in Arg(+) cell culture medium that was incubated with human PMN-S. As shown in Figure 3, PMN-S nearly completely depleted arginine from the medium and catalyzed the de novo production of ornithine. This enzymatic conversion could be inhibited by nor-NOHA (Figure 3A). The same results were observed when human recombinant arginase I was used to deplete arginine from the cell culture medium (Figure 3A). Human PMNs contain a multitude of enzymes and proteins that are liberated during cell death in vivo or cell sonication in vitro. We therefore analyzed how broadly the physiologic amino acid composition of cell culture medium is altered upon incubation with PMN-S. Interestingly, only the concentrations of arginine and ornithine were significantly altered by PMN-S while the concentrations of all other amino acids did not differ significantly (Figure 3B).
PMN arginase I is required for T-cell suppression
To confirm our data in an inhibitor-free experimental system, we prepared PMN-S of a patient with complete arginase I deficiency (Online Mendelian Inheritance in Man [OMIM] 207800). The PMN of this patient (arginase I-/-) did not contain arginase I or demonstrate arginase activity16 (see also later in Figure 7A). We analyzed the amino acid composition of Arg(+) medium that was preincubated with arginase I-/- PMN-S adjusted to the same amount of mean protein input as used in our previous experiments (Figure 2). In contrast to PMN-S of healthy donors (Figure 3), there was no arginine deprivation or ornithine synthesis when PMNs of an arginase I-/- patient were used (Figure 4A). We then analyzed the proliferation of T cells activated in this arginase I-/- PMN-S-preincubated medium (Figure 4B). As controls we used PMN-S of 3 healthy donors (arginase I+/+) and adjusted all sonicates to the same final protein concentration (160 μg/mL), corresponding to final arginase activities of control PMN-S from 100 to 200 mU/mL. In contrast to medium preincubated with arginase I+/+ sonicates, T cells proliferated normally when Arg(+) medium was preincubated with PMN-S from the arginase I-/- patient (Figure 4B), demonstrating that human PMN arginase I is required for the induction of T-cell suppression.
PMNs that lack arginase I do not suppress T-cell proliferation. (A-B) PMNs of an 18-year-old man with arginase I deficiency (arginase I-/-) were sonicated. RPMI 1640 cell culture medium containing 150 μM l-arginine (Arg(+) medium) was incubated at 37°C either alone or with an aliquot of the arginase I-/- PMN-S. Arginase I-/- PMN-S was diluted to a final protein concentration (160 μg/mL) that corresponds to an arginase activity of 200 to 300 mU/mL in the PMN-S of 3 healthy donors (arginase I+/+). (A) After 12 hours, the concentrations of arginine, ornithine, and urea were measured by ion exchange chromatography. The concentration of each substance in Arg(+) medium was used as control and set as 100%. The relative concentration (in percentage of control) after incubation with arginase I-/- PMN-S is shown. (B) T-cell proliferation assays were set up and analyzed as described in Figure 1. Purified human T cells were stimulated with platebound crosslinked anti-CD3 antibody (500 ng/mL), and proliferation was assessed by [3H]thymidine incorporation after 48 hours in triplicate wells. Representative data from 1 of 3 independent experiments are shown.
PMNs that lack arginase I do not suppress T-cell proliferation. (A-B) PMNs of an 18-year-old man with arginase I deficiency (arginase I-/-) were sonicated. RPMI 1640 cell culture medium containing 150 μM l-arginine (Arg(+) medium) was incubated at 37°C either alone or with an aliquot of the arginase I-/- PMN-S. Arginase I-/- PMN-S was diluted to a final protein concentration (160 μg/mL) that corresponds to an arginase activity of 200 to 300 mU/mL in the PMN-S of 3 healthy donors (arginase I+/+). (A) After 12 hours, the concentrations of arginine, ornithine, and urea were measured by ion exchange chromatography. The concentration of each substance in Arg(+) medium was used as control and set as 100%. The relative concentration (in percentage of control) after incubation with arginase I-/- PMN-S is shown. (B) T-cell proliferation assays were set up and analyzed as described in Figure 1. Purified human T cells were stimulated with platebound crosslinked anti-CD3 antibody (500 ng/mL), and proliferation was assessed by [3H]thymidine incorporation after 48 hours in triplicate wells. Representative data from 1 of 3 independent experiments are shown.
Human PMN arginase leads to down-regulation of the CD3ζ chain and also impairs T-cell activation independently of the TCR
Human and murine T-cell activation in the absence of arginine leads to down-regulation of the CD3ζ chain19 (Figure 5A), which compromises efficient T-cell signaling.3 We therefore analyzed the expression level of the CD3ζ chain in human T cells that were stimulated in cell culture medium depleted of arginine by human PMN-S (Figure 5B) or recombinant human arginase I (data not shown). Under both circumstances the CD3ζ chain was down-regulated comparably to activation in an arginine-free milieu (Figure 5A). Inhibition of PMN arginase within the PMN-S by nor-NOHA prevents down-regulation of the CD3ζ chain (Figure 5C). The different expression levels of the CD3ζ chain in 8 independent experiments are summarized in Figure 5D. We also assessed if arginase-mediated arginine depletion impairs T-cell receptor (TCR) signaling further downstream independently of proximal TCR signaling elements. For this purpose, human T cells were stimulated with PMA and ionomycin, which leads to direct activation of protein kinase C (PKC) and Ca2+ signaling without TCRζ chain phosphorylation. As demonstrated in Figure 5E, arginine depletion induces complete suppression of T-cell proliferation upon PMA/ionomycin activation, and this is reversible by arginase inhibition with nor-NOHA. Clearly, arginine depletion interacts with TCR activation at multiple signaling levels, possibly conferring a high degree of redundancy to this inhibitory system.
Human PMN arginase-mediated arginine depletion specifically alters T-cell activation
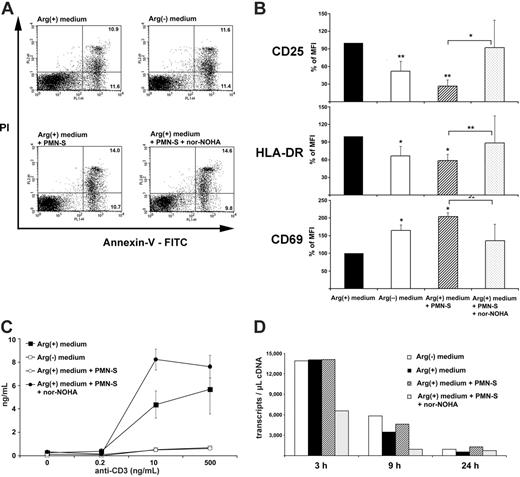
To confirm that T cells remained viable in arginine-depleted medium, cells were activated and then stained with annexin V-FITC and PI to determine the frequency of cells undergoing apoptosis (annexin V-FITC positive, PI negative) and of dead cells (annexin V-FITC positive, PI positive) by flow cytometry. As shown in Figure 6A, cell viability of anti-CD3-activated human T cells was not impaired by arginine depletion or the presence of PMN-S (mean PI-positive cells in Arg(+) medium, 14.7% ± 5.1%; in Arg(-) medium, 13.8% ± 5.1%; in Arg(+) medium + PMN-S, 15.2% ± 6.5%; in Arg(+) medium + PMN-S + nor-NOHA, 12.9% ± 7.4% in 3 independent experiments). Upon crosslinking of their TCR, T cells display various signs of activation and perform a series of different functions. To assess the degree of PMN arginase-mediated T-cell suppression, we first monitored the up-regulation of the activation markers CD69, CD25, and HLA-DR on human T cells by flow cytometry (Figure 6B). To allow for comparison between 5 separate experiments, we normalized the control mean fluorescence intensity (MFI) of anti-CD3-activated human T cells in Arg(+) medium to 100%. The up-regulation of CD25 and HLA-DR was clearly dependent on arginine in the micromilieu and could be suppressed by PMN arginase (CD25, 26.3% ± 10.8% of control MFI; HLA-DR, 58.7% ± 17.3% of control MFI). In contrast, the up-regulation of CD69 on human T cells was significantly increased by the presence of PMN-S (204.8% ± 37.4% of control MFI). The presence of nor-NOHA reversed the PMN-S-mediated differences in expression of the activation markers, thus verifying that liberated arginase was responsible for the observed effects.
Human PMN arginase-mediated arginine depletion induces down-regulation of CD3ζ in T cells. Purified human T cells were stimulated with antihuman CD3- and antihuman CD28-coupled paramagnetic microbeads. After 48 hours, cells were stained with FITC-labeled antihuman CD3, fixed, permeabilized, and stained with PE-labeled antihuman CD3ζ. Intracellular expression of CD3ζ was analyzed by flow cytometry. As control, CD3ζ expression in Arg(+) cell culture medium (150 μM l-arginine) is shown. The dashed lines represent stainings for the respective isotype control antibodies. The experimental conditions were as follows: (A) cell culture medium without arginine, (B) Arg(+) medium preincubated for 12 hours with human PMN-S (final arginase activity 300 mU/mL), and (C) as in panel B with or without inhibition of arginase by nor-NOHA (1 mM) from the start of the preincubation. (D) MFIs of the CD3ζ chain from 8 independent experiments are demonstrated as mean ± SD. CD3ζ expression upon stimulation in Arg(+) medium was set as 100% to allow for comparison between individual experiments. Data were analyzed with the paired Student t test. *P < .05 when comparing with T cells in Arg(+) medium; **P < .001 when comparing Arg(+) medium + PMN-S with or without nor-NOHA. (E) Purified human T cells were stimulated with different concentrations of PMA and ionomycin and in different cell culture conditions as indicated (see Figure 1 for explanation). After 48 hours, T-cell proliferation was assessed by [3H]thymidine incorporation in triplicate wells. Data are from 1 representative experiment (total, 4).
Human PMN arginase-mediated arginine depletion induces down-regulation of CD3ζ in T cells. Purified human T cells were stimulated with antihuman CD3- and antihuman CD28-coupled paramagnetic microbeads. After 48 hours, cells were stained with FITC-labeled antihuman CD3, fixed, permeabilized, and stained with PE-labeled antihuman CD3ζ. Intracellular expression of CD3ζ was analyzed by flow cytometry. As control, CD3ζ expression in Arg(+) cell culture medium (150 μM l-arginine) is shown. The dashed lines represent stainings for the respective isotype control antibodies. The experimental conditions were as follows: (A) cell culture medium without arginine, (B) Arg(+) medium preincubated for 12 hours with human PMN-S (final arginase activity 300 mU/mL), and (C) as in panel B with or without inhibition of arginase by nor-NOHA (1 mM) from the start of the preincubation. (D) MFIs of the CD3ζ chain from 8 independent experiments are demonstrated as mean ± SD. CD3ζ expression upon stimulation in Arg(+) medium was set as 100% to allow for comparison between individual experiments. Data were analyzed with the paired Student t test. *P < .05 when comparing with T cells in Arg(+) medium; **P < .001 when comparing Arg(+) medium + PMN-S with or without nor-NOHA. (E) Purified human T cells were stimulated with different concentrations of PMA and ionomycin and in different cell culture conditions as indicated (see Figure 1 for explanation). After 48 hours, T-cell proliferation was assessed by [3H]thymidine incorporation in triplicate wells. Data are from 1 representative experiment (total, 4).
We next assessed the ability of human T cells to secrete the cytokine IFN-γ in situations when arginine was limited or absent. T cells were again stimulated via their TCR, and the amount of IFN-γ in the supernatant was determined by ELISA (Figure 6C). In analogy to proliferation, cytokine secretion was severely impaired in arginine-free medium or in medium that was preincubated with human PMN-S. While human T cells secreted 7395 ± 4844 pg/mL IFN-γ (mean ± SD of 4 independent experiments), preincubation with PMN-S reduced this synthesis to 773 ± 293 pg/mL, corresponding to a reduction of 86.7% ± 4.9%. When arginase was inhibited with nor-NOHA during the preincubation, the production of IFN-γ was rescued (Figure 6C). Again, when human recombinant arginase I was used instead of PMN-S, a comparable suppression of T-cell IFN-γ synthesis was induced (data not shown). We then quantified the amount of different cytokine mRNA transcripts. While in unstimulated T cells only low copy numbers for IFN-γ, TNF-α, and IL-2 mRNA were detected at all time points (fewer than 100 transcripts per microliter of cDNA, data not shown), anti-CD3 stimulation induced pronounced transcription of the 3 cytokines (IFN-γ, Figure 6D; TNF-α and IL-2, data not shown), and arginine depletion did not lead to a quantitative reduction of various cytokine mRNA transcript numbers.
Human PMN arginase-mediated arginine depletion does not increase T-cell apoptosis or cell death but specifically alters T-cell activation. (A) Purified human T cells were stimulated with platebound crosslinked antihuman CD3 antibody for 48 hours in different cell culture conditions (nomenclature as in Figure 1). After double staining with annexin V-FITC and PI, cells were analyzed by flow cytometry. The percentages of T cells undergoing apoptosis (annexin V-FITC positive, PI negative) and of dead T cells (annexin V-FITC positive, PI positive) within the whole population are depicted within the respective quadrants. Data are representative of 3 individual experiments. (B) Human T cells were stimulated as in panel A and double stained with anti-CD3 and either anti-CD25, anti-HLA-DR, or anti-CD69. Analysis of the expression level of the different T-cell activation markers was done by flow cytometry after gating on CD3+ cells. To allow for comparison between 5 separate experiments, control MFI (activated human T cells in Arg(+) medium) was normalized to 100%. Data are shown as mean ± SD and were analyzed with the paired Student t test. *P < .05 and **P < .005. Arg(-) medium and Arg(+) medium + PMN-S are compared with Arg(+) medium. Arg(+) medium + PMN-S is also compared with Arg(+) medium + PMN-S + nor-NOHA, as indicated by bars. (C) Human PMN arginase suppresses T-cell IFN-γ synthesis by a posttranscriptional mechanism. Purified human T cells were stimulated with platebound crosslinked anti-CD3 antibody at the indicated concentrations. Cell culture media were as described in Figure 2. Supernatant was harvested after 48 hours, and IFN-γ was measured by ELISA. (D) A total of 3 × 106 human T cells were stimulated as in panel C. At the indicated time points (3 hours, 9 hours, and 24 hours) mRNA was prepared and reverse transcribed. The frequency of IFN-γ transcripts was quantified by LightCycler Technology.
Human PMN arginase-mediated arginine depletion does not increase T-cell apoptosis or cell death but specifically alters T-cell activation. (A) Purified human T cells were stimulated with platebound crosslinked antihuman CD3 antibody for 48 hours in different cell culture conditions (nomenclature as in Figure 1). After double staining with annexin V-FITC and PI, cells were analyzed by flow cytometry. The percentages of T cells undergoing apoptosis (annexin V-FITC positive, PI negative) and of dead T cells (annexin V-FITC positive, PI positive) within the whole population are depicted within the respective quadrants. Data are representative of 3 individual experiments. (B) Human T cells were stimulated as in panel A and double stained with anti-CD3 and either anti-CD25, anti-HLA-DR, or anti-CD69. Analysis of the expression level of the different T-cell activation markers was done by flow cytometry after gating on CD3+ cells. To allow for comparison between 5 separate experiments, control MFI (activated human T cells in Arg(+) medium) was normalized to 100%. Data are shown as mean ± SD and were analyzed with the paired Student t test. *P < .05 and **P < .005. Arg(-) medium and Arg(+) medium + PMN-S are compared with Arg(+) medium. Arg(+) medium + PMN-S is also compared with Arg(+) medium + PMN-S + nor-NOHA, as indicated by bars. (C) Human PMN arginase suppresses T-cell IFN-γ synthesis by a posttranscriptional mechanism. Purified human T cells were stimulated with platebound crosslinked anti-CD3 antibody at the indicated concentrations. Cell culture media were as described in Figure 2. Supernatant was harvested after 48 hours, and IFN-γ was measured by ELISA. (D) A total of 3 × 106 human T cells were stimulated as in panel C. At the indicated time points (3 hours, 9 hours, and 24 hours) mRNA was prepared and reverse transcribed. The frequency of IFN-γ transcripts was quantified by LightCycler Technology.
Arginase activity in vivo in the context of granulocytic inflammation
After having demonstrated that extracellular human PMN arginase I depletes arginine and induces a profound suppression of various T-cell functions, we finally studied its role in vivo in the context of granulocytic inflammation. A prototypical PMN-dominated inflammation in vivo leads to the generation of pus as a correlate of massive PMN cell death and liberation of intracellular molecules. We therefore prepared cellfree supernatant of pus (PUS-SN) derived from surgical abscess drainage of 3 patients. Arginase I protein could be detected in this cellfree supernatant by Western blot (Figure 7A). We then measured arginase activity and found a mean activity of 1245 ± 617 mU/mg protein or 48 644 ± 17 140 mU/mL within the PUS-SN. This activity in vivo is more than 150 times higher than the activity (300 mU/mL) that reproducibly leads to complete arginine depletion and T-cell suppression in vitro. To demonstrate the functional potential of arginase within human pus, we preincubated Arg(+) medium with PUS-SN (with or without nor-NOHA) diluted to a final arginase activity of 300 mU/mL. Human T cells were then activated by crosslinked anti-CD3, and proliferation was assessed after 48 hours. When the cells were activated in medium preincubated with PUS-SN, T-cell proliferation (Figure 7B) was completely suppressed. This suppression was due to liberated PMN arginase within the PUS-SN because proliferation could be completely rescued by exogenous supplementation of supraphysiologic amounts of arginine (1000 μM) at the beginning of the T-cell activation assay or by inhibition of arginase within PUS-SN by nor-NOHA from the start of the preincubation. The proinflammatory agonists IFN-γ and LPS (possible constituents of human pus) had no inhibitory influence on T-cell proliferation or TCRζ chain expression in our system (data not shown).
Discussion
Human PMN arginase constitutes a novel homeostatic immunoregulatory mechanism that limits excessive immune activation. During PMN-mediated inflammation, human arginase I is liberated. The enzyme depletes arginine in the surroundings, and this arginine depletion profoundly inhibits the expansion of T cells during purulent inflammation as well as certain T-cell effector functions. The mechanism of substrate depletion fully accounts for the observed immunosuppression whereas downstream metabolites of the arginase reaction (eg, ornithine, polyamines) are not involved. This anti-inflammatory role of human PMN arginase supports the concept that PMNs are not only proinflammatory cellular elements but can also contribute to the resolution of inflammation.1
Given the complex nature of a human PMN sonicate with multiple enzymatic effector systems, it is intriguing that among all tested amino acids only arginine concentration was altered in the context of PMN cell death. Furthermore, various potentially toxic mediators within a human PMN sonicate20 might have contributed to the suppression of T-cell functions. This was clearly not the case, because T-cell suppression could be completely reversed by inhibition of arginase with nor-NOHA and was not present when PMNs of a patient with arginase I deficiency were used. It remains to be analyzed if arginine depletion via liberated PMN arginase has even broader antiinflammatory consequences. In murine macrophages, induction of arginase I via IL-13 8 induces extracellular and intracellular arginine depletion, and this amino acid restriction suppresses the translation of inducible nitric oxide synthase.21 Also, downstream metabolic products of the arginase pathway might participate in other immunosuppressive effector functions or possibly other consequences of PMN cell death. While Th2-stimulated murine macrophages synthesize proline from arginase-generated ornithine,22 we did not observe the synthesis of proline in our in vitro human PMN cell death system. This question is highly relevant because proline is a necessary precursor amino acid for the synthesis of collagen and excessive proline generation during PMN-mediated inflammation might contribute to enhanced fibrotic tissue reactions.22 Also, ornithine decarboxylase might generate polyamines from ornithine, and these polycationic molecules possess antiinflammatory properties,23 regulate the survival of microbial pathogens,24,25 or induce apoptosis.25
Human PMN arginase I is liberated during PMN-dominated inflammation in vivo and suppresses T-cell proliferation. (A) Human pus derived from a parastomal skin abscess of a patient with Crohn disease was centrifuged 3 times and filtered through a 0.2 μm filter to remove cells and prepare cellfree supernatant (PUS-SN). Arginase I protein expression was analyzed by immunoblotting in PUS-SN and (as positive and negative controls) in PMN lysates of a healthy (Arg I+/+) or arginase I-deficient (Arg I-/-) blood donor or in normal human liver. The results of parallel determinations of arginase activities (in milliunits per milligram of protein) in aliquots of the same cell lysates are noted above the respective lanes of the immunoblot. (B) Human PUS-SN (arginase activity 31 380 mU/mL) was diluted to a final arginase activity of 300 mU/mL in Arg(+) medium (Figure 1), and the medium was incubated for 12 hours. When indicated, nor-NOHA (1 mM) was added from the start of the incubation to inhibit arginase activity within PUS-SN. Human T cells were then activated by crosslinked anti-CD3 in the respective media, and proliferation was assessed after 48 hours by [3H]thymidine incorporation. When indicated, l-arginine (1 mM) was supplemented at the start of the T-cell activation.
Human PMN arginase I is liberated during PMN-dominated inflammation in vivo and suppresses T-cell proliferation. (A) Human pus derived from a parastomal skin abscess of a patient with Crohn disease was centrifuged 3 times and filtered through a 0.2 μm filter to remove cells and prepare cellfree supernatant (PUS-SN). Arginase I protein expression was analyzed by immunoblotting in PUS-SN and (as positive and negative controls) in PMN lysates of a healthy (Arg I+/+) or arginase I-deficient (Arg I-/-) blood donor or in normal human liver. The results of parallel determinations of arginase activities (in milliunits per milligram of protein) in aliquots of the same cell lysates are noted above the respective lanes of the immunoblot. (B) Human PUS-SN (arginase activity 31 380 mU/mL) was diluted to a final arginase activity of 300 mU/mL in Arg(+) medium (Figure 1), and the medium was incubated for 12 hours. When indicated, nor-NOHA (1 mM) was added from the start of the incubation to inhibit arginase activity within PUS-SN. Human T cells were then activated by crosslinked anti-CD3 in the respective media, and proliferation was assessed after 48 hours by [3H]thymidine incorporation. When indicated, l-arginine (1 mM) was supplemented at the start of the T-cell activation.
T-cell suppression via arginase-mediated arginine depletion shares many features with the system of indoleamine dioxygenase (IDO)-mediated tryptophan degradation.26 Like human PMN arginase,16 IDO was also described initially as an antimicrobial effector system that operates via depletion of an amino acid essential for microbial growth.27 Besides its antimicrobial function, IDO also performs antiinflammatory tasks. Locally it suppresses invading maternal T cells during pregnancy and protects the murine semial-logeneic fetus.28 The regulation of complex functions of the immune system by amino acid availability has also been shown for cysteine. The depletion of cysteine induces cell cycle arrest in human T cells via intracellular depletion of glutathione and consecutively disturbed redox regulation.18
Human PMN arginase depletes arginine very efficiently under physiologic conditions (pH 7.4; 150 μM l-arginine, 4 μM Mn2+). This is especially important because mammalian arginases have an alkaline pH optimum and low substrate affinity (human arginase I: Km 0.02 mM at pH 9.5).29 Most importantly, we found that arginase activities in vivo in the context of purulent inflammation are 100 to 700 times higher (30 000 to 70 000 mU/mL) (Figure 7) than needed for complete T-cell suppression in vitro (100 to 300 mU/mL). While arginine was completely depleted in our cell culture model of PMN cell death, the situation in vivo is more complex due to the dynamic flux of arginine and arginase itself within the inflamed tissue. Most likely, a concentration gradient for arginase and reciprocally for arginine is found around the epicenter of PMN-dominated inflammation. Our results therefore support a model of transient T-cell nonresponsiveness in the context of PMN-dominated inflammation. Arginine depletion via liberated PMN arginase did not increase T-cell apoptosis or cell death, and the cells resumed their potential to proliferate and secrete cytokines when they were stimulated again in an arginine-supplemented environment (data not shown). Interestingly, arginine restriction increased the expression of CD69, a potential negative regulator of T-cell functions, which is up-regulated in chronic inflammatory infiltrates.30
T-cell hyporesponsiveness associated with a down-regulated TCRζ chain can be the consequence of various circumstances in the murine immune system.3 In humans, it is a recurrent finding in patients with cancer,31,32 autoimmunity,33,34 or chronic infections.35 The mechanism(s) and cells that induce the observed T-cell phenotype and/or the associated immunosuppression are largely unknown. Recently, arginase-expressing myeloid suppressor cells, which morphologically resemble PMNs, were described in patients with renal cell carcinoma,36 and arginase was shown to participate in the suppression of tumor-infiltrating lymphocytes in patients with prostate carcinoma.37 Also, reactive oxygen metabolites of activated human PMNs induce down-regulation of the CD3ζ chain and associated T-cell dysfunction in cancer patients.4 While stimulated human granulocytes might use additional mechanisms for T-cell suppression in vivo, arginase-mediated arginine depletion clearly fully accounts for the observed fundamental block in T-cell proliferation and cytokine synthesis in our in vitro system as well as in the context of human pus.
How does arginine depletion translate into suppression of T-cell function? The only known specific alteration so far is a down-regulation of the CD3ζ chain.3,19 We did reproduce this activation-dependent phenotype of the T cells when human PMN-S was used to deplete arginine in vitro. Besides this well-established mechanism of T-cell dysfunction, further alterations are likely present in human T cells that are activated in the absence of arginine. Because PMN arginase-mediated suppression of T-cell cytokine synthesis is not due to a defect on the level of transcription (Figure 6D), we hypothesize that it involves regulation also on the level of mRNA translation. Recently, it was demonstrated that the suppression of murine CD8+ T cells upon IDO-mediated depletion of tryptophan is due to activation of T-cell GCN2 kinase.38 Further studies must therefore be performed to analyze in depth the contribution of proximal and distal signal transduction alterations in human T cells upon arginine depletion.
In summary, we have shown that human PMNs have the potential to suppress T-cell immune reactions via liberation of arginase and consecutive arginine depletion. This immunosuppression might have evolved as a homeostatic immunoregulatory mechanism that limits excessive or inadequate immune activation. The pharmacologic inhibition of liberated PMN arginase in the context of PMN inflammatory reactions might therefore reconstitute T-cell immunity under circumstances of unwanted immunosuppression.
Prepublished online as Blood First Edition Paper, May 18, 2006; DOI 10.1182/blood-2006-11-010389.
Supported by the Deutsche Forschungsgemeinschaft (MU 1547/3-1) and grants 2PR04B002 (J.M.F., Junta of Extremadura) and PI040828 (J.M.F., FIS/ISCIII). M. Munder designed and performed experiments and wrote the manuscript; H.S. and C.L. performed experiments; T.G. performed experiments (mRNA quantification); C.D.L. performed experiments (amino acid measurements); J.M.F. generated and provided reagents (recombinant arginase); P.K., I.M., and A.D.H. assisted in designing experiments and writing the manuscript; A.K. provided patient material; and M. Modolell generated reagents (antibodies) and assisted in writing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Stephen D. Cederbaum (UCLA) for the gift of human arginase I cDNA, V. Daniel (Institute of Immunology, University of Heidelberg) and T. Luft (Department of Medicine, University of Heidelberg) for providing blood of healthy donors, and L. B. Nicholson (University of Bristol) for critical review of the manuscript.

![Figure 2. Human T-cell proliferation is suppressed by human PMN arginase. (A) Purified human T cells were stimulated with crosslinked anti-CD3 antibody at various concentrations, and proliferation was assessed by [3H]thymidine incorporation after 48 hours in triplicate wells. The cells were either stimulated in the presence (Arg(+) medium) or absence (Arg(-) medium) of 150 μM l-arginine. Alternatively, Arg(+) medium was preincubated for 12 hours with an aliquot of human PMN sonicate (PMN-S), corresponding to a final arginase activity of 300 mU/mL, in the presence or absence of the specific arginase inhibitor nor-NOHA, and T cells were then stimulated in these different media. Data are representative of 38 different experiments. (B) T-cell proliferation was analyzed as in panel A, but nor-NOHA and/or l-arginine (1 mM each) were added to arginine-depleted medium (Arg(+) medium + PMN-S) at the same time that the T cells were added. Results are expressed as mean cpm of triplicate cultures ± SD. (C) Human T-cell proliferation was assessed by cell division-mediated loss of intracellular CFSE fluorescence. T cells were labeled with CFSE and stimulated for 96 hours with antihuman CD3- and antihuman CD28-coupled microbeads in different cell culture media as described for panel A. Additionally, arginine was depleted in Arg(+) medium by 12 hours of preincubation with recombinant human arginase I (activity 300 mU/mL). Cell division (ie, loss of FL-1 fluorescence) was analyzed by flow cytometry. Data are representative of 4 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2006-11-010389/2/m_zh80170600600002.jpeg?Expires=1763464010&Signature=JFkdwDHG7fPwjut2PgEkiLguQ-5h~m~tHptdrATgjZILnYfxGhp2yxiOrhL--U2mNraFJheO0pzwpZNEJO81NZO8RPj1iXHt~PDvs4~rTo6izyJ6LWckQMxxHX9w~wMwQOSqr9lHga2~7qGh4u6jnpgmNvPYjihaunfbTRZbrFonWpjKkpBI5~dfMu1R1rB~kV6kWmH~JgABBEH8gfMm9g8KdHc96gypXRAXH-lejb8kSbua3uCRYhOHKlvg2Y-AHLSbdddmmtRTtb9BwUIBc6l~rq1~I54DbkkpPCmuegOiuYArPKqWg5fhqwV1tpxv-XrJdrhu1ctOaz~KTFkrVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. PMNs that lack arginase I do not suppress T-cell proliferation. (A-B) PMNs of an 18-year-old man with arginase I deficiency (arginase I-/-) were sonicated. RPMI 1640 cell culture medium containing 150 μM l-arginine (Arg(+) medium) was incubated at 37°C either alone or with an aliquot of the arginase I-/- PMN-S. Arginase I-/- PMN-S was diluted to a final protein concentration (160 μg/mL) that corresponds to an arginase activity of 200 to 300 mU/mL in the PMN-S of 3 healthy donors (arginase I+/+). (A) After 12 hours, the concentrations of arginine, ornithine, and urea were measured by ion exchange chromatography. The concentration of each substance in Arg(+) medium was used as control and set as 100%. The relative concentration (in percentage of control) after incubation with arginase I-/- PMN-S is shown. (B) T-cell proliferation assays were set up and analyzed as described in Figure 1. Purified human T cells were stimulated with platebound crosslinked anti-CD3 antibody (500 ng/mL), and proliferation was assessed by [3H]thymidine incorporation after 48 hours in triplicate wells. Representative data from 1 of 3 independent experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2006-11-010389/2/m_zh80170600600004.jpeg?Expires=1763464010&Signature=etrasZgBk9FtpzNe2cEjD2jFI1UnBjskUXthhk~0FhlDP~OUBw2PHRfzo8LzrZIaTxHELMiaBMAe54DL70qYwHdbHMb4rekYPKTVgig3r-2R3gdnCUnFA-82dQveie9-kP8K9zPW4l1iAM~nhVUr9DouL3J9gBQqfcHwLqAkj~16uYUHH-OEWb5PA0ZRZBW-blzypWnc-b952GM~XuwASb~irOnJmrXEDbhUCrEPaTqy2jx1eh4IhSX9BvJDVw2FXFgux2ZNM9RIJSBiy2gFSsPA3sq8r0nv4XibE0-c4i7AjfKBeBKq191eZg~cwmjv9P7iL~XYAgIgENiBw6hLtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Human PMN arginase-mediated arginine depletion induces down-regulation of CD3ζ in T cells. Purified human T cells were stimulated with antihuman CD3- and antihuman CD28-coupled paramagnetic microbeads. After 48 hours, cells were stained with FITC-labeled antihuman CD3, fixed, permeabilized, and stained with PE-labeled antihuman CD3ζ. Intracellular expression of CD3ζ was analyzed by flow cytometry. As control, CD3ζ expression in Arg(+) cell culture medium (150 μM l-arginine) is shown. The dashed lines represent stainings for the respective isotype control antibodies. The experimental conditions were as follows: (A) cell culture medium without arginine, (B) Arg(+) medium preincubated for 12 hours with human PMN-S (final arginase activity 300 mU/mL), and (C) as in panel B with or without inhibition of arginase by nor-NOHA (1 mM) from the start of the preincubation. (D) MFIs of the CD3ζ chain from 8 independent experiments are demonstrated as mean ± SD. CD3ζ expression upon stimulation in Arg(+) medium was set as 100% to allow for comparison between individual experiments. Data were analyzed with the paired Student t test. *P < .05 when comparing with T cells in Arg(+) medium; **P < .001 when comparing Arg(+) medium + PMN-S with or without nor-NOHA. (E) Purified human T cells were stimulated with different concentrations of PMA and ionomycin and in different cell culture conditions as indicated (see Figure 1 for explanation). After 48 hours, T-cell proliferation was assessed by [3H]thymidine incorporation in triplicate wells. Data are from 1 representative experiment (total, 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2006-11-010389/2/m_zh80170600600005.jpeg?Expires=1763464010&Signature=GBYf7t5PNaR0KO-k6tjBiO0Zed7RGN~NmScCqX-KHDrB2EOTAkyhrSPi7UpF8LCc7HvyhHlIMnRZCbuV8dcSi7MAIehOQFVK88eiz4zK582d~0LxG~rbzY7cF82Dv1~13XX62AN6bINOw~~cIoNYnssgKJTVcCl~Z4jVLBZsmcJJINJiBI6RbtaL29gzK3ATGXPGvyBqmVnjBMpjUY4ao9GNtFRk4yfdm9vNcTwS3O7RCtGjGiDNt6r0kpO7ZZkrrBpebffIKXXaX2a-mCl~mM-fQ5cxkOaOrjGjSeST~WbRaJ-EVafqeQNqukxIlV0uTlytbWcO~t12NFDCd1x0Gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Human PMN arginase I is liberated during PMN-dominated inflammation in vivo and suppresses T-cell proliferation. (A) Human pus derived from a parastomal skin abscess of a patient with Crohn disease was centrifuged 3 times and filtered through a 0.2 μm filter to remove cells and prepare cellfree supernatant (PUS-SN). Arginase I protein expression was analyzed by immunoblotting in PUS-SN and (as positive and negative controls) in PMN lysates of a healthy (Arg I+/+) or arginase I-deficient (Arg I-/-) blood donor or in normal human liver. The results of parallel determinations of arginase activities (in milliunits per milligram of protein) in aliquots of the same cell lysates are noted above the respective lanes of the immunoblot. (B) Human PUS-SN (arginase activity 31 380 mU/mL) was diluted to a final arginase activity of 300 mU/mL in Arg(+) medium (Figure 1), and the medium was incubated for 12 hours. When indicated, nor-NOHA (1 mM) was added from the start of the incubation to inhibit arginase activity within PUS-SN. Human T cells were then activated by crosslinked anti-CD3 in the respective media, and proliferation was assessed after 48 hours by [3H]thymidine incorporation. When indicated, l-arginine (1 mM) was supplemented at the start of the T-cell activation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2006-11-010389/2/m_zh80170600600007.jpeg?Expires=1763464010&Signature=1vluFr-T3V9S5mTfdOa~sVB9HGJnozGH0a~JX32eiWKq2sLdAa1q4jvz0l~5a3JzIqTXMj2dDy95g~k1c7rxwzvJs0ODROF2cB-YS9Vuuf577pn8DjSQ8TDGpbSOseclsqQy5ZDF17YN9ujEVUdUeeiTMUeuOSi-4s2A~-Xd6rOFq~s43oAFMJLvYPYBM1yQ~StV2SD1XtITasOeB068o-H0MQUoc7E1xJi-oDORnc2nzvgm2YYkxDP1FKg-VhBSKq877L2DugPIPde1TN7kVBa21LLD4azgKogNWgu5zegZs6mHF87EO8qNDlk~02tCjVWqn10LEgatraRZgaOtQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal