Circulating memory B cells are severely reduced in the peripheral blood of HIV-1-infected patients. We investigated whether dysfunctional serologic memory to non-HIV antigens is related to disease progression by evaluating the frequency of memory B cells, plasma IgG, plasma levels of antibodies to measles, and Streptococcus pneumoniae, and enumerating measles-specific antibody-secreting cells in patients with primary, chronic, and long-term nonprogressive HIV-1 infection. We also evaluated the in vitro production of IgM and IgG antibodies against measles and S pneumoniae antigens following polyclonal activation of peripheral blood mononuclear cells (PBMCs) from patients. The percentage of memory B cells correlated with CD4+ T-cell counts in patients, thus representing a marker of disease progression. While patients with primary and chronic infection had severe defects in serologic memory, long-term nonprogressors had memory B-cell frequency and levels of antigen-specific antibodies comparable with controls. We also evaluated the effect of antiretroviral therapy on these serologic memory defects and found that antiretroviral therapy did not restore serologic memory in primary or in chronic infection. We suggest that HIV infection impairs maintenance of long-term serologic immunity to HIV-1-unrelated antigens and this defect is initiated early in infection. This may have important consequences for the response of HIV-infected patients to immunizations.
Introduction
The ability to maintain an intact memory B-cell compartment is an essential component of the immune response to (re-) infections.1 Maintenance of serologic memory is carried out by plasma cells and memory B cells1-3 ; memory B cells play an essential role in the maintenance of antibody (Ab) levels by rapidly generating secondary immune responses upon reinfection or antigenic stimulation.4
One of the most deleterious effects of HIV-1 infection is B-lymphocyte hyperactivation, which manifests as hypergammaglobulinemia, increased expression of activation markers, high spontaneous Ab production in vitro, and increased incidence of B-cell lymphomas.5 Paradoxically, HIV-1-infected persons, especially those in advanced stages of disease, also have impaired humoral immune response to vaccination, and their B cells respond poorly to in vitro stimulation by common mitogens such as SAC and PWM.5 Earlier studies suggest that naive and memory B cells differentially contribute to B-cell dysfunctions in HIV-1 infection.6-10 Circulating memory B cells in peripheral blood from patients with chronic HIV-1 infection (CHI) are severely reduced and die by apoptosis.6,11 Serum Abs against measles, tetanus toxoid, and HIV-1 antigens are significantly reduced in patients with low memory B cells, indicating that this phenotypic alteration may severely affect memory B-cell functions.10 Recently, we reported that during primary HIV-1 infection (PHI), memory B cells are phenotypically and functionally altered though not significantly reduced in number.12 These alterations were only partially recovered upon antiretroviral therapy (ART), suggesting that PHI sets the stage for severe B-cell memory dysfunctions.
The clinical significance of depressed serologic memory during HIV-1 infection has not been thoroughly evaluated. Previous studies have suggested that a compromised memory B-cell response and/or low specific antibody titers may increase the risk of infections for the HIV-1 carrier10,13-15 and for children born to HIV-1-infected mothers.16-19 To further dissect the relationship between loss of memory B cells, reduction of specific antibody levels, and disease progression, we studied subjects at different stage of disease (ie, PHI, CHI, and long-term nonprogressor [LTNP]) and also evaluated the effects of ART. In order to cover the spectrum of currently held views on maintenance of B-cell memory, we measured Ab titers against a T-cell-dependent antigen (measles) and a T-cell-independent antigen (pneumococcal polysaccharide capsule antigen [PPS]). Measles remains a serious public health issue around the world,20,21 especially in pediatric patients in measles- and HIV-1-endemic regions of the world and despite the availability of a measles vaccine.22,23 Infection with Streptococcus pneumoniae causes severe morbidity and mortality in HIV-1-infected individuals, who have a considerably higher risk of acquiring serious pneumococcal infection,24,25 even in the era of potent antiretroviral therapy.26 By studying individuals at varying stages of infection, we give a comprehensive picture of the effects of HIV-1 infection on maintenance of serologic memory to clinically relevant pathogens.
Patients, materials, and methods
Study population
Three groups of HIV-1-infected patients were included in the study: 41 patients with PHI and 20 with CHI enrolled at the San Raffaele Institute, Milan, Italy, and 20 patients with CHI and 7 LTNP patients enrolled at the Karolinska University Hospital, Stockholm, Sweden (Table 1).
Study population baseline characteristics
. | Patients with PHI . | Patients with CHI . | LTNP patients . |
|---|---|---|---|
| No. patients | 41 | 40 | 7 |
| Median age, y (range) | 32 (17-53) | 43 (30-79) | 42 (36-47) |
| Median CD4+ T-cell counts (range) | 645 (204-1412) | 591 (68-850) | 736 (520-908) |
| Median HIV RNA, log10 copies/mL (range) | 4.57 (2.69-5.46) | 2.28 (1.7-4.5) | 2.49 (1.7-3.34) |
| Median time from ARS, d (range) | 32 (10-84) | NA | NA |
| Duration of HIV infection | 3 mo | ≥ 7 y | ≥ 10 y |
| ART | Yes | Yes | No |
. | Patients with PHI . | Patients with CHI . | LTNP patients . |
|---|---|---|---|
| No. patients | 41 | 40 | 7 |
| Median age, y (range) | 32 (17-53) | 43 (30-79) | 42 (36-47) |
| Median CD4+ T-cell counts (range) | 645 (204-1412) | 591 (68-850) | 736 (520-908) |
| Median HIV RNA, log10 copies/mL (range) | 4.57 (2.69-5.46) | 2.28 (1.7-4.5) | 2.49 (1.7-3.34) |
| Median time from ARS, d (range) | 32 (10-84) | NA | NA |
| Duration of HIV infection | 3 mo | ≥ 7 y | ≥ 10 y |
| ART | Yes | Yes | No |
Data in the table are expressed as median (range)
PHI indicates primary HIV infection; CHI, chronic HIV infection; LTNP, long-term nonprogressors; ARS, antiretroviral syndrome; ART, antiretroviral therapy; and NA, not available
We have previously described27 the PHI cohort that consisted of seropositive subjects who had to fulfill at least one clinical criterion (signs/symptoms of ARS at visit or during the previous 60 days; HIV-1 exposure in previous 3 months; and negative test in previous 6 months) and 1 laboratory criterion (detectable plasma HIV-1 RNA; detectable HIV-1 p24; gp120, gp160 ± p24 bands at Western blotting; low positive enzyme-linked immunosorbent assay [ELISA] with increasing reactivity over time), according to international guidelines. Twenty-six of the 41 patients with PHI were sampled at diagnosis and 6 months after antiretroviral therapy.
The inclusion criteria for the patients with CHI were certified seroconversion 7 or more years prior to sampling and receiving antiretroviral therapy at the time of enrollment. Nineteen patients from the Swedish CHI cohort undergoing ART were followed up for 2 years with samples collected every 6 months from start of therapy. These patients have also been described elsewhere.28
The 7 LTNP patients included in this study were defined as subjects who maintained normal CD4+ T-cell counts and who had stable viral loads, with no clinical disease progression during more than 10 years of infection, in the absence of any ART.29,30 The LTNP patients were followed up for 7 years. Cell samples were collected twice during the follow-up (at the beginning and at the end of the study), and 4 plasma samples were collected from each subject at 2-year intervals during the follow-up period.
Thirty healthy blood donors of similar age were also enrolled to serve as control subjects.
More than 95% of all subjects included in this study acquired measles through natural infection.10 Plasma viral load and CD4+ T-cell counts were determined in all patients. The ethical committees of Karolinska Institute and San Raffaele Institute approved the study.
Quantification of specific antibodies in plasma
Total plasma IgG was measured by nephelometric analysis. Specific antibody titers in plasma were measured by ELISA.
Antimeasles antibodies. Antimeasles antibodies were measured using the Enzygnost antimeasles virus/IgG ELISA kit (Dade Behring, Marburg, Germany). Microtitration test plate wells were coated with inactivated measles antigen derived from permanent simian kidney cells infected with measles virus, and control wells were coated with control antigen (noninfected cells). The World Health Organization 2nd International Standard for antimeasles serum was used for quantification, and the cut-off for protective levels was set at more than 0.2 IU/mL.10
Antipneumococcus antibodies. Anti-PPS antibody titers were measured using the ELIZEN Pneumococcus IgG immunopotency level ELISA kit (Zentech, Brussels, Belgium). Test plates provided in the kit were coated with polysaccharide capsules of the 23 most seroprevalent pneumococcus strains.
Anti-HIV-1 gp41 antibodies. Determination of anti-HIV-1 gp41 antibody titers was performed by using the Enzygnost HIV ½ Plus ELISA (Dade Behring) following the manufacturer's instructions. Five-fold dilutions (from 1:100 to 1:62 500) of plasma were prepared, and the antibody levels for each sample were calculated at the fixed OD450 value of 0.5 after interpolation.
Cell preparation and flow cytometry
Peripheral blood mononuclear cells (PBMCs) from study subjects were purified from 30 to 40 mL EDTA-treated whole blood by centrifugation over a Ficoll-Hypaque density gradient. Plasma samples were collected and stored at -70°C until analysis. PBMCs were preserved in liquid nitrogen for subsequent analysis.
Flow cytometric analysis on cryopreserved PBMCs was used to analyze memory B cells defined as CD19+CD27+ lymphocytes. The following antibodies were used: anti-CD19 CyChrome, anti-CD27 fluorescein isothiocyanate (FITC), anti-IgD phycoerythrin (PE), anti-IgM allophycocyanin (APC), and isotype control antibodies (Pharmingen, San Diego, CA) as previously described.6 The analysis was performed with a FACScan instrument (Becton Dickinson, Mountain View, CA) using Cellquest software (Becton Dickinson). Forward/side scatter dot plot was used to gate the live lymphocyte population, and at least 25 000 live cells/sample were analyzed.
Activation of B cells in PBMC cultures
PBMCs from patients were stimulated as reported elsewhere.31 Briefly, cryopreserved PBMCs were quickly thawed, washed in sterile phosphate-buffered saline (PBS), and resuspended in X-Vivo serum-free culture medium (Cambrex Bio Science, Walkersville, MD). Cells were cultured in 48-well plates at a concentration of 0.5 × 106 cells/well with the following: 10 μg/mL CpG ODN 2006 (Microsynth, Balgach, Switzerland), 1 μg/mL anti-CD40 mAb (Diaclone, Besancon, France), 100 ng/mL IL-2, and 50 ng/mL IL-10 (Peprotech, London, United Kingdom). Cells were cultured for 6 days at 37°C, 5% CO2. Cell culture supernatants were collected on day 6 and stored at -20°C until needed.
Quantification of measles- and pneumococcus-specific Abs in culture supernatants
Anti-measles IgM and IgG Abs were measured in 6-day culture supernatants as previously described,32 with slight modifications. Briefly, polystyrene microplates (Nunc, Roskilde, Denmark) were coated with 5 μg/mL measles virus purified from a lysate of measles virus-infected Vero cells prepared in 0.05 M sodium carbonate-hydrogen carbonate buffer, pH 9.6, at 4°C overnight. For pneumococcus-specific Abs, a 10-μg/mL solution of a mixture of 4F, 6, 14, 19, 23 pneumococcus polysaccharide Ags in PBS was used to coat the plates. Plates were washed 5 times and blocked using ovalbumin for 1 hour at room temperature. Plates were then washed 3 times with PBS containing 0.05% Tween-20 (Sigma, St Louis, MO); samples diluted 1:2 in PBS-Tween were added to the plates and incubated for 1.5 hours at 30°C. Plates were then washed 3 times with PBS-Tween, and HRP-conjugated rabbit anti-human IgG or IgM (Dako, Copenhagen, Denmark) was added and incubated for 1 hour at room temperature. After a final wash, the plates were incubated with HRP substrate buffer for 1 hour at room temperature and the reaction was stopped using 1 M H2SO4, and the absorbances were read at 450 nm with an automated spectrophotometer (Labsystems Multiscan, Helsinki, Finland).
Enzyme-linked immunospot (ELISPOT) assay for enumeration of IgG- and measles-specific antibody-secreting cells
Five days after the PBMC cultures were started, separate wells of 96-well filtration plates (MSIPS 4510; Millipore, Billerica, MA) were coated overnight at 4°C with 0.5 μg/well affinity-purified goat anti-human IgG (Jackson Laboratories, West Grove, PA), 0.5 μg/well keyhole limpet hemocyanin (KLH; Pierce Biotechnology, Rockford, IL), or 5 μg/well measles grade 2 antigen (Microbix Biosystems, Toronto, ON), with Na2CO3 coating buffer. Optimal antigen concentrations were determined after multiple titration experiments, and samples were run in triplicate for each test antigen. Plates were washed and blocked with 5% FCS/RPMI-1640 (Sigma) for at least 1.5 hours at 37°C, 7% CO2. Stimulated PBMCs were washed and 3 × 105 cells were added to wells of the coated plates and incubated overnight at 37°C, 7% CO2. Plates were again washed 3 times with PBS and 150 μL PBS containing 0.05% Tween-20. For detection, 1 μg/mL biotinylated goat anti-human IgG detection antibody (Tago Immunologicals, Camarillo, CA) was added to the wells and the plate was incubated at 1.5 hours at room temperature (RT). After another wash with PBS-Tween, alkaline phosphatase-conjugated avidin (AAF; Dako) was added to the plates followed by 1.5 hours of incubation at RT. The plates were then washed with distilled water and developed with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) in the dark at RT for 10 minutes. Plates were washed 6 times with distilled water and air-dried overnight. Spots were enumerated with an AID ELISPOT reader using AID software version 3.2.3 (Cell Technology, Columbia, MD).
Data analysis
Statistical analyses were performed using Sigma Stat for Windows software (SPSS, Chicago, IL). Differences between groups were analyzed by parametric (t test, 1-way ANOVA followed by multiple comparisons by Dunn method) or nonparametric (Mann-Whitney or Wilcoxon test, one-way ANOVA on ranks) tests as appropriate. Correlation between variables was tested using linear regression and Spearman rank tests. Data in the text are expressed as median (25th-75th percentile) or mean ± SEM.
Results
Frequency of memory B cells as a marker of disease progression
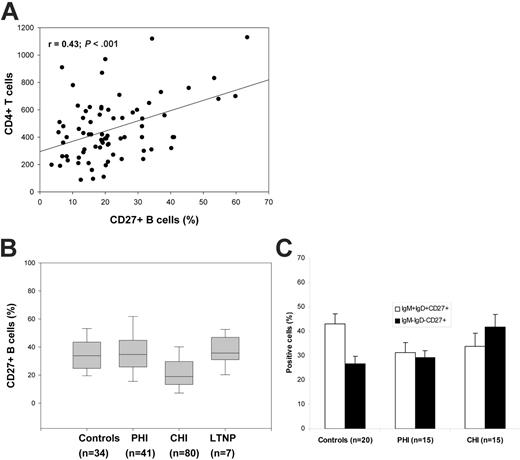
We have previously shown a dramatic reduction of memory (CD19+CD27+) B-cell percentage and numbers in individuals with CHI.6 In order to study the correlation between loss of memory B cells and CD4+ T-cell counts, we pooled the data on memory B-cell percentages in all CHI subjects (n = 80), including those CHI subjects we have studied in our previously published work.6,10,11 We found a strong correlation (r = 0.43, P < .001) between memory B cells and CD4+ T-cell counts (Figure 1A), suggesting that memory B cells may be a marker of disease progression. We did not find such a correlation in the healthy controls (r = -0.06; P = .8).
In a recent study, we showed that memory B cells in PHI subjects, though phenotypically altered and functionally compromised, were similar to controls in percentage.12 We then compared the memory B-cell percentages among the groups and found that the CHI subjects had the lowest median percentage of memory B cells (19% [13.3%-29.05%]) compared with controls (33.8% [25%-43.1%]; P < .001), while the PHI and LTNP groups were comparable with the controls (38.9% [26.9%-44.9%] and 35.7% [28.7%-40.6%], respectively) (Figure 1B).
We previously found that the percentage of IgM memory (IgM+IgD+CD27+) B cells was decreased in 5 patients with PHI.12 In the present study, we analyzed 15 patients with PHI and 15 patients with CHI and found that the percentage of IgM+IgD+CD27+ B cells in PHI (26.6% ± 4.3%) and CHI (24.6% ± 5.6%) patients was lower than in controls (32.8% ± 4.1%), though these differences were not statistically significant. Conversely, the percentage of IgD-IgM-CD27+ B cells in patients with CHI (42% ± 5.3%) was significantly higher than that in patients with PHI (29.2% ± 2.9%) and controls (26.8% ± 2.9%; P = .02) (Figure 1C).
Hypergammaglobulinemia is present throughout the course of HIV-1 infection
Hypergammaglobulinemia is a major feature of B-cell dysfunction in HIV-1 infection,10,33 mainly described in the context of CHI. We recently found that the levels of IgG are already elevated during PHI.12 As previously reported,34 the LTNP group also presented with hypergammaglobulinemia. Plasma IgG levels in all HIV groups were comparable (PHI, 13.2 g/L [11.4-15.1 g/L]; CHI, 14.2 g/L [12.8-18.2 g/L]; LTNP, 16.2 g/L [12.7-24.4 g/L]) and all significantly higher compared with controls (9.6 g/L [7.9-11.9 g/L]; P < .001).
Memory (CD27+) B cells and progression of HIV infection. (A) Relationship between memory B-cell percentages and CD4+ T-cell counts. The relationship between memory B cells and CD4+ T cells in 80 subjects with CHI was tested by linear regression. (B) Comparison of memory B-cell percentages at different stages of HIV infection. PBMCs from HIV-negative controls and individuals with PHI or CHI, and LTNP patients were analyzed for percentage of cells staining double positive for CD19 and CD27 (CD19+CD27+) using fluorescence-activated cell sorter (FACS) analysis. The box plot for LTNP patients represents data pooled from 7 subjects, each sampled twice, with a 7-year interval between samples. (C) IgM+IgD+CD27+ and IgM-IgD-CD27+ B cells in controls, patients with PHI, and patients with CHI. Expression of IgM and IgD was analyzed in CD19+CD27+ (memory B cells) from HIV-negative controls and individuals with PHI or CHI.
Memory (CD27+) B cells and progression of HIV infection. (A) Relationship between memory B-cell percentages and CD4+ T-cell counts. The relationship between memory B cells and CD4+ T cells in 80 subjects with CHI was tested by linear regression. (B) Comparison of memory B-cell percentages at different stages of HIV infection. PBMCs from HIV-negative controls and individuals with PHI or CHI, and LTNP patients were analyzed for percentage of cells staining double positive for CD19 and CD27 (CD19+CD27+) using fluorescence-activated cell sorter (FACS) analysis. The box plot for LTNP patients represents data pooled from 7 subjects, each sampled twice, with a 7-year interval between samples. (C) IgM+IgD+CD27+ and IgM-IgD-CD27+ B cells in controls, patients with PHI, and patients with CHI. Expression of IgM and IgD was analyzed in CD19+CD27+ (memory B cells) from HIV-negative controls and individuals with PHI or CHI.
Hypergammaglobulinemia in LTNP patients remained stable during a 7-year follow-up period (not shown).
Serologic memory is impaired in patients with PHI and those with CHI but preserved in LTNP patients
Antimeasles Ab titers were decreased in both PHI (5.3 IU/mL; [3.5-9.4 IU/mL]) and CHI (4.9 IU/mL; [1.4-9.4 IU/mL]) patients compared with the control group (8.9 IU/mL [3.3-12.7 IU/mL]). This difference was not statistically significant for the patients with PHI (P = .16) but reached statistical significance in the CHI group (P = .01). Plasma samples were collected from each LTNP patient 4 times over the course of a 7-year follow-up period, and titers of antimeasles Ab in LTNP patients were comparable with controls (7.8 IU/mL; range, 4.9-8.7 IU/mL; P = .68) (Figure 2A).
Anti-PPS Ab titers were dramatically decreased both in PHI (582 mU/mL [412-779 mU/mL]) and CHI (505 mU/mL [351-833 mU/mL]) patients compared with controls (1743 mU/mL [715-2508 mU/mL]; P < .001). Titers of anti-PPS in LTNP patients (1236 mU/mL [676-1554 mU/mL]) were comparable with controls (P = .24) and significantly higher than CHI levels (P = .02) (Figure 2B).
Production of measles-and pneumococcus-specific Abs in cell culture supernatants
Since protection against pathogens is important at the time of infection, we determined whether the production of antigen-specific IgM and IgG antibodies was impaired in B cells from HIV-infected patients. Following 6 days of in vitro polyclonal stimulation, patients with CHI produced significantly lower amounts of IgM and IgG Abs to both measles (P < .001 for both) and pneumococcus (P = .002 for IgM and P < .001 for IgG) compared with controls. Production of Ab in LTNP patients was comparable with that in controls (Figure 3).
Comparison of antimeasles and antipneumococcus antibody titers in uninfected and HIV-infected subjects. Antimeasles (A) and antipneumococcus (B) antibody titers were determined in plasma samples from 30 HIV-negative (controls), 26 PHI (16 in B), 40 CHI, and 7 LTNP subjects. The box plots for LTNP patients represents data from 7 subjects, each sampled 4 times, with an average of 2 years between samples. The ends of the boxes define the 25th and 75th percentiles, with a line at the median and error bars defining the 10th and 90th percentiles.
Comparison of antimeasles and antipneumococcus antibody titers in uninfected and HIV-infected subjects. Antimeasles (A) and antipneumococcus (B) antibody titers were determined in plasma samples from 30 HIV-negative (controls), 26 PHI (16 in B), 40 CHI, and 7 LTNP subjects. The box plots for LTNP patients represents data from 7 subjects, each sampled 4 times, with an average of 2 years between samples. The ends of the boxes define the 25th and 75th percentiles, with a line at the median and error bars defining the 10th and 90th percentiles.
Measles-specific antibody-secreting cells are reduced in HIV infection
In order to determine whether the reduction in antigen-specific antibodies was a consequence of reduction in antigen-specific memory B cells, we randomly selected (based on sample availability) 8 control samples, 9 PHI samples, and 20 CHI samples for enumeration of total IgG and measles-specific antibody-secreting cells (ASCs) by ELISPOT assay. We first expressed the ELISPOT assay results as number of spots/106 stimulated PBMCs. The control samples responded best to stimulation with the mitogen cocktail (described in detail in “Materials and methods”) as evidenced by blast formation in culture and the development of IgG spots (5243/106 [4407-6605/106] stimulated PBMCs) and measles spots (67/106 [42-70/106] stimulated PBMCs). patients with PHI had significantly lower numbers of both IgG spots (1716/106 [1076-2640/106] stimulated PBMCs; Figure 4A) and measles spots (4/106 [4-35/106] stimulated PBMCs; Figure 4B) compared with controls (P < .001) and patients with CHI (P = .02). patients with CHI also had significantly lower numbers of both IgG spots (3520/106 [2198-5435/106] stimulated PBMCs; P = .02) (Figure 4A) and measles spots (14/106 [4-35/106] stimulated PBMCs; P = .02; Figure 4B) compared with controls. The number of spots does not directly reflect the number of antigen-specific memory B cells/ASCs, due to in vitro proliferation induced during the PBMC stimulation step.31 We therefore analyzed the percentage of Ag-specific memory B cells by relating the measles spots to IgG spots. The median percentage of measles-specific memory B cells in the PHI (0.2% [0.08%-0.8%]) was comparable with that in CHI (0.5%; [0.12%-1.21%]; P = .52) patients, but levels in both patient groups were lower than in the controls (1.01% [0.73%-1.42%]). This difference achieved statistical significance only in the PHI group (P = .04). The ELISPOT assay data thus consistently reflected the data on the plasma levels of antimeasles antibodies.
Correlation between memory B cells, measles antibody titers, and measles-specific ASCs
We found a strong correlation between antimeasles antibody titers in patients with CHI and the number of antimeasles ASCs (Figure 5A), a moderately strong correlation between antimeasles Ab titers and the percentage of measles-specific memory B cells (Figure 5B), and a strong correlation between the number of antimeasles ASCs/106 stimulated PBMCs and total memory B cells in patients with CHI (Figure 5C). We did not find similar correlations in the PHI group, but this may be due to the smaller number of patients in the PHI group (n = 9), as well as to the fact that loss of total memory B cells is evident in CHI but not in patients with PHI.12
Antiretroviral therapy does not restore antigen-specific antibodies in PHI and patients with CHI
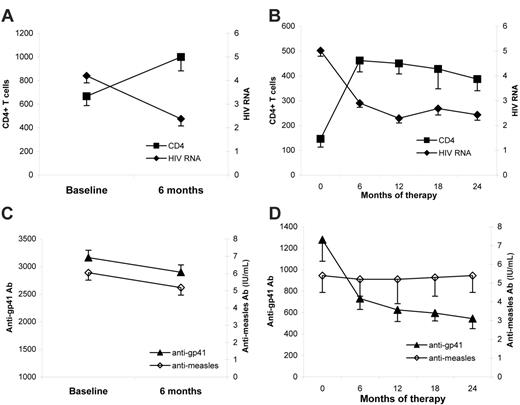
The effect of ART on serologic memory was evaluated in patients with PHI treated for 6 months and in patients with CHI treated for 24 months. In both PHI and patients with CHI, CD4+ T-cell counts increased and viral load significantly decreased after 6 and 24 months of therapy, respectively (P < .001) (Figure 6A-B).
Production of specific Abs in culture. PBMCs from healthy controls, patients with CHI, and LTNP patients were stimulated with CpG ODN + IL-2 + IL10 + CD40 mAb. Cell culture supernatants were collected after 6 days of culture and used to measure anti-pneumococcus IgG and IgM, and anti-measles IgG and IgM Abs.
Production of specific Abs in culture. PBMCs from healthy controls, patients with CHI, and LTNP patients were stimulated with CpG ODN + IL-2 + IL10 + CD40 mAb. Cell culture supernatants were collected after 6 days of culture and used to measure anti-pneumococcus IgG and IgM, and anti-measles IgG and IgM Abs.
Following 6 months of antiretroviral therapy in patients with PHI, antimeasles titers remained low compared with controls. In fact, the levels of antimeasles Ab tended to further decrease after ART in patients with PHI (from 6.0 ± 1 to 5.1 ± 1.1 IU/mL; P = .08) (Figure 6C). Similarly, plasma IgG levels after 6 months of therapy were slightly reduced but remained elevated compared with controls (data not shown).
In the patients with CHI followed up for 24 months, ART had no effect on antimeasles titers, which remained significantly lower than control titers throughout the follow-up period (Figure 6D). These patients experienced a significant decrease in plasma IgG titers (from 20.3 ± 2.5 to 13.6 ± 1.2 g/L; P < .01), although levels did not return to normal.
Since antibody titers to HIV-1 glycoproteins are reduced in patients undergoing ART,35-37 we also analyzed the titers of antibodies to gp41 in PHI and patients with CHI during ART. The levels of anti-gp41 (Figure 6C-D) antibodies gradually and significantly decreased during follow-up in both groups. The changes in anti-gp41 antibodies followed the changes in viral load.
Total IgG-secreting and anti-measles IgG-secreting cells in HIV infection. PBMCs from 8 HIV-negative controls, 9 PHI subjects, and 20 CHI subjects were stimulated for 6 days with a combination of CpG, anti-CD40 mAb, IL-2, and IL-10. Stimulated PBMCs were then added to ELISPOT plate wells precoated with goat anti-human IgG or measles antigen. Results are expressed as number of total IgG-secreting (A) or anti-measles IgG-secreting (B) cells per 106 stimulated PBMCs. (C) Percentages of measles-specific ASCs were determined by dividing the number of measles IgG-secreting cells by the number of total IgG-secreting cells in controls, patients with PHI, and patients with CHI. The ends of the boxes define the 25th and 75th percentiles, with a line at the median and error bars defining the 10th and 90th percentiles.
Total IgG-secreting and anti-measles IgG-secreting cells in HIV infection. PBMCs from 8 HIV-negative controls, 9 PHI subjects, and 20 CHI subjects were stimulated for 6 days with a combination of CpG, anti-CD40 mAb, IL-2, and IL-10. Stimulated PBMCs were then added to ELISPOT plate wells precoated with goat anti-human IgG or measles antigen. Results are expressed as number of total IgG-secreting (A) or anti-measles IgG-secreting (B) cells per 106 stimulated PBMCs. (C) Percentages of measles-specific ASCs were determined by dividing the number of measles IgG-secreting cells by the number of total IgG-secreting cells in controls, patients with PHI, and patients with CHI. The ends of the boxes define the 25th and 75th percentiles, with a line at the median and error bars defining the 10th and 90th percentiles.
Discussion
HIV patients have impaired ability to mount and/or maintain a serologic response to immunizing antigens, and this is often related to the immune system status and/or disease progression.15,38-44 A severe dysfunction of long-term serologic memory has been suggested by vaccination studies15,45 and by studies of the memory B-cell compartment of patients with HIV-1 infection.6,8,10 Moreover, in vivo studies with simian immunodeficiency virus (SIV) showed that cytomegalovirus (CMV) reactivation-associated disease in AIDS is also associated with impairment of CMV-specific CD4+ T-cell help and suppression of CMV-specific antibodies, suggesting a functional defect in CMV-specific memory B cells.46 These observations indicate the importance of a functional memory B-cell response for protection against opportunistic infections in HIV patients. We investigated the status of serologic memory to viral (measles) and bacterial (S pneumoniae) infections of potential clinical relevance in HIV-1-infected persons. Patients with primary, chronic, and long-term nonprogressive infection were studied in order to evaluate the influence of disease progression and the effect of antiretroviral treatment on serologic memory maintenance.
Relationship between antimeasles ASCs, antimeasles antibody titers, and memory B-cell percentages in chronic HIV-1 infection. The strengths of association between (A) antimeasles antibody titers and anti-measles IgG ASCs per 106 stimulated PBMCs, (B) antimeasles antibody titers and percentages of measles-specific memory B cells, and (C) memory B cells and anti-measles IgG ASCs were tested using the Spearman rank order correlation test. Figures represent scatter plots of the results of the correlation test, with corresponding regression lines. Percentages of measles-specific memory B cells in panel B were determined by dividing the number of anti-measles IgG ASCs by the number of total IgG ASCs.
Relationship between antimeasles ASCs, antimeasles antibody titers, and memory B-cell percentages in chronic HIV-1 infection. The strengths of association between (A) antimeasles antibody titers and anti-measles IgG ASCs per 106 stimulated PBMCs, (B) antimeasles antibody titers and percentages of measles-specific memory B cells, and (C) memory B cells and anti-measles IgG ASCs were tested using the Spearman rank order correlation test. Figures represent scatter plots of the results of the correlation test, with corresponding regression lines. Percentages of measles-specific memory B cells in panel B were determined by dividing the number of anti-measles IgG ASCs by the number of total IgG ASCs.
We found that memory B-cell loss is a progressive event occurring in the course of HIV-1 infection, not readily apparent at the early stages of PHI but very significant in CHI. This is indicated by 2 observations: (1) the percentage of memory B cells is low and correlated to the CD4+ T-cell count in a large cohort of patients with CHI and (2) the percentage of memory B cells is still preserved in both PHI and LTNP patients. Thus, the percentage of circulating memory B cells represents a novel marker of HIV-1 disease progression.
The finding of relatively normal levels of circulating memory B cells in both PHI and LTNP patients, likely accompanied by a relatively preserved helper T-cell function, might explain previous studies showing that a good antibody response to vaccination occurs in patients with PHI and in patients with high CD4+ T-cell counts.15,43,44,47 Accordingly, the LTNP patients followed up for 7 years showed not only a normal frequency of memory B cells but also normal levels of antibodies to measles and PPS, suggesting that memory B cells are functionally preserved in LTNP patients. Our results clearly show that a fall in specific antibodies is already detectable during PHI, well in line with our previous observation that peripheral memory B cells during PHI are functionally altered.12 Equally interesting is the dramatic reduction in antibodies to S pneumoniae, which is generally considered to be a T-cell-independent antigen. This indicates that an intrinsic defect may be occurring in the B-cell compartment and may lead to a decline of serologic immune response early during HIV-1 infection.
Effect of ART on CD4+ T-cell counts, HIV RNA, and specific antibody titers in primary and chronic HIV infection. Twenty-six individuals with PHI were sampled at baseline and after 6 months of ART, while 19 with CHI were sampled at baseline and 4 more times over a 24-month period of ART. (A) Changes in CD4+ T-cell counts and HIV RNA in subjects with PHI at baseline and after 6 months of therapy. (B) Changes in CD4+ T-cell counts and HIV RNA in subjects with CHI. (C) Antimeasles and anti-gp41 antibody titers in subjects with PHI at baseline and after 6 months of therapy. (D) Antimeasles and anti-gp41 antibody titers in subjects with CHI.
Effect of ART on CD4+ T-cell counts, HIV RNA, and specific antibody titers in primary and chronic HIV infection. Twenty-six individuals with PHI were sampled at baseline and after 6 months of ART, while 19 with CHI were sampled at baseline and 4 more times over a 24-month period of ART. (A) Changes in CD4+ T-cell counts and HIV RNA in subjects with PHI at baseline and after 6 months of therapy. (B) Changes in CD4+ T-cell counts and HIV RNA in subjects with CHI. (C) Antimeasles and anti-gp41 antibody titers in subjects with PHI at baseline and after 6 months of therapy. (D) Antimeasles and anti-gp41 antibody titers in subjects with CHI.
The number of measles-specific ASCs was significantly reduced in patients with CHI and strongly correlated to both the memory B-cell percentages and the plasma antimeasles Ab titers. The correlation between total circulating memory B cells, measles-specific ASCs, and measles antibodies in patients with CHI suggested that the reduced numbers of circulating memory B cells reflects an actual functional defect in serologic memory. Of interest, the percentage of measles-specific memory B cells was significantly reduced in PHI despite the fact that the total number of circulating memory B cells was comparable with normal.12 This may be explained by the fact that even though no massive loss of memory B cells is observed in PHI, the comparatively few memory B cells that are lost are likely those specific for non-HIV-1 antigens such as measles, since HIV-1-specific memory B cells are still in the process of being formed. Also, memory B cells may be redistributed to other lymphoid compartments, making it more difficult to detect them in peripheral blood. Given the high amounts of circulating HIV-1 Ags, generation of anti-HIV memory B cells may also overshadow that of other noncirculating Ags. Although the occurrence of measles reinfection has not been reported in HIV-1-infected adults, an increased rate of measles infections and deaths occur in infants born to HIV-1-infected mothers living in geographic areas with poor access to measles vaccination.16,17,19,23 Moreover, although not exhaustively addressed here, it is conceivable that memory B cells specific to other relevant antigens are reduced in HIV-1 infection. This would, for instance, include pathogens such as S pneumoniae, which has a well-characterized clinical relevance for HIV-1 patients. The strong association between loss of serologic memory and disease progression also raises the question whether such B-cell deficiency may be corrected by therapy. After 6 and 24 months of ART in PHI and patients with CHI, respectively, plasma levels of measles antibodies were still significantly lower than control levels. Of interest, these patients experienced a reduction of both hypergammaglobulinemia and HIV-1-specific antibodies. ART administered during PHI significantly reduces plasma viremia and restores the number of CD4+ T cells.12 However, ART did not improve serologic memory irrespective of when therapy was initiated (early in PHI or after established infection), suggesting that primary infection might be crucial for the elimination of antigen-specific pools of memory B cells. The mechanisms leading to the establishment and/or maintenance of HIV-specific memory B cells during HIV-1 infection may be defective and/or differentially regulated, as demonstrated by the disappearance of HIV-1-specific antibodies in patients on ART.35-37
A possible scenario for impairment of serologic memory in HIV-1 infection might be the following: massive HIV-1 replication during the early phases of infection is likely responsible for depletion of antigen-specific memory B cells (with specificity to eg, measles, S pneumoniae, tetanus toxoid), through apoptosis and altered expression of TNF receptors,48 lack of survival factors, and poor cross-talk with other cells.5 In the subsequent phase of chronic infection, naive B cells would still be available to differentiate into memory cells, with help from CD4+ T cells, though constant HIV-1 replication drives CD4+ T cells to undergo apoptosis.49 Progressive destruction of helper CD4+ T cells in the chronic phase of infection may eventually impair differentiation of naive B cells into Ag-specific memory B cells (ie, an indirect consequence of CD4+ T-cell depletion or “collateral damage”). Our results also indicate that long-term responses to different Ags may have differential requirement for the presence of circulating Ag: for example, production of Abs to measles (noncirculating Ag) may require intermittent exposure of memory B cells to measles Ag. The Ab response to HIV-1-specific Ags seems to require the presence of Ags since suppression of viral replication by ART leads to suppressed humoral immune response.35,37
In conclusion, we have reported that HIV-1-infected patients experience impairment in maintenance of long-term serologic memory. Such an important immune dysregulation cannot be overlooked in the timing and design of therapy and immunization strategies.
Prepublished online as Blood First Edition Paper, April 27, 2006; DOI 10.1182/blood-2005-11-013383.
Supported by grants from the Swedish Medical Research Council, the Swedish International Development Agency (SIDA-SAREC), the Hedlund Foundation, the Swedish Physicians against AIDS Research Foundation, and the Swedish Association for Medical Research (SSMF).
K.T. and A.D.M. designed and performed research, analyzed data, and wrote the paper; A.C. and A.N. performed research; R.T., S.G., A.A., B.H., F.P.K., and L.L. contributed analytical tools; and F.C. designed research and wrote the paper.
K.T. and A.D.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Therese Wallerskog for helpful assistance with the ELISPOT.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal