Abstract
ULBPs are human ligands for NKG2D, an activating receptor expressed on natural killer (NK) cells, NK1.1+ T cells, and T cells. ULBPs are expressed by a variety of leukemias, carcinomas, melanomas, and tumor cell lines. ULBP expression correlates with improved survival in cancer patients, however, the nature of the immune response that ULBPs elicit is not well understood. We report that ectopic expression of ULBP1 or ULBP2 on murine EL4 or RMA tumor cells elicits potent antitumor responses in syngeneic C57BL/6 and SCID mice. Although binding of ULBP3 to murine NKG2D could not be demonstrated in vitro, ULBP3 can also stimulate antitumor responses, suggesting that ULBP3 binds to murine NKG2D or possibly another receptor in vivo. ULBP expression was found to recruit NK cells, NK1.1+ T cells, and T cells to the tumor. IL-15 was found to strongly enhance the immune response directed against ULBP-expressing tumors. Tumors can evade NKG2D immunity by down-regulating expression of NKG2D. Our data suggest that IL-15 may be useful for overcoming this tumor-evasion strategy. Together, these results demonstrate that ULBP expression can elicit a potent immune response and suggest that ULBPs, alone or in combination with IL-15, can be exploited for antitumor therapy.
Introduction
The ULBPs are a family of human cell-surface molecules distantly related to classic MHC class I molecules. ULBP1, ULBP2, and ULBP3 are GPI-linked proteins with 55% to 60% sequence identity,1 whereas the more divergent ULBP4/Letal is a transmembrane protein.2,3 The ULBPs are ligands for NKG2D, an activation receptor that is a key regulator of both innate and adaptive immune responses.4 In the mouse, NKG2D transmits its signal by associated adaptor proteins, DAP10 and DAP12, whose cytoplasmic domains contain binding sites for PI3-kinase, and Syk and Zap70 kinases, respectively.5 In humans, only DAP10 is associated with NKG2D.6 NKG2D is expressed on natural killer (NK) cells, CD8+ αβ T cells, γδ T cells, some NK-T cells, and a rare population of CD4+/CD28– T cells.7,8 NKG2D can deliver an activation signal to NK cells and, under some conditions, can act as a costimulatory receptor for TCR-mediated activation of T cells, in a similar manner as CD28.9-11
NKG2D is unusual in that it binds to several, highly divergent families of nonclassic MHC class I–like molecules. In addition to ULBPs, in humans MICA and MICB have been described, and in mice Rae-1α, Rae-1β, Rae-1γ, Rae-1δ, Rae-1ϵ, H60, and MULT-1 proteins are recognized by NKG2D.5 NKG2D ligands have very low sequence homology, however, X-ray crystallographic and binding studies have identified several shared key residues that are responsible for binding to NKG2D.12
Under normal circumstances in the adult, NKG2D ligand expression is generally low or absent but is induced under conditions of transformation. MICs are frequently expressed by epithelial tumors, whereas H60 and RAE-1 are expressed on many tumor cell lines and are induced by carcinogen treatment of murine skin.13-15 Natural or engineered expression of NKG2D ligands on tumor cells greatly enhances sensitivity to killing by NK cells in vitro, even when the tumor cells express normal levels of MHC class I molecules.1,7,16 These in vitro findings have been extended by mouse models showing that ectopic expression of RAE-1 or H60 results in rejection of MHC class I–bearing tumors by NK and/or CD8+ T cells.17-19 Studies in mice also suggest a role for NKG2D in protecting the host from spontaneous malignancy.20 Together, these studies support a general model in which NKG2D ligand expression serves as a way to mark transformed cells for destruction by immune effector cells.4 In addition to being overexpressed in certain tumors, dysregulated expression of the MICs or RAE-1 has been associated with autoimmune disease8,21 and with Celiac disease.22
Compared with the MICs and murine NKG2D ligands, much less is known about the ULBPs and their possible roles in vivo. Given that they are less than 20% homologous in sequence to other NKG2D ligands, it is possible that they may have unique properties or functions in vivo. ULBP message is detectable in many normal and transformed tissues1 ; however, protein expression has been difficult to detect, suggesting that ULBP expression may be tightly regulated.1 ULBPs are expressed by a variety of tumors including neuroblastomas,23 malignant gliomas,24 leukemias,25 and ovarian carcinomas,26 and correlations have been made between ULBP expression and a better prognosis.25,26 These findings suggest that ULBPs may have a role in mediating antitumor immune responses in vivo.
In this study, we asked whether ectopic expression of ULBP1, ULBP2, or ULBP3 can induce an antitumor immune response in vivo and determined what types of cells are recruited to ULBP-expressing tumors. One way in which tumors can evade immune responses is by shedding NKG2D ligands, which in turn down-regulate NKG2D expression and function on NK and T cells.27-29 IL-15 can up-regulate NKG2D on NK and T cells,30,31 even in the presence of shed NKG2D ligands,8 and could possibly be used to combat these tumor-evasion strategies. With this in mind, we determined if IL-15 could enhance the ULBP-mediated antitumor response in vivo. Our findings demonstrate that ULBP expression can induce a potent immune response in vivo and suggest the potential use of ULBPs and IL-15 in the development of antitumor vaccines.32,33
Materials and methods
Cell lines and antibodies
EL4, a murine thymoma, was obtained from ATCC (TIB-39; Manassas, VA). RMA, a murine T lymphoma, was provided by Dr Lewis Lanier (University of California, San Francisco, CA). These NKG2D ligand–negative cell lines were grown in RPMI-1640 containing 5% FBS, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 50 μM 2-mercaptoethanol. CV-1/EBNA cells were grown as described.34 CTLL-2.4 murine T lymphocyte cells were cultured in IMDM containing 5% FBS, 2 mM l-glutamine, MEM essential amino acids and MEM vitamin solution (both from Invitrogen, Carlsbad, CA), 50 μM 2-mercaptoethanol, and 2 ng/mL hIL-2. M315 Rat IgG1 anti–murine NKG2D monoclonal antibody and M139 anti-KLH isotype control are from Amgen (Seattle, WA).
Ectopic expression of ULBPs
Amphotropic retroviruses expressing ULBPs or RAE-1β were generated as described.2 The retroviral vector did not contain any selection marker. After retroviral transduction, EL4 and RMA cells were sorted for NKG2D ligand expression and single-cell cloned. Clones expressing ULBPs were identified by staining with the following murine IgG1 monoclonal antibodies: M295 anti-ULBP1; M310 and M312 anti-ULBP2; and M550 anti-ULBP3.1 M230 anti-UL16 was used as an isotype control.35 Specific binding was detected with PE-conjugated F(ab′)2 goat anti–mouse IgG (Jackson Immunoresearch, West Grove, PA). RAE-1β–expressing clones were identified by staining with murine NKG2D-Fc or control Fc fusion protein, p7.5-Fc,36 followed by PE-conjugated F(ab′)2 goat anti–human IgG, Fc specific (Jackson Immunoresearch). Negative control mock EL4 cells were generated by infecting EL4 with retrovirus carrying empty LZRS vector. Mock clones were identified by polymerase chain reaction (PCR) with primers corresponding to 5′ and 3′ UTRs in LZRSpBMN-Z (forward primer: 5′-CGAGTCGGCGACACAGTGTGG-3′; reverse primer: 5′-CCATCTGTTCTTGGCCCTGAGC-3′). For each NKG2D ligand and for mock, 20 identified clones were pooled and used for experimentation.
In vitro binding and cytotoxicity assays
To determine if soluble forms of ULBPs bind to cells transfected with murine NKG2D, plasmids encoding FLAG-DAP101 and murine NKG2D or human NKG2D control were transfected into CV-1/EBNA cells. The construction of the plasmids containing human or murine NKG2D are available upon request. Cells (5 × 105) were stained with ULBP-Fc or p7.5-Fc control, as described.1 To determine if a soluble form of murine NKG2D binds ULBPs, 1 μg murine NKG2D-Fc or human NKG2D-Fc control35 was incubated with 5 × 105 ULBP-expressing EL4 cells. Binding of Fc proteins was detected with PE-conjugated F(ab′)2 goat anti–mouse IgG, Fc specific (Jackson Immunoresearch).
Generation of tumor cells expressing IL-15
cDNA consisting of the human IL-2 signal sequence, followed by the murine IL-15 sequence was adapted for Gateway cloning technology from Invitrogen by PCR-amplification using pDC409/huIL-2/muIL-15 vector (Amgen) as template (forward primer: 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGGGCCGCCAC CATGGCCCTG-3′ [bold denotes start codon, IL-2 sequence is underlined]; reverse primer: 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTCAGGACGTGTTGATG-3′ [bold denotes stop codon, IL-15 sequence is underlined]). The amplified sequence was cloned into a Gateway-adapted LZRSpBMN-Z vector. Amphotropic retroviruses were generated as described2 and used to infect mock EL4 and ULBP1-expressing EL4 cells.
Proliferation assays
The CTLL-2 mouse cell line, known to proliferate in response to IL-15,37 was used to determine IL-15 activity. Activity was assessed by adding Alamar Blue (BioSource International, Camarillo, CA) for the last 6 hours of a 24-hour period of incubation with cell supernatants and reading fluorescence at 560 nm. Serial dilutions of murine recombinant IL-15 (R&D Systems, Minneapolis, MN) ranging from 1 ng/mL to 15.6 pg/mL and containing a specific activity of 1.8 × 105 units/μg were used as a standard. Supernatants were obtained by culturing 1 × 105 transfected EL4 cells/mL for 48 hours. The concentration of IL-15 in the supernatants was calculated by dividing the activity/mL by the specific activity.37 The average IL-15 concentration was 16.0 ± 3.7 pg/mL for EL4 mock, 764.0 ± 34.0 pg/mL for EL4 mock/IL-15+, 19.0 ± 1.0 pg/mL for ULBP1+ EL4, and 440.0 ± 8.1 pg/mL for ULBP1+ EL4/IL-15+. Data represent the average ± standard deviation for triplicate wells from a typical experiment from 4 separate experiments.
Mice and tumor inoculation
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Female C57BL/6 SCID mice were purchased from Jackson Labs. All mice were between 8 and 12 weeks of age. EL4 or RMA tumor cells (3 × 105) were injected subcutaneously in PBS into the right flank. Six to 10 mice per group were used in each experiment. Tumor size was measured with calipers and represents the product of 2 perpendicular diameters. When tumor burden became too large, generally 150 mm2 or larger, mice were humanely killed. Tumor size is expressed as the average for surviving mice within each treatment group.
Characterization of tumor-infiltrating leukocytes
Mock, ULBP1-, or ULBP3-expressing EL4 cells (1 × 107) were injected subcutaneously into the right flank of C57BL/6 mice. Tumors were excised 2 or 4 days after injection and dispersed by fine mincing followed by digestion with Liberase enzyme blend (Roche, Nutley, NJ). Cells were blocked with PBS containing 2% FBS and 10 μg/mL 2.4G2 rat anti–murine Fc receptor and then stained with anti–NK1.1-FITC and anti–TCRβ-PE (Pharmingen, San Diego, CA).
Results
Generation of ULBP-expressing tumor cell lines
Mouse cell lines were transduced with retroviruses that carry only the ULBP gene and no selection marker. We chose EL4 and RMA cells, as these lines do not normally express NKG2D ligands.33 EL4 and RMA cells expressing high levels of ULBP1, ULBP2, ULBP3, or RAE-1β were generated by multiple rounds of cell sorting.
Binding of ULBP1 and ULBP2 to murine NKG2D
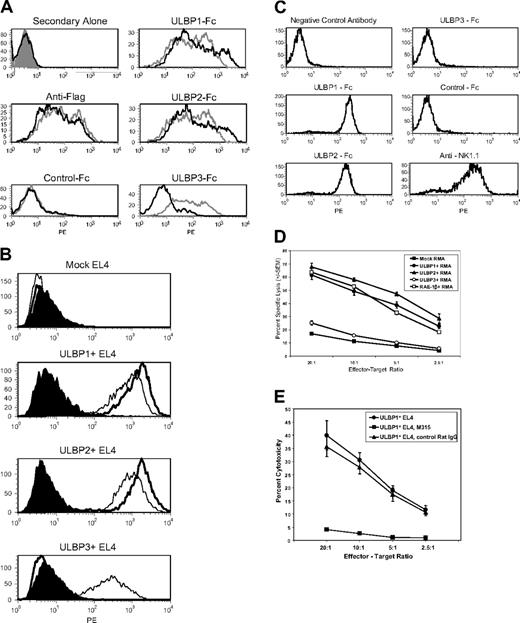
To compare the ability of ULBP1, ULBP2, and ULBP3 to bind to murine NKG2D, fusion proteins in which the extracellular regions of the ULBPs were fused to the Fc portion of human IgG1 were used. As expected, all 3 ULBP-Fc proteins bound well to CV-1/EBNA cells transfected with human NKG2D/DAP10 (Figure 1A). In contrast, only ULBP1 and ULBP2 fusions bound to CV-1/EBNA cells transfected with murine NKG2D/DAP10, whereas the ULBP3 fusion showed no detectable binding above the negative control. To determine if this result might be an artifact of expressing the ULBPs in soluble form, binding of murine NKG2D-Fc to EL4 cells transfected with full-length ULBPs was tested. Murine NKG2D-Fc was able to bind to ULBP1- or ULBP2-expressing EL4 cells but not to ULBP3-expressing cells (Figure 1B). We found that Fc versions of ULBP1 and ULBP2, but not ULBP3, bind to primary murine NK cells (Figure 1C) and that this binding was completely blocked by an anti-NKG2D monoclonal antibody (N.J.C., unpublished data, August 2, 2004). Together, these results demonstrate that binding of ULBP1 and ULBP2, but not ULBP3, to murine NKG2D can be detected in vitro.
ULBP1 and ULBP2, but not ULBP3, bind to murine NKG2D and elicit killing by murine NK cells in vitro. (A) Soluble forms of ULBP1 and ULBP2, but not ULBP3, bind to cells transfected with murine NKG2D/DAP10. CV-1 cells were cotransfected with cDNAs encoding human (gray histograms) or murine (black histograms) NKG2D and flag-tagged human DAP10. Cells were stained with anti-FLAG followed by anti–murine IgG-PE, anti–murine IgG-PE alone, or with ULBP1-Fc, ULBP2-Fc, ULBP3-Fc, or p7.5-Fc negative control protein followed by anti–human IgG-PE, and analyzed by flow cytometry. (B) Murine NKG2D-Fc binds to cells transfected with ULBP1 or ULBP2, but not ULBP3. EL4 cells were transduced with cDNAs encoding ULBP1, ULBP2, or ULBP3. Cells were incubated with murine NKG2D-Fc (thick line), human NKG2D-Fc (thin line), or negative control p7.5-Fc (filled histogram) followed by anti–human IgG-PE and analyzed by flow cytometry. (C) Soluble forms of ULBP1 and ULBP2, but not ULBP3, bind to murine NK cells. Splenocytes from C57BL/6 SCID mice were cultured in rhuIL-15 to enrich NK cells. Cells were incubated with the indicated Fc proteins, followed by anti–human IgG-PE and analyzed by flow cytometry. More than 90% of the cells in the live gate were positive for NK1.1, an NK-cell marker, but did not stain with isotype-matched negative control antibody. (D) Enhanced in vitro cytotoxicity of murine NK cells against RMA cells transduced with ULBP1 or ULBP2, but not ULBP3. RMA cells expressing vector alone (▪), ULBP1 (•), ULBP2 (▴), ULBP3 (○), or RAE-1β (□) as a positive control were tested as targets in cytotoxicity assays using murine NK cells as effectors. (E) In vitro cytotoxicity of murine NK cells against EL4 cells transduced with ULBP1 (•) is blocked by 20 μg/mL M315 rat IgG1 anti–murine NKG2D monoclonal antibody (▪) but not by 20 μg/mL M139 isotype control (▴). Each data point in panels D-E represents the average of triplicate samples, and error bars represent 1 SD from the mean for triplicate wells. Results shown in panels A-E are each representative of 2 or more independent experiments.
ULBP1 and ULBP2, but not ULBP3, bind to murine NKG2D and elicit killing by murine NK cells in vitro. (A) Soluble forms of ULBP1 and ULBP2, but not ULBP3, bind to cells transfected with murine NKG2D/DAP10. CV-1 cells were cotransfected with cDNAs encoding human (gray histograms) or murine (black histograms) NKG2D and flag-tagged human DAP10. Cells were stained with anti-FLAG followed by anti–murine IgG-PE, anti–murine IgG-PE alone, or with ULBP1-Fc, ULBP2-Fc, ULBP3-Fc, or p7.5-Fc negative control protein followed by anti–human IgG-PE, and analyzed by flow cytometry. (B) Murine NKG2D-Fc binds to cells transfected with ULBP1 or ULBP2, but not ULBP3. EL4 cells were transduced with cDNAs encoding ULBP1, ULBP2, or ULBP3. Cells were incubated with murine NKG2D-Fc (thick line), human NKG2D-Fc (thin line), or negative control p7.5-Fc (filled histogram) followed by anti–human IgG-PE and analyzed by flow cytometry. (C) Soluble forms of ULBP1 and ULBP2, but not ULBP3, bind to murine NK cells. Splenocytes from C57BL/6 SCID mice were cultured in rhuIL-15 to enrich NK cells. Cells were incubated with the indicated Fc proteins, followed by anti–human IgG-PE and analyzed by flow cytometry. More than 90% of the cells in the live gate were positive for NK1.1, an NK-cell marker, but did not stain with isotype-matched negative control antibody. (D) Enhanced in vitro cytotoxicity of murine NK cells against RMA cells transduced with ULBP1 or ULBP2, but not ULBP3. RMA cells expressing vector alone (▪), ULBP1 (•), ULBP2 (▴), ULBP3 (○), or RAE-1β (□) as a positive control were tested as targets in cytotoxicity assays using murine NK cells as effectors. (E) In vitro cytotoxicity of murine NK cells against EL4 cells transduced with ULBP1 (•) is blocked by 20 μg/mL M315 rat IgG1 anti–murine NKG2D monoclonal antibody (▪) but not by 20 μg/mL M139 isotype control (▴). Each data point in panels D-E represents the average of triplicate samples, and error bars represent 1 SD from the mean for triplicate wells. Results shown in panels A-E are each representative of 2 or more independent experiments.
Enhanced in vitro cytotoxicity against ULBP1- and ULBP2-expressing tumor cells
In cytotoxicity assays, expression of ULBP1 or ULBP2 rendered EL4 and RMA tumor cells, shown here for RMA, sensitive to lysis by IL-15–activated murine NK cells. The magnitude of killing was comparable with RMA cells expressing the positive control murine NKG2D ligand, RAE-1β (Figure 1D). In contrast, cells expressing ULBP3 were killed at the same level as cells carrying vector alone (Figure 1D). The enhanced killing of ULBP-expressing cells, shown here for EL4, was completely blocked by anti-NKG2D monoclonal antibody indicating specificity (Figure 1E). Together, these data indicate that ULBP1 and ULBP2 are capable of binding to and activating murine NKG2D effector activity in vitro, whereas ULBP3 is not. We also observed that freshly isolated murine splenic NK cells that have not been cultured in IL-15 can kill ULBP-expressing tumor cells (C.L.S., unpublished data, June 16, 2003).
Tumor cells that express ULBPs are rejected in C57BL/6 mice
Initially, we injected EL4 cells that were FACS-sorted for ULBP expression into C57BL/6 mice and found that the tumors were not rejected. When we excised the tumors and analyzed them, we found that the ULBPs were not expressed at the cell surface, RNA, or genomic levels (C.L.S., unpublished data, September 20, 2003). This suggested that the ULBP-expressing tumor cells had been rapidly killed in vivo and the tumors resulted from small populations of parental cells that had escaped the sorting. To remedy this situation, single-cell clones were made from each population, screened by flow cytometry for ULBP expression, and pooled (n = 20) for future use (Figure 2).
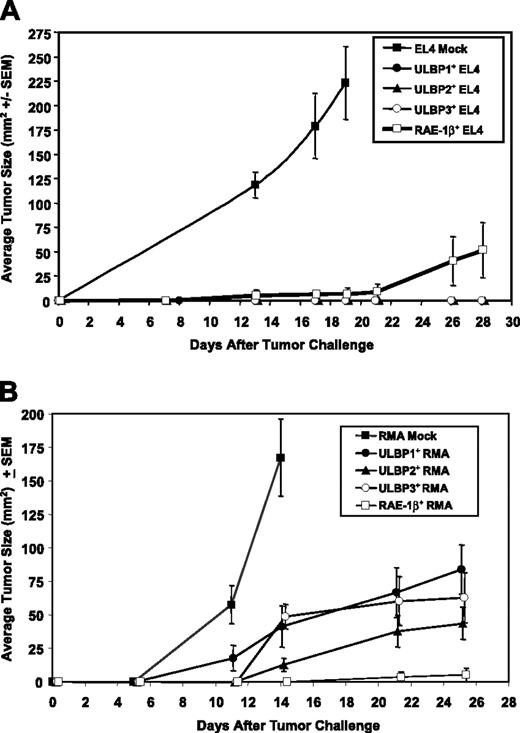
To determine if ectopic expression of ULBPs can mediate an antitumor immune response, pooled EL4 clones expressing ULBP1 or ULBP2 were injected subcutaneously into C57BL/6 mice and tumor growth was monitored. While mock EL4 cells carrying empty vector rapidly grew into tumors, EL4 cells expressing ULBP1 or ULBP2 were rejected in C57BL/6 mice at least as well as RAE-1β–expressing control cells (Figure 3A). To determine if ULBP expression could enhance tumor rejection in another tumor model, we analyzed the RMA tumor cell line. RMA tumors expressing ULBP1 or ULBP2 were also targeted in vivo, although to a lesser extent than ULBP-expressing EL4 cells (Figure 3B).
Generation of ULBP-expressing tumor cell lines. EL4 (A) and RMA (B) tumor cells expressing either empty vector (mock), ULBP1, ULBP2, ULBP3, or the murine NKG2D ligand RAE-1β were generated by retroviral-mediated gene transfer. Cells were single-cell sorted to obtain clones. Mock-transduced clones were identified by reverse-transcription (RT)–PCR. ULBP-expressing clones were stained with anti-ULBP1, anti-ULBP2, anti-ULBP3–specific (black histograms), or M230 isotype control antibody (white histograms), followed by anti–mouse IgG-PE. RAE-1β–expressing clones were stained with murine NKG2D-Fc (black histograms) or p7.5-Fc control (white histograms), followed by anti–human IgG Fc. Clones were analyzed by flow cytometry. Twenty positively expressing clones for each construct were pooled and used for future experiments. Staining of the pooled clones is shown.
Generation of ULBP-expressing tumor cell lines. EL4 (A) and RMA (B) tumor cells expressing either empty vector (mock), ULBP1, ULBP2, ULBP3, or the murine NKG2D ligand RAE-1β were generated by retroviral-mediated gene transfer. Cells were single-cell sorted to obtain clones. Mock-transduced clones were identified by reverse-transcription (RT)–PCR. ULBP-expressing clones were stained with anti-ULBP1, anti-ULBP2, anti-ULBP3–specific (black histograms), or M230 isotype control antibody (white histograms), followed by anti–mouse IgG-PE. RAE-1β–expressing clones were stained with murine NKG2D-Fc (black histograms) or p7.5-Fc control (white histograms), followed by anti–human IgG Fc. Clones were analyzed by flow cytometry. Twenty positively expressing clones for each construct were pooled and used for future experiments. Staining of the pooled clones is shown.
Since we could not detect binding of ULBP3 to murine NKG2D in vitro, we expected that tumor cells expressing ULBP3 would not be rejected in vivo. However, ULBP3 expression rendered EL4 (Figure 3A) and RMA tumors (Figure 3B) sensitive to killing in vivo to a similar extent as ULBP1 and ULBP2 expression. To determine if the xenogeneic nature of the ULBPs was responsible for rejection, serum samples from mice that had rejected ULBP1-, ULBP2-, or ULBP3-expressing EL4 tumors were collected 10 days after a booster shot that was identical to, but given 3 weeks after, the initial injection, and tested for an IgG response to the specific ULBP protein. Using enzyme-linked immunosorbent assays (ELISAs) sensitive to a lower limit of 20 ng/mL, we did not detect IgG antibodies directed toward ULBP1, ULBP2, or ULBP3 (C.L.S., unpublished data, January 25, 2005). These findings suggest that an anti-ULBP immune response was not likely a major contributor to the tumor rejection.
We found that C57BL/6 mice vaccinated with live ULBP1, ULBP2, or ULBP3 EL4 cells could generate a memory response that protected against challenge with parental EL4 cells. (C.L.S., unpublished data, November 18, 2004). Although we are unable to rule out the possibility of a xenogeneic response to ULBP protein, these results are consistent with the possible use of ULBP molecules in the design of tumor vaccines. There is no evidence, however, that the strategy described will be effective with tumors of epithelial origin.
ULBP expression retards tumor growth in C57BL/6 SCID mice, with enhancement by IL-15
To determine what types of cells may mediate the rejection of the ULBP-expressing tumor cells, and to further rule out the involvement of an antihuman response, we determined if SCID mice, which lack T and B cells, are capable of mediating an antitumor response against ULBP-expressing tumor cells. Growth of ULBP1-, ULBP2-, and ULBP3-expressing EL4 cells was greatly retarded compared with EL4 mock cells in SCID mice (Figure 4A), however, the magnitude of rejection was lower than that observed in C57BL/6 wild-type mice (Figure 3A). These findings are consistent with the interpretation that NKG2D-expressing NK cells mediate a large portion of the ULBP-mediated antitumor response, but that T cells, when present, may also contribute to the response.
IL-15 can exert antitumor effects in vivo by augmenting NK and cytotoxic T-cell (CTL) responses.38-42 IL-15 can also upregulate NKG2D on NK cells and effector CD8+ CTLs.30,31 To determine if IL-15 can enhance ULBP-mediated antitumor responses, ULBP1-expressing or mock EL4 control cells were transduced with a modified cDNA encoding the human IL-2 leader sequence and the murine IL-15 protein sequence. IL-15 message is expressed by a variety of tissues, however, several posttranscriptional regulatory mechanisms tightly control IL-15 expression, and it is often difficult to demonstrate IL-15 in the supernatants of cells that express IL-15 message.43 We found that substitution of the IL-15 leader sequence with the human IL-2 leader sequence results in increased secretion of IL-15 (Amgen). In C57BL/6 SCID mice, expression of IL-15 had only a modest effect on retarding growth of mock EL4 cells (Figure 4B). In contrast, expression of IL-15 greatly enhanced the antitumor response against ULBP1-expressing EL4 cells (Figure 4B). Given that IL-15 alone had little effect on enhancing the immune response against mock EL4 tumors, these data suggest that IL-15 augments tumor immunity in a manner dependent on the coexpression of a ULBP and thus is likely also NKG2D dependent.
Tumor cells that express ULBPs are rejected in syngeneic mice. C57BL/6 mice were challenged subcutaneously with 3 × 105 EL4 (A) or RMA (B) tumor cells expressing ULBP1 (•), ULBP2 (▴), ULBP3 (○), RAE-1β (□), or vector alone (▪). Tumor sizes were measured and mean tumor size (± SEM) was calculated from all surviving mice in each group. (A) Ten of 10 mice that received EL4 mock cells developed tumors and were humanely killed on day 18 due to tumor burden; none of the mice (n = 10/group) that received ULBP1-, ULBP2-, or ULBP3-expressing EL4 cells grew tumors; 3 of 10 mice that received RAE-1β–expressing EL4 cells developed tumors, and one was humanely killed at day 26 due to tumor burden. (B) Six of 6 mice that received RMA mock cells developed tumors and were humanely killed on day 14; 7 of 9, 5 of 7, 6 of 8, and 1 of 8 mice that received ULBP1-, ULBP2-, ULBP3-, or RAE-1β–expressing RMA cells, respectively, grew tumors. Data are representative of 4 independent experiments.
Tumor cells that express ULBPs are rejected in syngeneic mice. C57BL/6 mice were challenged subcutaneously with 3 × 105 EL4 (A) or RMA (B) tumor cells expressing ULBP1 (•), ULBP2 (▴), ULBP3 (○), RAE-1β (□), or vector alone (▪). Tumor sizes were measured and mean tumor size (± SEM) was calculated from all surviving mice in each group. (A) Ten of 10 mice that received EL4 mock cells developed tumors and were humanely killed on day 18 due to tumor burden; none of the mice (n = 10/group) that received ULBP1-, ULBP2-, or ULBP3-expressing EL4 cells grew tumors; 3 of 10 mice that received RAE-1β–expressing EL4 cells developed tumors, and one was humanely killed at day 26 due to tumor burden. (B) Six of 6 mice that received RMA mock cells developed tumors and were humanely killed on day 14; 7 of 9, 5 of 7, 6 of 8, and 1 of 8 mice that received ULBP1-, ULBP2-, ULBP3-, or RAE-1β–expressing RMA cells, respectively, grew tumors. Data are representative of 4 independent experiments.
EL4 cells expressing ULBPs are partially rejected in C57BL/6 SCID mice; IL-15 enhances the rejection. (A) C57BL/6 SCID mice were challenged subcutaneously with 3 × 105 EL4 cells expressing vector alone (▪), ULBP1 (•), ULBP2 (▴), or ULBP3 (○). The number of tumor-bearing animals at the end of the experiment was 10 of 10 for EL4 mock, 8 of 10 for ULBP1+ EL4, 8 of 10 for ULBP2+ EL4, and 9 of 10 for ULBP3+ EL4. The EL4 mock mice were humanely killed on day 19 due to tumor burden. (B) C57BL/6 SCID mice were challenged as in panel A with EL4 mock cells (▪), EL4 mock–expressing IL-15 (□), EL4-expressing ULBP1 (•), or EL4-coexpressing ULBP1 and IL-15 (○). Mean tumor size (± SEM) was calculated from all of the mice. The number of tumor-bearing animals at the end of the experiment was 8 of 9 for EL4 mock, 7 of 7 for EL4 mock/IL-15+, 7 of 8 for ULBP1+, and 3 of 7 for ULBP1+/IL-15+. The EL4 mock and EL4 mock/IL-15+ mice were humanely killed on day 21 due to tumor burden. Data are representative of 3 independent experiments.
EL4 cells expressing ULBPs are partially rejected in C57BL/6 SCID mice; IL-15 enhances the rejection. (A) C57BL/6 SCID mice were challenged subcutaneously with 3 × 105 EL4 cells expressing vector alone (▪), ULBP1 (•), ULBP2 (▴), or ULBP3 (○). The number of tumor-bearing animals at the end of the experiment was 10 of 10 for EL4 mock, 8 of 10 for ULBP1+ EL4, 8 of 10 for ULBP2+ EL4, and 9 of 10 for ULBP3+ EL4. The EL4 mock mice were humanely killed on day 19 due to tumor burden. (B) C57BL/6 SCID mice were challenged as in panel A with EL4 mock cells (▪), EL4 mock–expressing IL-15 (□), EL4-expressing ULBP1 (•), or EL4-coexpressing ULBP1 and IL-15 (○). Mean tumor size (± SEM) was calculated from all of the mice. The number of tumor-bearing animals at the end of the experiment was 8 of 9 for EL4 mock, 7 of 7 for EL4 mock/IL-15+, 7 of 8 for ULBP1+, and 3 of 7 for ULBP1+/IL-15+. The EL4 mock and EL4 mock/IL-15+ mice were humanely killed on day 21 due to tumor burden. Data are representative of 3 independent experiments.
ULBP expression enhances recruitment of NK cells, NK1.1+ T cells, and T cells to the tumor
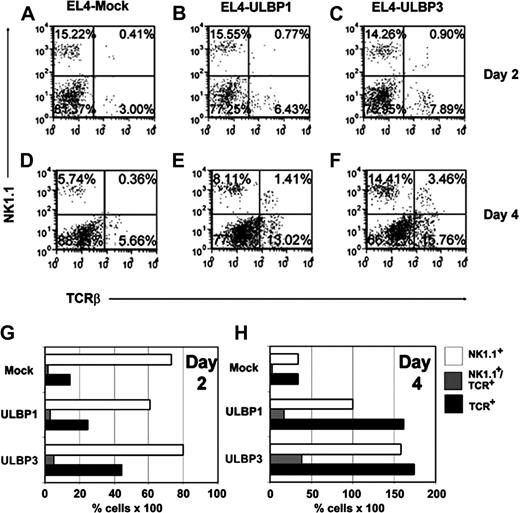
We analyzed whether ULBP expression affects the types and numbers of tumor-infiltrating lymphocytes obtained from resected tumors. Since we could not detect binding of ULBP3 to murine NKG2D in vitro, we were especially interested in determining if there were differences in the types or numbers of immune effector cells recruited by ULBP3 compared with ULBP1. C57BL/6 mice were injected with 1 × 107 EL4 cells transduced with vector alone, ULBP1, or ULBP3. At this dose, the tumor burden was so large that the ULBP-expressing tumors initially grew as rapidly as the mock EL4 cells. As shown in Figure 5, as early as 2 days after tumor injection, tumor-infiltrating or tumor-associated NK cells, NK1.1+ T cells, and T cells could be detected in the mock, ULBP1, and ULBP3 tumors. However, both ULBP1- and ULBP3-expressing tumors contain much higher percentages of NK cells, NK1.1+ T cells, and T cells compared with mock EL4 tumors. ULBP3 tumors were as effective at recruiting all 3 effector-cell types as the ULBP1 tumors.
Discussion
We show that ectopic expression of ULBPs, human NKG2D ligands, can induce robust antitumor immune responses in both C57BL/6 (Figure 3) and C57BL/6 SCID (Figure 4) mice. ULBPs can induce recruitment of NK cells, NK1.1+ T cells, and T cells to the tumor as early as 2 days after tumor injection (Figure 5). Together, these data suggest that NK cells are important for mediating the response against ULBP-expressing tumors but that NK1.1+ T and T cells may also be involved. The mechanism by which NK cells, NK1.1+ T cells, and T cells are recruited to ULBP+ tumors could be direct, through stimulation of NKG2D on the surface of these cells. Another possibility is that engagement of NKG2D on NK cells induces secretion of cytokines and chemokines, as has been described,1 that cause recruitment of effector cells to the tumor area. In patients with advanced ovarian carcinoma, expression of ULBP4 is associated with higher levels of CD8+/NKG2D+ lymphocytes infiltrating the carcinoma and is a prognosticator of improved survival.26 Our findings indicate that in addition to T cells, ULBPs can recruit NK cells, and NK1.1+ T cells to the tumor.
In contrast to ULBP1 and ULBP2, we could not detect binding of ULBP3 to the murine form of NKG2D or induction of NK-cell effector function in vitro (Figure 1A-D). In mice, however, ULBP3 could induce antitumor responses (Figures 3, 4, 5). A possible explanation for this discrepancy is that although we were unable to detect ULBP3 binding to murine NKG2D in vitro, ULBP3 can bind murine NKG2D at low, but effective, levels in vivo. BiaCore analysis indicates that ULBP3 binds much more weakly than ULBP1 and ULBP2 to human NKG2D (N.J.C., unpublished data, February 2, 2001), and in cytotoxicity assays ULBP3-transduced human target cells deliver a much weaker killing signal to human NK cells than do ULBP1 and ULBP2.1 Thus, at least with human NKG2D, there is precedence in the literature for ULBP3 having much lower affinity than ULBP1 and ULBP2 for NKG2D. There are also reports of wide variances in the binding affinities of the different murine NKG2D ligands for murine NKG2D.1,44
We attempted to determine whether anti–mouse NKG2D blocked the antitumor response to ULBP3-expressing tumor cells in vivo. In extensive studies, neither of the anti–mouse NKG2D antibodies known to be blockers in vitro and available to try, our M315 Rat IgG1 and CX5 Rat IgG1 from eBioscience (San Diego, CA), blocked rejection of ULBP- or RAE-1–expressing EL4 or RMA cells in vivo. So, from these studies, we are unable to make any conclusions about whether another receptor is triggering ULBP3 in vivo. The idea that ULBPs may bind to receptors other than NKG2D is supported by findings that the NKG2D ligands, H60 and MICA, can mediate immunosuppressive effects on T-cell proliferation, and that this response involves a receptor distinct from NKG2D.45 Given that ULBP3 binds so poorly to NKG2D, it may be that the true receptor for this molecule is yet to be identified. We have preliminary data that soluble versions of ULBP3, but not ULBP1 or ULBP2, can bind to primary human macrophages and to macrophage cell lines. Studies are in progress to address the possibility of a non-NKG2D receptor for ULBP3 on macrophages.
ULBP-expressing tumors resected after subcutaneous implantation contain higher percentages of NK cells, NK1.1+Tcells, and T cells. EL4 cells (1 × 107) transduced with vector alone (A,D), ULBP1 (B,E), or ULBP3 (C,F) were injected subcutaneously into C57BL/6 mice (n = 3; a representative experiment is shown). Two days (A-C) or 4 days (D-F) after implantation, tumors were resected, dispersed, and stained for the presence of T cells, NK cells, and NK1.1+ T cells. Dot plots portray NK1.1 expression versus TCR-β expression on lymphocytes that were gated based on forward and side scatter. Increased percentages of infiltrating NK cells, NK1.1+ T cells, and T cells were observed on day 4 when EL4 cells expressed either ULBP1 or ULBP3 compared with mock. When the percentage of NK cells (□), NK1.1+ T cells (▦), and T cells (▪) was adjusted to compensate for the relative percentage of infiltrating lymphocytes on days 2 (G) and 4 (H), the expression of ULBP1 or ULBP3 on EL4 cells resulted in approximately a 3- to 5-fold increase in infiltration of T cells and NK cells to the tumor relative to the EL4 mock tumors on day 4. NK1.1+ T-cell infiltration was augmented by as much as 5- to 6-fold.
ULBP-expressing tumors resected after subcutaneous implantation contain higher percentages of NK cells, NK1.1+Tcells, and T cells. EL4 cells (1 × 107) transduced with vector alone (A,D), ULBP1 (B,E), or ULBP3 (C,F) were injected subcutaneously into C57BL/6 mice (n = 3; a representative experiment is shown). Two days (A-C) or 4 days (D-F) after implantation, tumors were resected, dispersed, and stained for the presence of T cells, NK cells, and NK1.1+ T cells. Dot plots portray NK1.1 expression versus TCR-β expression on lymphocytes that were gated based on forward and side scatter. Increased percentages of infiltrating NK cells, NK1.1+ T cells, and T cells were observed on day 4 when EL4 cells expressed either ULBP1 or ULBP3 compared with mock. When the percentage of NK cells (□), NK1.1+ T cells (▦), and T cells (▪) was adjusted to compensate for the relative percentage of infiltrating lymphocytes on days 2 (G) and 4 (H), the expression of ULBP1 or ULBP3 on EL4 cells resulted in approximately a 3- to 5-fold increase in infiltration of T cells and NK cells to the tumor relative to the EL4 mock tumors on day 4. NK1.1+ T-cell infiltration was augmented by as much as 5- to 6-fold.
We demonstrate that IL-15 can enhance the rejection of NKG2D ligand–expressing tumors (Figure 4B). IL-15 is a key cytokine for effector and memory T-cell activation and survival, and for NK-cell differentiation, proliferation, and effector activity.43 It is possible that the enhanced rejection of IL-15/ULBP1–expressing EL4 cells is a result of a general, nonspecific increase in immune effector function rather than being dependent on NKG2D-mediated responses. To address this question, SCID mice were injected with IL-15–secreting mock EL4 cells that do not express NKG2D ligands, and tumor growth was measured. The presence of IL-15 in the absence of ULBPs resulted in little to no protective effect on these cells, and the tumors grew at a similar rate to EL4 mock cells (Figure 4B). These results suggest that the effect of IL-15 is dependent on the presence of ULBP1 on the tumor. Given that IL-15 can up-regulate NKG2D,30,31 one possibility for the mechanism by which IL-15 enhances rejection of ULBP-expressing tumors in SCID mice may be by up-regulating NKG2D expression on NK cells.
Tumors have evolved ways to subvert the NKG2D/NKG2D ligand system in order to evade immune detection. Soluble forms of NKG2D ligands are shed by tumors into the blood of patients and can interfere with NKG2D function either by acting as decoy ligands or by down-regulating NKG2D expression on CD8+ T cells and NK cells.27,28,46 Our finding that IL-15 expression can augment NKG2D ligand–mediated antitumor responses suggests that IL-15 may be a useful treatment for overcoming these tumor-evasion mechanisms.5 Taken together, the results from this study suggest that ULBPs may be exploited for antitumor therapy, and that IL-15 administration may be useful to enhance the immune response against NKG2D ligand–expressing tumors.
Prepublished online as Blood First Edition Paper, April 18, 2006; DOI 10.1182/blood-2005-11-011320.
C.L.S., B.R., N.J.C., and R.M. participated in performing the research. C.L.S., B.R., P.B., and D.C. designed the research. C.L.S. wrote the paper and all authors checked the final version of the paper.
One of the authors (D.C.) holds a patent related to the work that is described in the present study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Annie Rein-Weston for assistance in measuring tumors; Elizabeth Bell for flow cytometry; Karen Longin and June Eisenman for assistance with the CTLL.2 assay; Roberta Hanna, Courtney Beers, and William Fanslow for helpful discussion; and Anne-Renee van der Vuurst De Vries for reading the paper.
Abbreviations used in this article: ULBP indicates UL16-binding protein; LZ, leucine zipper; KIR, killer-cell immunoglobulin-like receptor; RAE-1, retinoic acid early inducible-1; MIC, MHC class I-related chain; and MULT-1, murine UL16-binding protein–like transcript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal