Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is characterized by lymphoproliferation and autoimmune clinical manifestations and is generally caused by defective Fas-mediated apoptosis. This report describes the first homozygous FASL gene mutation in a woman with clinical and immunologic features of ALPS. T-cell blasts from the patient did not induce FasL-mediated apoptosis on Fas-transfected murine L1210 or on Jurkat cells, and activation-induced cell death was impaired. Furthermore, Fas-dependent cytotoxicity was drastically reduced in COS cells transfected with the mutant FasL. In addition, FasL expression on T-cell blasts from the patient was similar to that observed in a healthy control, despite its bearing the high-producer genotype –844C/C in the FASL promoter. Sequencing of the patient's FASL gene revealed a new mutation in exon 4 (A247E). The location of A247E in the FasL extracellular domain and the conservation of the protein sequence of that region recorded in 8 species different from humans support the essential role of FasL COOH terminal domain in Fas/FasL binding. These findings provide evidence that inherited nonlethal FASL abnormalities cause an uncommon apoptosis defect producing lymphoproliferative disease, and they highlight the need for a review of the current ALPS classification to include a new ALPS type Ic subgroup.
Introduction
FasL/CD178 and Fas/CD95 are transmembrane proteins that belong to the tumor necrosis factor (TNF) and the TNF receptor family, respectively. Fas is expressed on many cell types, whereas FasL is mainly expressed on T lymphocytes.1 Like other members of the TNF family of molecules, the biologically active FasL molecule exists as a homotrimerized complex. Membrane-bound FasL induces the high-order oligomerization of preassembled Fas multimers, initiating a cascade of events that ultimately result in programmed cell death or apoptosis.2,3 This process is initiated when Fas triggers the formation of the death-inducing signaling complex by recruiting Fas-associated death domain (FADD) caspase 8 and caspase 10 proteins. The molecular complex that forms cleaves and activates several downstream proteins in a cascade that leads to apoptotic cell death characterized by structural changes such as DNA fragmentation and chromatin condensation.4
The Fas-FasL pathway plays an important role in the homeostasis of mature lymphocytes by limiting lymphocyte accumulation and minimizing reactions against self-antigens. This was clearly shown in the lymphoproliferative syndromes observed in lpr (lymphoproliferation) or gld (generalized lymphoproliferative disease) mice,5-7 which carry autosomal recessive mutations in the FAS gene or in the gene encoding FASl, respectively.
More than 30 years ago, Canale and Smith8 reported a condition characterized by nonmalignant lymphadenopathies associated with autoimmune features in children. Prompted by the discovery of FAS and FASl mutations in animals, defects of the Fas pathway were identified in patients in subsequent years, and the disease was named autoimmune lymphoproliferative syndrome (ALPS). In most patients, the disease is revealed early, usually before 5 years of age. ALPS associates lymphoproliferative manifestations, such as lymphadenopathies and hepatosplenomegaly, with a specific immunologic disorder consisting of hypergammaglobulinemia G (sometimes associated with hyper IgA) and an expanded population of TcRαβ+CD4–CD8– double-negative (DN) T lymphocytes. Autoimmune manifestations are observed in most patients. Lymphoproliferative manifestations resolve with age, whereas immunologic disorders frequently persist. The prognosis for patients with ALPS appears good, but they are at increased risk for malignant lymphoma.9-12
ALPS (Online Mendelian Inheritance in Man [OMIM] no. 60185934 ) is classified according to the underlying genetic defect.13 In ALPS type 0, a homozygous FAS mutation usually causes complete deficiency of the Fas protein and a severe form of the disease.10 In ALPS type I, a heterozygous FAS mutation (ALPS type Ia)14 and, more rarely, a heterozygous mutation in the gene for FASL in patients with systemic lupus erythematosus (SLE) (ALPS type Ib)15 are usually associated with a partial defect in apoptosis. ALPS type II is characterized by resistance to Fas-mediated apoptosis by mutations in caspase 10 (ALPS type IIa)16 and caspase 8 (ALPS type IIb)17 genes. In ALPS type III, Fas-mediated apoptosis is normal in vitro, and the genetic defect is unknown.18 Recently, Holzelova et al19 have found heterozygous dominant somatic FAS mutations in some populations of hematopoietic cells in patients with ALPS type III. These patients belong to a subgroup with mosaic ALPS type I, or ALPS type Im.
This report describes the first patient with a homozygous mutation in the FASL gene that causes a new type of ALPS. Here we show the spectrum of clinical, immunologic, and functional studies of apoptosis and the genetic features of this patient with lymphoproliferative syndrome but without SLE.
Patient, materials, and methods
The protocols of this study were approved by the Institutional Review Board of Hospital 12 de Octubre (Madrid, Spain).
Patient
This patient was first seen in our hospital when she was 11 years of age because of generalized lymphadenopathy, massive hepatosplenomegaly, and persistent hypergammaglobulinemia that had been present since she was a few months old. When she was 6 weeks of age, she had jaundice, acute otitis media, and anemia of unknown cause that required erythrocyte transfusion. At 6 months, she was referred to a local hospital because of a history of recurrent acute otitis media, frequent episodes of fever, generalized lymphadenopathy, and hepatosplenomegaly. On examination the patient had enlarged occipital, anterior, and posterior cervical and axillary lymph nodes. Liver and spleen were felt 2 and 5 cm below the costal margin, respectively. Bone marrow examination results were normal, and lymph node biopsy revealed hyperplasia with increased numbers of histiocytes and eosinophils. A diagnosis of Langerhans histiocytosis was made, and the patient received several courses of chemotherapy for 8 years. During this time, she had recurrent infections, as follows: 4 episodes of pneumonia at 8 months, 3 years, 3.5 years, and 4 years; Salmonella spp and Klebsiella pneumoniae sepsis at 11 months; hemorrhagic varicella at 4 years; postvaccine meningoencephalitis at 5 years; recurrent suppurative acute otitis media requiring placement of tympanostomy tubes; and recurrent dermatitis. When she was 8 years old, a thorough review of lymph node biopsy specimens obtained at 6 months of age ruled out Langerhans histiocytosis. After chemotherapy was stopped (when she was 11-15 years of age), the girl continued to have massive lymphadenopathy (cervical, axillary, and intraabdominal), hepatomegaly (2-4 cm below costal margin), and splenomegaly (7-15 cm below the costal margin). She also had herpes zoster, autoimmune thrombopenia, and hemolytic anaemia. She had persistent polyclonal hypergammaglobulinemia with IgG levels ranging from 40 to 50 g/L. Her lymph nodes showed histopathologic features characteristic of ALPS. The most prominent finding was a marked paracortical expansion by lymphocytes in varying stages of immunoblastic transformation with some intermingled lymphocytes and polyclonal plasma cells. Immunophenotypic examination showed that 80% to 90% of the paracortical cells were CD3+ T cells with a CD4/CD8 ratio of 3:1 and MIB-1 positivity suggesting a high proliferation index. Markers for Langerhans histiocytosis (C1a and S100) were persistently negative. Liver biopsy revealed portal lymphocytic infiltrates. Lymphadenopathy, splenomegaly, and hypergammaglobulinemia have gradually diminished, and the patient has been free of infections and autoimmune phenomena. During her last outpatient visit, at 26 years of age, some anterior and posterior lymph nodes measured less than 1 cm in diameter. Spleen was felt 2 cm below the left costal margin. She has no family history of lymphoproliferative or autoimmune diseases or systemic lupus. Informed consent was obtained according to the World Medical Association Declaration of Helsinki.
Cells and cell culture
Human peripheral-blood mononuclear cells (PBMCs) were obtained from the blood of healthy controls, from the mother of the patient, and from the patient herself by Ficoll-Paque density centrifugation, and 6-day T-cell blasts were generated from them by PHA stimulation in the presence of exogenous IL-2 under the same conditions indicated elsewhere.20
Quantification of serum proteins
Serum immunoglobulin levels (IgG, IgA, IgM) were measured by nephelometry (Immage; Beckman, Brea, CA). Serum IL-10 (Bender MedSystems, Vienna, Austria) and soluble CD25 (sCD25; R&D, Abingdon, United Kingdom) were measured in duplicate by ELISA.
Apoptosis assay after Fas stimulation
T-cell blasts from the patient and healthy controls were cultured with different concentrations of anti-Fas mAb (50, 100, 200, 500, and 1000 ng/mL) (Clon CH11; Coulter Clone, Hialeah, FL) for the induction of Fas-mediated apoptosis in the presence of IL-2 for 48 hours. Apoptotic cells were detected by flow cytometry with the use of annexin V–FITC/propidium iodide (PI).
Activation-induced cell-death assay
Sensitivity to activation-induced cell death (AICD) was tested by the incubation of 2 × 106 cells/mL T-cell blasts with different PHA concentrations for 12 hours. After incubation, cell death was determined by trypan blue staining and microscopic inspection and, in parallel, by PI staining and flow cytometry.
Constructs and transfections
The cDNA sequence for the FasL protein was cloned into the pIRES2-DsRed2 vector (Clontech Laboratories, Mountain View, CA). RNA from the patient and control were used as template in a one-step reverse transcription–polymerase chain reaction (RT-PCR). An EcoRI site in the forward primer (5′-AAAGAATTCAGCTGCCATGCAGCAGCCCTTCA-3′) and a BamHI site in the reverse primer (5′-AAAGGATCCTTAGAGCTTATATAAGCCGAAAAACG-3′) were introduced. The resultant PCR products and the pIRES2-DsRed2 vector were EcoRI (New England Biolabs, Ipswich, MA) and BamHI (Promega, Madison, WI) digested, purified (Qiagen, Hilden, Germany), and ligated with T4 DNA ligase (New England Biolabs) according to the manufacturer's recommendations. DH5α-competent bacteria were transformed by the heat shock method and grown on LB-kanamycin plates. Colonies were selected and checked for insertion and orientation by PCR and sequencing.
COS-7 cells were grown in DMEM with 10% FCS. They were split every 2 to 3 days and were seeded in 6-well plates for 24 hours before transfection (8 × 105 cells/well), and then they were transfected in DMEM with 10 μL lipofectamine 2000 (Invitrogen, Paisley, United Kingdom) with 4 μg wild-type and mutant DNA according to the adherent-cell transient-transfection protocol. After transfection, cells were grown for 36 hours before evaluation. Transfection efficiency, based on microscopic evaluation of DsRed2 expression, varied between 20% and 25%. No significant differences in transfection efficiency were found between the wild-type and the mutant construct.
Cytotoxicity assays
For FasL-mediated cytolysis, blast T cells were stimulated for 3 hours with 10 ng/mL phorbol myristate acetate (PMA; Sigma, St Louis, MO) plus 600 nM ionomycin (Sigma) at 37°C to induce FasL surface expression, secretion, or both. Control COS-7 cells, or COS-7 transfected with either the wild-type or the mutant FasL constructs indicated, were also used as effectors in cytotoxicity assays. Cells were then tested at different effector-to-target ratios against 3H-thymidine–labeled Fas-transfected murine L1210 cells (L1210Fas) or against untransfected L1210 cells negative for Fas expression in 6-hour or 16-hour assays.21,22 Cytotoxicity assays were performed in the presence of 1 mM EGTA plus 1.5 mM MgCl2 to block perforin-mediated cytolysis. For spontaneous and maximum lysis, target cells were incubated alone or in 2% SDS, respectively. The percentage of specific DNA fragmentation was calculated as follows: experimental 3H-thymidine release – spontaneous 3H-thymidine release × 100/maximum 3H-thymidine release – spontaneous 3H-thymidine release. Spontaneous 3H-thymidine release was typically less than 15%.
For cell-free toxicity assays, supernatants of blast T cells stimulated as indicated were collected and tested for toxicity on Jurkat cells by a modification of the MTT reduction method for microplates.20
Immunofluorescence
For direct immunofluorescence, peripheral whole blood was incubated using the corresponding monoclonal antibodies: anti–CD3-TC, anti–CD4-TC, anti–CD8-TC, anti–CD19-TC (Caltag, Burlingame, CA); anti–TCRαβ-PE, anti–TCRγδ-FITC (Immunotech, Marseille, France); and anti–CD4-PE, anti–CD3-PE, anti–HLA-DR-PE, anti–CD3-FITC/16 + 56-PE, anti–CD8-FITC, anti–CD3-FITC, anti–CD4-FITC, anti–CD38-PE, anti–CD8-PE, and anti–CD95-PE (Becton Dickinson, San Diego, CA).
To analyze surface FasL expression of human T cells blasts, 5 × 105 cells were stained for FACS analysis in cold PBS containing 0.2% bovine serum albumin with anti–CD3-phycoerytrin (PE) (Caltag), washed, and stained with FITC-labeled rat anti–human FasL mAb H11 (Bender Medsystems). To analyze the intracellular FasL expression of human T-cell blasts, 5 × 105 cells were stained for FACS analysis in cold PBS containing 0.2% serum bovine albumin with anti–CD3-PE, washed, fixed with 1% paraformaldehyde, and permeabilized with 0.1% saponin. Permeabilized cells were then stained with H11-FITC. Labeling of microvesicles/exosomes secreted by human T-cell blasts to detect FasL expression was also performed with H11-FITC, and it was detected by flow cytometry.23,24
Molecular genetics
DNA, RNA extraction, and mutation analysis of theFASLandFASgenes. DNA and cytoplasmic RNA were extracted from peripheral-blood lymphocytes from the patient and her mother by standard methods. Reverse transcription was performed on 0.5 μg cytoplasmic RNA by one-step RT-PCR (Invitrogen) with specific primers for the reaction that cover all the coding sequences of FASL and FAS genes. Primers used for PCR amplification of the FASL gene were15 5′-TGACTCACCAGCTGCCATGCAG and 5′-GGAAAGAATCCCAAAGTGCTTCTC. Primers used for PCR amplification of the FAS gene were 5′-CCCGCTCAGTACGGAGTTGG-3′ for FAS-D and 5′-CCAAGCAGTATTTACAGCCAGC-3′ for FAS-R. PCR conditions for cDNA amplification of the FASL and FAS genes were initial denaturation at 94°C for 5 minutes, 38 cycles with denaturation at 94°C for 15 seconds, annealing at 57°C for 30 seconds and 1 minute 20 seconds of extension at 72°C, and a final 10-minute cycle of extension at 72°C. PCR products were purified with the QIA quick PCR purification kit according to the manufacturer's protocols (Qiagen). Purified DNA products were directly sequenced (Sanger dideoxy chain terminator method with dye-labeled dideoxy terminators; Applied Biosystem, Foster City, CA) and analyzed in a DNA sequencer (3100 Avant; Applied Biosystem).
Mutation analysis ofFASgene in DN T cells. DNA extracted from magnetic-sorted DN T lymphocytes (Miltenyi Biotec, Auburn, CA) (purification of DN T cells exceeded 90%) was amplified and sequenced with specific primers spanning the 9 exons and the exon–intron boundaries of the FAS gene (PCR conditions and primers sequences are available on request).
Competitive PCR. Competitive PCR amplification was performed on genomic DNA from the patient, her mother, and 2 healthy controls (male and female). Two oligonucleotide primer pairs were used in the same PCR reaction, the first for amplification of part of exon 4 of the FASL gene and the second corresponding to an amplification of exon 13 of the X-linked dystrophin gene. Reaction was limited to 20 cycles of PCR, and a limiting amount of Taq polymerase (0.8 U) was added to the PCR mixture (the dye used to end-label PCR primers was FAM). Under these conditions, PCR was quantitative and did not reach a plateau. The DNA was electrophoresed at 1400 V for 14 hours, and the data were automatically analyzed using the GeneScan Fluorescent Fragment Analyzer (3100-Avant Genetic Analyzer; Applied Biosystems). Ratios between the 2 band areas (FASL/dystrophin) were calculated and compared among the patient, her mother, and the controls.25
PCR amplification and sequencing ofFASLpromoter region. The FASL promoter region was amplified with the primers 5′-CTACGATAGCACCACTGCAC for FasL-Promoter-F, annealing to nucleotide positions –954 to –935, and 5′-CTGGGGATATGGGTAATTGAAGG for FasL-Promoter-R, annealing to positions +11 to +33 (+1 site corresponds to A of the ATG translation start codon). PCR conditions were initial denaturation at 94°C for 5 minutes, then 40 cycles with denaturation at 94°C for 30 seconds, annealing at 62°C for 45 seconds and 1 minute of extension at 72°C, and a final 10-minute cycle of extension at 72°C. PCR products were purified and sequenced in both directions.
Control and SLE population
DNA was obtained from peripheral-blood leukocytes from 104 racematched healthy donors and 51 race-matched SLE patients, fulfilling the revised American College of Rheumatology criteria for SLE.26
Statistical analysis
Statistical comparisons of mean ± SD data were performed with the Mann-Whitney U test. P values less than .05 were considered statistically significant.
Results
Immunologic characteristics
The most prominent immunologic abnormalities in the patient included increased percentage and numbers of circulating CD4–CD8– DN T lymphocytes that expressed the αβ T-cell receptor, increased percentage and numbers of T cells expressing the activation markers HLA-DR and CD38 (level of sCD25 as an activation marker was also elevated), polyclonal hyperimmunoglobulinemia G, overexpression of IL-10, and elevated titles of antinuclear autoantibodies (ANAs) in February 1993 (1/5120) and February 1995 (1/1280) that coincided with the autoimmune manifestations of autoimmune thrombopenia and hemolytic anemia (Table 1). Immunologic parameters for the mother of the patient were normal.
Immunologic features of patient and her mother at last revision (March 2005)
. | Patient . | Mother . | Reference value . |
|---|---|---|---|
| Total lymphocytes, × 109/L | 2.073 | 1.542 | 1-4 |
| T lymphocytes, % | |||
| CD3+ | 91 | 80 | 61-82 |
| CD3+CD4+ | 25 | 50 | 36-56 |
| CD3+CD8+ | 39 | 24 | 13-31 |
| CD4/CD8 ratio | 0.64 | 2.18 | 1-2.5 |
| CD3+TcR-αβ+ | 88 | 74 | 55-75 |
| CD3+TcR-γδ+ | 1 | 5 | 0-7 |
| TcR-αβ+CD4-CD8- (DN) | 25* | 1 | 0-2 |
| CD3+CD95+ | 81 | 65 | 60¶ |
| T-activation markers, % | |||
| CD3+CD38+ | 22 | 6 | 0-8 |
| CD3+HLA-DR+ | 18† | 6 | 0-10 |
| CD19+ B lymphocytes, % | 5 | 9 | 5-18 |
| CD16+CD56+CD3-NK lymphocytes, % | 3 | 10 | 3-18 |
| Immunoglobulins, g/L | |||
| IgG | 17.6‡ | 11.9 | 7-16 |
| IgA | 2.11 | 3.04 | 0.7-4 |
| IgM | 0.29 | 0.84 | 0.4-2.3 |
| Cytokines, pg/mL | |||
| IL-10 | 85§ | 0 | 0-5 |
| sCD25 | 15740 | 1107 | 0-1900 |
| Autoantibodies | |||
| ANA | Negative∥ | Negative | NA |
| Anti-cardiolipin IgG, UFL/mL | < 0.5 | ND | 0-16 |
| Anti-cardiolipin IgM, UFL/mL | 3.9 | ND | 0-25 |
| Other laboratory test: direct Coombs test | Negative | ND | NA |
. | Patient . | Mother . | Reference value . |
|---|---|---|---|
| Total lymphocytes, × 109/L | 2.073 | 1.542 | 1-4 |
| T lymphocytes, % | |||
| CD3+ | 91 | 80 | 61-82 |
| CD3+CD4+ | 25 | 50 | 36-56 |
| CD3+CD8+ | 39 | 24 | 13-31 |
| CD4/CD8 ratio | 0.64 | 2.18 | 1-2.5 |
| CD3+TcR-αβ+ | 88 | 74 | 55-75 |
| CD3+TcR-γδ+ | 1 | 5 | 0-7 |
| TcR-αβ+CD4-CD8- (DN) | 25* | 1 | 0-2 |
| CD3+CD95+ | 81 | 65 | 60¶ |
| T-activation markers, % | |||
| CD3+CD38+ | 22 | 6 | 0-8 |
| CD3+HLA-DR+ | 18† | 6 | 0-10 |
| CD19+ B lymphocytes, % | 5 | 9 | 5-18 |
| CD16+CD56+CD3-NK lymphocytes, % | 3 | 10 | 3-18 |
| Immunoglobulins, g/L | |||
| IgG | 17.6‡ | 11.9 | 7-16 |
| IgA | 2.11 | 3.04 | 0.7-4 |
| IgM | 0.29 | 0.84 | 0.4-2.3 |
| Cytokines, pg/mL | |||
| IL-10 | 85§ | 0 | 0-5 |
| sCD25 | 15740 | 1107 | 0-1900 |
| Autoantibodies | |||
| ANA | Negative∥ | Negative | NA |
| Anti-cardiolipin IgG, UFL/mL | < 0.5 | ND | 0-16 |
| Anti-cardiolipin IgM, UFL/mL | 3.9 | ND | 0-25 |
| Other laboratory test: direct Coombs test | Negative | ND | NA |
Reference values were obtained from 50 unrelated healthy adults.
NA indicates not applicable; ND, not done.
Percentages of DN T lymphocytes: 29% in January 1990, 35% in March 1991, 23% in February 1995, 21% in October 2000, 23% in September 2003.
Percentages of CD3+HLA-DR+: 34% in February 1990, 20% in March 1991, 22% in March 1992, 17% in February 1995, 23% in February 1998, 12% in September 2003.
IgG concentrations: 50.6 g/L in January 1990; 40.9 g/L in March 1991; 32.2 g/L in March 1992; 28.4 g/L in February 1993; 26.4 g/L in February 1995; 23.3 g/L in January 1996; 21.0 g/L in February 1998; 19.1 g/L in October 2000; 26.2 g/L in September 2003.
IL-10 concentration: 96 pg/mL in September 2003.
ANA: 1/5120 in February 1993, 1/1280 in February 1995, negative in February 1998, 1/40 in September 2003.
Percentage of CD3+CD95+ in one healthy control.
Fas-induced apoptosis in the patient is normal, but AICD is impaired
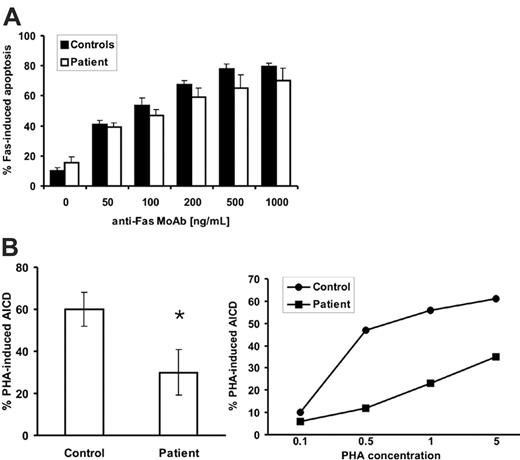
The immunologic abnormalities and clinical features described led us to suspect that cells from this patient were defective in their ability to undergo Fas-mediated apoptosis. To investigate this possibility, T-cell blasts were generated and analyzed for apoptosis assays after exposure to different concentrations of anti-Fas mAb. T-cell blasts from the patient showed only a slight, but not statistically significant, decrease of Fas-mediated apoptosis in vitro at all concentrations tested (Figure 1A). However, when PHA-induced AICD was tested, though approximately 60% of cell death was observed in T-cell blasts from healthy controls, only approximately 30% of cell death was observed in T-cell blasts from the patient (Figure 1B, left). The level of AICD observed in the healthy controls in this study was in the normal range observed in more than 50 donors (70% ± 10%) under the same experimental conditions previously reported by our group.27 The reduction in AICD in T-cell blasts from the patient was observed at all doses of PHA used (0.1-5 μg/mL) in a dose-response experiment (Figure 1B, right). These results indicate that the defect probably lies on FasL because T-cell AICD depends, at least in part, on the Fas/FasL interaction.28
Impaired FasL-induced cytotoxicity in the patient
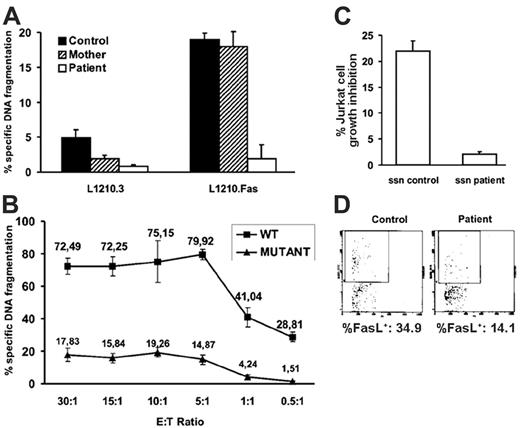
To analyze whether FasL has lost its cytotoxic activity in the patient, cytotoxicity assays were performed using T-cell blasts from the patient, her mother, or a healthy male control. T-cell blasts from the patient previously stimulated for 3 hours with PMA/ionomycin to induce FasL surface expression21 exerted little cytolysis on L1210Fas. By contrast, T cells from the mother of the patient exerted levels of cytolysis that were comparable to those of T cells from the healthy control, suggesting that FasL from the patient was inactive (Figure 2A). As a control, no significant cytotoxicity was exerted in the same conditions on Fas-negative L1210.3 cells (Figure 2A).
To confirm the defect in FasL functionality in the patient, FasL-negative COS-7 cells were transfected with either the wild-type or the mutated FASL genes and were used as effectors in cytotoxicity assays, as described in “Cytotoxicity assays.” As shown in Figure 2B, although COS-7 cells transfected with wild-type FasL clearly induced DNA fragmentation in L1210Fas cells at all effector-target (E/T) ratios used (from 30:1 to 0.5:1), COS-7 cells transfected with mutant FasL induced much (at least 4-fold) lower cytotoxicity in the same conditions. In both cases, cytotoxicity titrated from E/T ratios of 5:1 and below indicated that at higher ratios, Fas receptors in the targets were saturated by FasL expressed by the effectors. In the same conditions, untransfected COS-7 cells did not induce cytotoxicity (data not shown).
It has been reported that activated human T-cell blasts can secrete preformed FasL-loaded microvesicles/exosomes that retain cytolytic activity.23 To analyze the cytotoxic activity of FasL released on exosomes by T cells after activation, effector-cell–free cytolysis was measured in the supernatants from the stimulated cultures by the MTT reduction test with Jurkat cells as targets (see “Patient, materials, and methods”). Results showed that supernatants from the healthy control exerted 22% of cell-growth inhibition, whereas supernatants from the patient were not toxic at all against Jurkat cells (Figure 2C). This result is in agreement with the result shown in Figure 2A.
Finally, we wanted to characterize FasL secretion associated with exosomes after the reactivation of T-cell blasts from the patient in comparison with control T-cell blasts. T-cell blasts from the control and from the patient released the same amount of exosomes (data not shown), indicating that no obvious exocytosis problems were evident in T cells from the patient. Percentages of exosomes positive for FasL staining were 34.9% in the control and 14.1% in the patient in the representative experiment shown in Figure 2D. Although FasL expression in exosomes of the patient was lower than in the healthy control, the complete absence of cytotoxicity in the supernatants obtained from reactivated T-cell blasts from the patient (Figure 2C) did not correlate with this partial reduction in FasL+ exosomes.
Normal Fas-induced apoptosis but defective AICD in T-cell blasts from the patient. (A) Fas-mediated apoptosis was induced in T-cell blasts from 3 healthy controls and from the patient using different concentrations of anti-Fas mAb. Results are mean ± SD on T-cell blasts from 3 healthy controls or in 3 experiments performed with T-cell blasts from the patient. (B) Day 6 T-cell blasts from healthy controls or from the patient, as indicated, were incubated at 2 × 106 cells/mL during 12 hours with control medium, 5 μg/mL PHA (left panel), or different concentrations of PHA (0.1-5.0 μg/mL) (right panel), always in the presence of 30 UI/mL IL-2. Left panel: Cell death was estimated by trypan blue and PI staining, with identical results, and results are the mean ± SD of triplicate determinations on cells from 4 healthy controls or in 4 different experiments performed with T-cell blasts from the patient. *P = .011. Right panel: In one experiment, ΔΨm was determined by DiOC63 staining and flow cytometry.
Normal Fas-induced apoptosis but defective AICD in T-cell blasts from the patient. (A) Fas-mediated apoptosis was induced in T-cell blasts from 3 healthy controls and from the patient using different concentrations of anti-Fas mAb. Results are mean ± SD on T-cell blasts from 3 healthy controls or in 3 experiments performed with T-cell blasts from the patient. (B) Day 6 T-cell blasts from healthy controls or from the patient, as indicated, were incubated at 2 × 106 cells/mL during 12 hours with control medium, 5 μg/mL PHA (left panel), or different concentrations of PHA (0.1-5.0 μg/mL) (right panel), always in the presence of 30 UI/mL IL-2. Left panel: Cell death was estimated by trypan blue and PI staining, with identical results, and results are the mean ± SD of triplicate determinations on cells from 4 healthy controls or in 4 different experiments performed with T-cell blasts from the patient. *P = .011. Right panel: In one experiment, ΔΨm was determined by DiOC63 staining and flow cytometry.
Fas/FasL-mediated cytotoxicity. (A) T-cell blasts from a healthy control, the mother, and the patient were stimulated with PMA plus ionomycin (P+I) and tested for cell-mediated FasL-induced cytolytic activity using L1210 cells transfected (L1210.Fas) or not (L1210.3) with Fas as targets at a fixed 5:1 E/T ratio in 16-hour cytotoxicity assays. (B) COS-7 cells were transfected with an expression vector containing the wild-type (WT) FASL allele or the mutant FASL allele from the patient. Forty-eight hours after transfection, transfected COS-7 cells were tested against L1210 Fas target cells at the indicated E/T ratios in 6-hour cytotoxicity assays. (C) Supernatants (ssn) from P+I-stimulated T-cell blasts from the healthy control and the patient were collected and tested for cell-free FasL-induced toxicity on Jurkat cells, as described in “Patient, materials, and methods.” (A-C) Results are the mean ± SD of at least 3 different experiments. (D) Exosomes secreted by human T-cell blasts from the healthy control and the patient after P+I stimulation were labeled with H11-FITC and analyzed by flow cytometry using a gating protocol specific for microvesicles/exosomes. Percentage of FasL+ exosomes in each case is shown.
Fas/FasL-mediated cytotoxicity. (A) T-cell blasts from a healthy control, the mother, and the patient were stimulated with PMA plus ionomycin (P+I) and tested for cell-mediated FasL-induced cytolytic activity using L1210 cells transfected (L1210.Fas) or not (L1210.3) with Fas as targets at a fixed 5:1 E/T ratio in 16-hour cytotoxicity assays. (B) COS-7 cells were transfected with an expression vector containing the wild-type (WT) FASL allele or the mutant FASL allele from the patient. Forty-eight hours after transfection, transfected COS-7 cells were tested against L1210 Fas target cells at the indicated E/T ratios in 6-hour cytotoxicity assays. (C) Supernatants (ssn) from P+I-stimulated T-cell blasts from the healthy control and the patient were collected and tested for cell-free FasL-induced toxicity on Jurkat cells, as described in “Patient, materials, and methods.” (A-C) Results are the mean ± SD of at least 3 different experiments. (D) Exosomes secreted by human T-cell blasts from the healthy control and the patient after P+I stimulation were labeled with H11-FITC and analyzed by flow cytometry using a gating protocol specific for microvesicles/exosomes. Percentage of FasL+ exosomes in each case is shown.
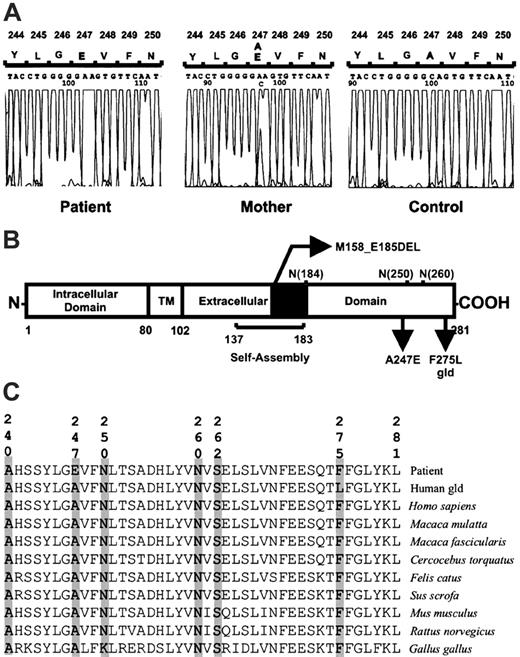
Schematic representation of the FasL protein and alignment of sequences. (A) Exon 4 sequence analysis of FASL. The homozygous and heterozygous mutation (A247E) is shown in the patient and her mother, respectively. (B) In the extracellular part of the protein are shown the self-assembly domain32 ; the heterozygous deletion of 28 amino acid (M158_E185DEL)15 ; 3 potential N-glycosylation sites at asparagines 184, 250, and 260 residues; the mutation characterized that changes an alanine for a glutamic acid (A247E); and the gld human FasL construct prepared with the corresponding mutation found in gld mice (F275L in the human sequence).32 (C) Alignment of the COOH terminal portion of the FasL protein, demonstrating the highly conserved amino acids A240, A247, N260, S262, and F275. Aligned FasL sequences are from the patient (GenBank accession no. AY858799), gld human FasL construct, Homo sapiens (GenBank accession no. AY225406), Macaca mulatta (GenBank accession no. AB035139), Macaca fascicularis (GenBank accession no. AB035138), Cercocebus torquatus (GenBank accession no. AF344847), Felis catus (GenBank accession no. NM_001009352), Sus scrofa (GenBank accession no. NM_213806), Mus musculus (GenBank accession no. NM_010177), Rattus norvegicus (GenBank accession no. NM_012908), and Gallus gallus (GenBank accession no. AJ890143).
Schematic representation of the FasL protein and alignment of sequences. (A) Exon 4 sequence analysis of FASL. The homozygous and heterozygous mutation (A247E) is shown in the patient and her mother, respectively. (B) In the extracellular part of the protein are shown the self-assembly domain32 ; the heterozygous deletion of 28 amino acid (M158_E185DEL)15 ; 3 potential N-glycosylation sites at asparagines 184, 250, and 260 residues; the mutation characterized that changes an alanine for a glutamic acid (A247E); and the gld human FasL construct prepared with the corresponding mutation found in gld mice (F275L in the human sequence).32 (C) Alignment of the COOH terminal portion of the FasL protein, demonstrating the highly conserved amino acids A240, A247, N260, S262, and F275. Aligned FasL sequences are from the patient (GenBank accession no. AY858799), gld human FasL construct, Homo sapiens (GenBank accession no. AY225406), Macaca mulatta (GenBank accession no. AB035139), Macaca fascicularis (GenBank accession no. AB035138), Cercocebus torquatus (GenBank accession no. AF344847), Felis catus (GenBank accession no. NM_001009352), Sus scrofa (GenBank accession no. NM_213806), Mus musculus (GenBank accession no. NM_010177), Rattus norvegicus (GenBank accession no. NM_012908), and Gallus gallus (GenBank accession no. AJ890143).
To rule out that the defective Fas-mediated cytotoxicity in T-cell blasts from the patient could have been caused by a reduced level of FasL expression, protein expression was analyzed by immunoblot and flow cytometry in CD3+ cells through analyses of surface and whole-cell expression. Results indicated that the level of FasL expression was similar to that observed in T-cell blasts from healthy controls or from the mother of the patient (data not shown).
Molecular analysis of FASL gene and promoter
We excluded mutations in the FAS gene by analyzing RNA from PHA-activated T-cell blasts and DNA from purified DN T lymphocytes. No germline or somatic FAS mutations were found in the patient.
After observing that FasL-mediated cytolytic activity was impaired, we suspected that the patient had a defect in the FASL gene. We performed a mutation search in PCR-amplified transcripts of the FASL gene. Sequencing analysis in the patient revealed a novel homozygous C-to-A substitution at cDNA nucleotide 740 (GenBank accession number AY858799), which produces a nonconservative mutation of alanine for glutamic acid (A247E) in exon 4 affecting the extracellular domain of the protein (Figure 3A). The mutation was confirmed in cDNA and in genomic DNA. Both parents of the patient were white and were not related. The mother is healthy and heterozygous for the mutation described. The father of the patient died of metastatic melanoma, and his DNA was not available.
We did not find the A247E FASL mutation in DNA samples from 104 race-matched healthy unrelated donors and the 51 race-matched patients with SLE. This fact, combined with the observed defects in FasL cytotoxicity, strongly argues that the mutation detected in this ALPS patient was functionally abnormal and did not represent a normal polymorphism of the FASL gene.
Competitive PCR did not show extensive FASL deletion in the patient. PCR amplification of exon 4 of the patient corresponded to 2 copies because FASL/dystrophin (X-linked) ratios were identical to those obtained in the patient's mother and the female control. The male control showed a FASL/dystrophin ratio twice that of values obtained in the patient, her mother, and the control female, as expected.
The proximal 950 bp of FASL promoter region was sequenced in the patient, her mother, and a healthy donor. We did not find any alteration of the promoter except the single nucleotide polymorphism (SNP) in position –844, described previously.29 The patient was –844C/C homozygous, her mother was –844C/T heterozygous, and the healthy control was –844T/T homozygous. These genotypes are associated with high, intermediate, and low FasL expression levels, respectively.29
Discussion
We describe the first human homozygous mutation in the FASL gene (A247E) associated with ALPS phenotype. The absence of clinical features in her heterozygous mother suggests autosomal recessive inheritance of the defect in the patient. This differs from the first FasL deficiency described in a patient with SLE that was caused by a heterozygous deletion within exon 4 of the FASL gene with a dominant-negative effect (Figure 3B).15,30
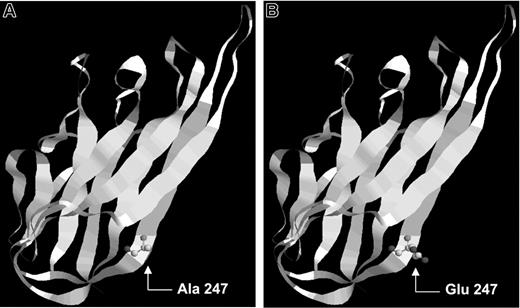
The patient's mutation in position 247 changed the original alanine, a neutral hydrophobic amino acid, by a glutamic acid with a negatively charged R group. FasL protein is structurally formed by 8 β-strands (A-H), and residue A247 was placed in the β-strand F of FasL. β-Strand conformation is favored by the presence of amino acids with neutral R-group (no net charge). If the R-group of a residue is charged (as in glutamic acid), it could destabilize the β-strand by establishing new interactions between the residues. Therefore, the mutation found (A247E) could disturb FasL trimerization because it was located at the subunit interface31 and could cause subtle local, structural changes that affected the folding of the individual subunit and prevented efficient receptor recognition.
Representation of the location of the described FasL mutation. FasL protein is formed by 8 β-strands: A, B, C, D, E, F, G, and H. (A) Wild-type FasL protein showing alanine in position 247. (B) Mutant FasL protein showing glutamic acid in residue 247.
Representation of the location of the described FasL mutation. FasL protein is formed by 8 β-strands: A, B, C, D, E, F, G, and H. (A) Wild-type FasL protein showing alanine in position 247. (B) Mutant FasL protein showing glutamic acid in residue 247.
Several positions surrounding the A247E mutation may be involved in receptor binding (A240, N260, S262) and in N-glycosylation of FasL (residues 250 and 260).31,32 We found that residue 247 and other adjacent amino acids are conserved in 8 species different from humans, which reinforces the importance of this domain in the binding function (Figures 3C, 4). Data from other groups also support that the COOH-terminal region of FasL is essential for receptor binding. The constructed gld mutation in the human FASL (F275L), located 7 amino acids from the COOH terminus, displays significantly attenuated receptor binding.32 Therefore, the disturbance of FasL function by the A247E mutation, located 34 amino acids from the COOH terminus, might support the essential role of this part of the protein. The identification of residues potentially involved in receptor recognition could help in the design of mutagenesis experiments for structure–function relationship studies. We are using this model to decipher the precise nature of the structural defect induced by point mutations in inactive FasL. FasL function was severely impaired in the patient; therefore, we propose that the homozygous mutation found in FASL gives rise to a nonfunctional protein that affects the signaling necessary to maintain lymphocyte homeostasis.
The SNP identified by Wu et al29 at nucleotide position –844 in the 5′ promoter of the FASL gene affects FasL protein expression level in vitro and ex vivo and is associated with SLE in African American patients. In luciferase promoter activity assay, it was reported that the –844C genotype had twice the basal activity of the –844T construct and that basal expression of FasL on peripheral-blood fibrocytes was also significantly higher in –844C than in –844T homozygous donors.29 Hence, to rule out the possibility that the low FasL expression found in the patient was an effect of the –844 genotype, the promoter DNA was sequenced in the patient, her mother, and a control. The control had the low-producer –844T/T genotype, and the patient's mother had –844C/T. The patient had the high-producer –844C/C genotype; thus, hypothetically she should have had FasL expression twice that of the control. However, her actual FasL level was similar to that of the control. This suggests that the A247E mutation itself alters not only the function but also the translation and the subsequent processing of the FasL protein, leading to a lower level of FasL protein expression.
In the light of our findings, the classification of ALPS requires revision. The patient reported here carried the same mutation on both alleles and therefore was homozygous for this recessive FASL gene mutation, whereas her heterozygous mother did not show clinical symptoms. The patient could be classified as having ALPS type I or ALPS type Ic because, though the clinical phenotype was indistinguishable from that of ALPS type I, the underlying genetic defect (FASL autosomal recessive inheritance) is different from that of type Ia (heterozygous FAS mutations) or SLE-associated type Ib (heterozygous FASL deletion). When she was very young, lymphoproliferative and autoimmune manifestations were manifested in our patient, but, in contrast to the first FasL-deficient patient described15 (ALPS type Ib), she has never had SLE. Most patients with SLE do not have FAS/FASL mutations.33 However, the fact that SLE patients do, though rarely, have FAS or FASL mutations and the discovery of the functional polymorphism in the FASL promoter (–844C/T) associated with SLE in African American patients (–844C/C)29 suggest that further research is needed to understand the precise role of apoptosis in SLE.
Prepublished online as Blood First Edition Paper, April 20, 2006; DOI 10.1182/blood-2006-04-015776.
Supported in part by grant 2004-008 from Mutua Madrileña (E.P.-A.) and grant SAF2004-03058 from Ministerio de Educación y Ciencia (Spain)/Fondo Europeo de Desarollo Regional (FEDER) Program (European Union) and by the Gobierno de Aragón (A.A.). A.B. was supported by a Formación Profesorado Universitario (FPU) Fellowship from Ministerio de Educación y Ciencia.
J.R.-C., E.P.-A., and L.M.A. designed the research. M.D.-R., A.B., S.C., A.S., and A.A. performed the research. A.B., J.G.-R., E.R., A.S., and A.A. designed analytic tools. M.D.-R., P.M., and L.M.A. analyzed the data. J.R.-C., A.A., E.P.-A., and L.M.A. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Carlos Rodríguez-Gallego and Miguel A. García-Pérez for their initial research in this study; Paloma de Pablos, Silvia Cortezón, Luis González, Marcela Castillo, and Miguel Angel Moreno-Pelayo for excellent technical assistance; and Patricia Carreira for sharing genomic DNA samples of SLE patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal