Abstract
Neuropilin 2 (NRP2) is a receptor for the vascular endothelial growth factor (VEGF) and the semaphorin (SEMA) families, 2 unrelated ligand families involved in angiogenesis and neuronal guidance. NRP2 specifically binds VEGF-A and VEGF-C, although the biological relevance of these interactions in human endothelial cells is poorly understood. In this study, we show that both VEGF-A and VEGF-C induce the interaction of NRP2 with VEGFR-2. This interaction correlated with an enhancement of the VEGFR-2 phosphorylation threshold. Overexpression of NRP2 in primary human endothelial cells promoted cell survival induced by VEGF-A and VEGF-C. In contrast, SEMA3F, another ligand for NRP2, was able to inhibit human endothelial cell survival and migration induced by VEGF-A and VEGF-C. Moreover, a siRNA targeting specifically NRP2 was a potent inhibitor of human endothelial cell migration induced by VEGF-A and VEGF-C. Thus, our data indicate that NRP2 acts as a coreceptor that enhances human endothelial cell biological responses induced by VEGF-A and VEGF-C.
Introduction
Members of the vascular endothelial cell growth factor (VEGF) family are the most important mediators of angiogenesis and lymphangiogenesis.1 These factors recognize at least 2 cell surface receptor families, the tyrosine kinase receptors (VEGFR) and the neuropilin (NRP) receptors. Three VEGFRs have been identified: VEGFR-1 (Flt-1), VEGFR-2 (Flk-1/KDR), and VEGFR-3 (Flt-4). All 3 receptors are vital for the development of the vasculature during embryogenesis, because inactivation of one of these genes leads to defective blood or lymphatic vessel development and to embryonic death.2-4 Binding of VEGFs to these receptors induces conformational changes, followed by receptor dimerization,5-7 which, in turn, triggers kinase activation and autophosphorylation on tyrosine residues.8,9 The phosphorylated receptor subsequently induces the activation of multiple substrates, leading to cell permeability, migration, and proliferation of endothelial cells.10
The neuropilins are structurally related molecules, which are expressed at the cell surface. They have a short cytoplasmic domain devoid of enzymatic activity. Only 2 neuropilins (NRP1 and NRP2) have been described until now. Initially, neuropilins were identified as receptors for axonal guidance factors belonging to the class-3 semaphorin subfamily including SEMA3A and SEMA3F.11-13 Several lines of evidence from various systems, including in vitro and in vivo experiments, clearly indicate that neuropilins also play an essential role in the development of vascular and lymphatic systems.14-16
In vitro results have demonstrated that neuropilins bind the VEGF members in an isoform-specific manner. NRP1 binds VEGF-A165, VEGF-B, VEGF-E, and PLGF, whereas NRP2 binds VEGF-A165, VEGF-A145, VEGF-C, and PLGF.17 It has been reported that NRP1 is able to form complexes with VEGFR-1 and VEGFR-2,18-20 but the biological function of these complexes is still unclear. However, it has been shown that VEGF-A165–induced proliferation and migration of cells expressing VEGFR-2 is enhanced in the presence of NRP1.19,21,22 On the contrary, SEMA3A, a competitor of VEGF-A165 binding to NRP-1, inhibited endothelial cell migration and sprouting from an aortic ring assay.23
In vivo, it has been demonstrated that zebrafish embryos in which Sema3A is either knocked-down or overexpressed show abnormal vasculogenesis.24 Similarly, malformations can be observed in the cardiovascular system of Sema3A knock-out mice,25 indicating a physiologic role of this neuronal ligand in the vascular network. NRP1-deficient mice die at the embryo stage at E14 with heart vasculature and nervous system defects.16,26,27 Selected deletion of NRP1 in the developing endothelial cells also causes marked vascular abnormalities.28 Conversely, overexpression of NRP1 results in an excess of capillary blood vessels29 and increased tumor growth,30 confirming that NRP1 expression in endothelial cells is essential to normal vascular development.
With regard to NRP2, it has been reported that the expression of this protein at the vascular cell membrane is restricted to veins and lymphatic vessels.14,31,32 Although they are viable, homozygous NRP2 mutants show an absence or a severe reduction of small lymphatic vessels and capillaries during development.14 Moreover, mice, in which both NRP1 and NRP2 were targeted, died in utero earlier than NRP1 knock-out mice (E8.5).33 These results suggest that NRPs are early genes involved in embryonic vessel development and that both NRP1 and NRP2 are required. Although NRP2 has been shown to interact with VEGFR-1,34 its role in angiogenesis is poorly understood. Some evidence indicates that SEMA3F, which binds NRP2 and induces neurite retraction, is also able to mediate repulsion of endothelial cells solely if they express NRP2.35 In addition, injection of recombinant SEMA3F in mice reduces angiogenesis.35 Consistent with these data, Kessler et al36 showed that SEMA3F is also able to inhibit endothelial cell survival and angiogenesis induced by VEGF-A.36
NRPs are not able to initiate signaling pathways by themselves and require an interaction with coreceptors such as plexins or VEGFRs. In the vascular system, NRP2 and VEGFR-3 are expressed almost exclusively on lymphatic vessels.14,37,38 However, it has been demonstrated that VEGFR-3 activity requires heterodimerization with VEGFR-2, which is also expressed on lymphatic vessels.39-41 In this study, we have investigated the ability of NRP2 to interact with VEGFR-2 and VEGFR-3 and analyzed the consequences of such interactions on human endothelial cell survival and migration.
Materials and methods
Antibodies and growth factors
Rabbit polyclonal anti–Flk-1/KDR (VEGFR-2), anti–Flt-4 (VEGFR-3), mouse monoclonal anti-NRP2, goat polyclonal anti-NRP1, agarose-conjugated monoclonal antihemagglutinin (HA) antibody, and the horseradish peroxidase (HRP)–conjugated anti-HA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-SEMA3F was from Chemicon (Temecula, CA). The HRP-conjugated monoclonal antibody anti–phosphotyrosine PY20 was from Zymed (San Francisco, CA). Agarose-conjugated monoclonal anti-Flag antibody, the HRP-conjugated anti-Flag, the secondary HRP-conjugated goat antimouse, goat antirabbit, and rabbit antigoat were from Sigma-Aldrich (St Louis, MO). Recombinant human VEGF-A was obtained from R&D Systems (Minneapolis, MN) and VEGF-C from ReliaTech (Braunschweig, Germany).
Sema3F, neuropilin 2 cDNA, and siRNA
An expression vector encoding human SEMA3F was purchased from Origene (Rockville, MD), and its sequence was confirmed prior to use. The NRP2 cDNA was amplified using the following oligonucleotides: 1, CCCAAGCTTCACCATGGATATGTTTCCTCT CACCTGG, and 2, AAAGTCGACTCATGCCTCGGAGCAGCACTTTTGG, cloned into the pcDNA-3-V5-Topo (Invitrogen, Carlsbad, CA), sequenced, and then subcloned into pCDNA3hygro. For the generation of Flag-tagged NRP2, a stop codon–deleted form of NRP2 was amplified with oligonucleotides 1 and 3, AAAGTCGACTGCCTCGGAGCAGCACTTTTGG; the PCR product was then digested with HindIII and SalI and cloned into HindIII and XhoI sites of pCMV4a (Stratagene, La Jolla, CA), in frame with the C-terminal Flag-tag and sequenced. NRP2 siRNA (catalog D-017721-03) and nontargeting control siRNA (catalog D-001210-01-20) were purchased from Dharmacon (Chicago, IL).
Cell culture and transfection
Human embryonic kidney 293T (HEK293T) cells were maintained in MEM supplemented with 10% fetal calf serum (FCS) and glutamine. The day before transfection, 106 cells were seeded on 10-cm Petri dishes, and transfection was done using Fugene-6 (Roche, Basel, Switzerland).
Porcine aortic endothelial cells (PAECs) were grown in DMEM supplemented with 5% FCS, glutamine, sodium pyruvate, and nonessential amino acid. For the generation of VEGFR-2/NRP-2–expressing stable PAEC clones, VEGFR-2 PAE cells (described previously by Alam et al40 ) were transfected with human pCDNAhygro–NRP-2, using the Lipofectamine method (Invitrogen-Life Technologies, Carlsbad, CA) and selected using the hygromycin agent (1 mg/mL). Clones were then established by limited dilutions, and expression of NRP2 was monitored by Western blot analysis.
Human microvascular endothelial cells (HMVECs; Cambrex Biosciences, Paris, France) and human umbilical endothelial cells (HUVECs; TEBU, Le Perray en Yvelines, France) were grown and transfected as previously described.38 Briefly, cells were mock transfected with 4 μg pCDNA3 plasmid or with pCDNA3-NRP2, using an Amaxa Nucleofector Kit (Amaxa, GmbH, Cologne, Germany). The day after electroporation, cells were harvested and plated for cell-survival assay. NRP-2 overexpression was confirmed by Western blot analysis.
For siRNA transfections, HMVECs were transfected with 100 nM of either control siRNA or NRP2 siRNA, using Amaxa Nucleofector Kit (Amaxa). Cells were then incubated for 36 hours at 37°C, 5% CO2 prior to the starting of endothelial cell migration assay.
Cell stimulation, immunoprecipitation, and Western blotting
Twenty-four hours after transfection, HEK293T cells were washed and starved overnight in a serum-free medium supplemented with 0.01 mg/mL BSA. Cells were stimulated or not for 10 minutes at 37°C, with the indicated concentration of VEGF-A or VEGF-C. After washing with ice-cold PBS, cells were resuspended in lysis buffer containing 150 mM NaCl, 20 mM Tris-HCl, pH 7.4, 10 mM EDTA, 1% Triton-100, 100 μM Na3VO4, and protease inhibitor cocktail (Sigma-Aldrich). For analysis of VEGFR-2 phosphorylation and interaction with NRP2 in PAECs, cells were seeded in Petri dishes (106 cells per dish) and incubated overnight at 37°C, 5% CO2. Cells were serum-starved for 2 hours and then incubated with the indicated doses of VEGF-A or VEGF-C for 10 minutes at 37°C. After stimulation, cells were washed with PBS containing 100 μM Na3VO4 and lysed in lysis buffer: 150 mM NaCl, 20 mM HEPES, 2.5 mM EDTA, 10% glycerol, 1% NP40, 100 μM Na3VO4, and protease inhibitor cocktail. The cell lysates were centrifuged at 10 000g for 15 minutes, and the supernatants were collected and subjected to immunoprecipitation and Western blot analysis.
Flag-tagged NRP2 was immunoprecipitated with anti–Flag-agarose (Sigma-Aldrich). Immunoprecipitation of VEGFR-2 was performed by using rabbit polyclonal anti–VEGFR-2 and protein-A/G-Agarose (Santa Cruz Biotechnology). Immunoprecipitates were washed 5 times with 1 mL lysis buffer. Proteins were eluted by incubation with sample buffer, boiling, electrophoresed on Novex polyacrylamide gels (Invitrogen, Carlsbad, CA), and transferred onto Hybond ECL nitrocellulose membranes (Amersham Pharmacia Biosciences, Piscataway, NJ). Following incubation with 5% nonfat milk in PBS, the membranes were incubated overnight with HRP–anti-Flag, HRP–anti-HA, HRP-PY20, anti-NRP2, or anti–VEGFR-2. NRP2 and VEGFR-2 were, respectively, revealed by using HRP-conjugated antimouse and antirabbit secondary antibodies. Membranes were revealed with enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biosciences).
Production of SEMA3F
HEK293T cells were transiently transfected with empty vector or SEMA3F expression vector using Fugene-6 (Roche). After 24 hours, medium was replaced with serum-free RPMI and incubated at 37°C for an additional 24 hours. Control medium and SEMA3F–containing medium were then collected; protease inhibitor cocktail (Sigma-Aldrich) was added and cleared by filtration through 0.2-μm filter unit (Nalgene, Rochester, NY). Control and SEMA3F media were concentrated 50 times by centrifugation on Centricon with a 30-KDa cutoff (Millipore, Billerica, MA) and immediately used for survival and migration assay.
Survival assay
HMVECs were seeded in 96-well plates coated with 0.3% gelatin (5 × 103 cells/well). Cells were incubated in RPMI 0.1% FCS with VEGF-A or VEGF-C at the indicated concentrations. Recombinant proteins were added again after 2 days. For survival assay concentrated conditioned medium, containing SEMA3F, or an equal volume of concentrated control medium (from empty vector transfected cells) was added after the cells adhered. Conditioned medium was added again after 2 days. Five days later, viable cells were quantified with the cell Titer-glo luminescent cell viability assay (Promega, Madison, WI).
Migration assay
The migration assay was performed with the BD BioCoat Angiogenesis System-Endothelial Cell Migration (BD Biosciences, San Jose, CA). HMVECs (1 × 105 cells/well in RPMI) were added in triplicate in the upper chambers with SEMA3F medium or an equal volume of empty vector control medium. The lower chamber was loaded with RPMI alone, RPMI with VEGF-A 50 ng/mL, or VEGF-C 100 ng/mL. After 24 hours at 37°C cells were labeled with calcein AM (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Fluorescence of cells that had migrated was measured on a TECAN GENios microplate reader (TECAN, Maennedorf, Switzerland).
Quantification of VEGFR-2 phosphorylation by ELISA
Quantification of VEGFR-2 phosphorylation by enzyme-linked immunosorbent assay (ELISA) was performed using VEGFR-2 and phospho–VEGFR-2 detection kits (R&D Systems) as described by the manufacturer. For PAECs, cells were seeded, stimulated, and lysed as described for immunoprecipitation. In the case of HMVECs, cells were transfected with control or NRP2 siRNA. The following day, 7 × 105 cells were seeded in 10-cm Petri dishes coated with 0.3% gelatin and incubated overnight at 37°C. Cells were then stimulated and lysed as described above under “Cell stimulation, immunoprecipitation, and Western blotting.” Total cell lysates were used for the quantification of the total amount of VEGFR-2 and the level of VEGFR-2 phosphorylation. The phosphorylation threshold was normalized to the total amount of VEGFR-2. Bars represent the ratio between normalized values of unstimulated and stimulated cells.
Results
Neuropilin 2 interacts with VEGFR-2 and VEGFR-3 in HEK293T cells
The fact that NRP2 and VEGFR-3 are coexpressed in lymphatic endothelial cells and that both receptors bind to VEGF-C has led to the speculation that NRP2 may act as a coreceptor for VEGFR-3.14 We recently showed that heterodimerization with VEGFR-2, which is also expressed on lymphatic cells and binds VEGF-C, is required for VEGFR-3 activity.40 Therefore, we evaluated the ability of NRP2 to associate with VEGFR-2 and VEGFR-3 on VEGF-A and -C stimulation.
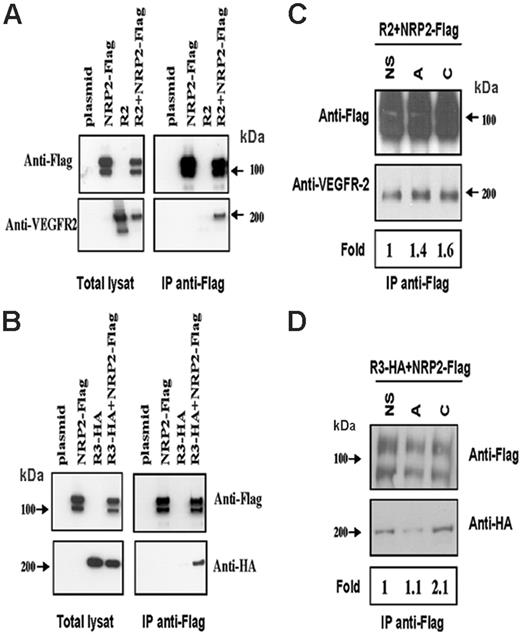
It has been described that NRP1 is able to associate with VEGFR-2 in a ligand-independent manner.19 A first set of experiments was designed to verify whether NRP2 would be able to associate spontaneously with either VEGFR-2 or VEGFR-3. For this purpose, Flag–NRP-2 was cotransfected with VEGFR-2 or with HA–VEGFR-3 in HEK293T cells. Flag-NRP2 was immunoprecipitated, and proteins were submitted to Western blot analysis. As shown in Figure 1A-B, anti-Flag antibodies were able to coimmunoprecipitate VEGFR-2 and VEGFR-3 from cells coexpressing NRP2-Flag but not from cells expressing either VEGFR-2 or VEGFR-3 alone.
We next tried to determine whether VEGF-A and VEGF-C are able to modulate these spontaneous interactions. To this end, cotransfected HEK293T cells were either left untreated or stimulated with VEGF-A or -C. The resulting Western blots and the quantification of the bands shown in Figure 1C indicate that stimulation with VEGF-A or VEGF-C slightly increases the VEGFR-2/Flag-NRP2 association. However, HA-VEGFR-3/Flag–NRP2 association was slightly increased in the presence of VEGF-C but not in the presence of VEGF-A (Figure 1D). Similar results were obtained when specific antibodies against HA–VEGFR-3 were used for immunoprecipitation (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
NRP-2 associates with VEGFR-2 and VEGFR-3 in a ligand-dependent manner in HEK cells. HEK cells were transfected with an empty vector (plasmid), NRP-2–Flag, VEGFR-2, HA–VEGFR-3 or cotransfected with NRP-2–Flag and either VEGFR-2 or VEGFR-3HA. Cells were either left unstimulated (A-B) or stimulated (C-D) with VEGF-A 10 ng/mL (lane A) or VEGF-C 100 ng/mL (lane C) for 10 minutes at 37°C. After immunoprecipitation with anti-Flag antibody, the receptors were immunoblotted with anti-Flag and anti–VEGFR-2 (A,C) or with anti-Flag and anti-HA (B,D). Bands were quantified and normalized to total amount of NRP-2 and then compared with unstimulated cells. The resulting fold increase of VEGFR-2/NRP-2 and VEGFR-3/NRP-2 association in response to VEGF-A and VEGF-C is indicated below the Western blots. Results are representative of 3 independent experiments with similar results.
NRP-2 associates with VEGFR-2 and VEGFR-3 in a ligand-dependent manner in HEK cells. HEK cells were transfected with an empty vector (plasmid), NRP-2–Flag, VEGFR-2, HA–VEGFR-3 or cotransfected with NRP-2–Flag and either VEGFR-2 or VEGFR-3HA. Cells were either left unstimulated (A-B) or stimulated (C-D) with VEGF-A 10 ng/mL (lane A) or VEGF-C 100 ng/mL (lane C) for 10 minutes at 37°C. After immunoprecipitation with anti-Flag antibody, the receptors were immunoblotted with anti-Flag and anti–VEGFR-2 (A,C) or with anti-Flag and anti-HA (B,D). Bands were quantified and normalized to total amount of NRP-2 and then compared with unstimulated cells. The resulting fold increase of VEGFR-2/NRP-2 and VEGFR-3/NRP-2 association in response to VEGF-A and VEGF-C is indicated below the Western blots. Results are representative of 3 independent experiments with similar results.
These results clearly indicate that NRP2 can associate with both VEGFR-2 and VEGFR-3 in a ligand-dependent as well as in a ligand-independent manner. Even though the level of the VEGF-induced heterodimerization is not very high, it is experimentally reproducible. This may be due to the high level of spontaneous association between NRP2 and VEGFR-2 or VEGFR-3, resulting from the transient overexpression in HEK cells. This high amount of basal interaction might be therefore difficult to enhance.
Neuropilin 2 enhances the VEGFR-2 phosphorylation threshold induced by VEGF-A and VEGF-C in endothelial cells
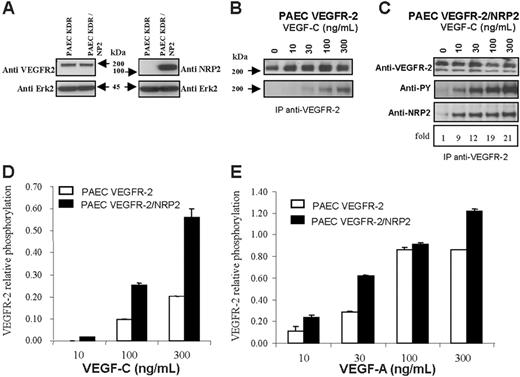
We next investigated whether the association between VEGFR-2 and NRP2 could be more efficiently induced in endothelial cells than in HEK cells. For this purpose, we used human VEGFR-2–expressing PAECs that we further stably transfected with NRP2. Western blot analysis revealed that the level of VEGFR-2 was unaffected by NRP2 transfection (Figure 2A).
PAEC–VEGFR-2 and PAEC–VEGFR-2/NRP2 cells were left untreated or stimulated with increasing concentrations of VEGF-C. VEGFR-2 was immunoprecipitated, and proteins were submitted to Western blot analysis. In PAEC VEGFR-2/NRP2 transfectants, the association between VEGFR-2 and NRP2 was very low in the absence of ligand. This association was highly enhanced in a dose-dependent manner, up to 20 times, by VEGF-C (Figure 2C). This result confirmed those obtained in HEK cells and indicated that this ligand-dependent association is much more efficient in endothelial cells than in HEK cells.
NRP2/VEGFR-2 interaction enhances VEGFR-2 phosphorylation induced by VEGF-A and VEGF-C in PAECs. PAECs stably expressing VEGFR-2 were transfected with NRP2. (A) Western blot analysis of VEGFR-2 and NRP2 expression in VEGFR-2– and VEGFR-2/NRP-2–expressing cells. The blot was cut around 70 kDa; the top part was incubated with either anti–VEGFR-2 or anti-NRP2 and the bottom part with anti-Erk antibodies. (B-C) Phosphorylation of VEGFR-2 induced by VEGF-C in cells coexpressing NRP2 (C) or not (B). Cells were stimulated with the indicated concentration of VEGF-C for 10 minutes at 37°C. Cell lysates were immunoprecipitated with anti–VEGFR-2 antibodies. Samples from PAEC VEGFR-2 and PAEC VEGFR-2/NRP2 were run on 2 separate gels, but membranes were exposed together on the same film for the same amount of time. Proteins were immunoblotted with anti-phosphotyrosine (PY), anti–VEGFR-2, or anti-NRP2 (C). The values below the anti-NRP2 indicate the fold increase in the association between NRP2 and VEGFR-2 in response to VEGF-C. (D-E) Phosphorylation of VEGFR-2 was quantified by ELISA, and specific signal was normalized to the total amount of VEGFR-2 which was also measured by ELISA. Cells were stimulated with the indicated concentration of either VEGF-A or VEGF-C as described in panels B and C. Results are representative of 2 independent experiments, and data are shown as mean ± SEM.
NRP2/VEGFR-2 interaction enhances VEGFR-2 phosphorylation induced by VEGF-A and VEGF-C in PAECs. PAECs stably expressing VEGFR-2 were transfected with NRP2. (A) Western blot analysis of VEGFR-2 and NRP2 expression in VEGFR-2– and VEGFR-2/NRP-2–expressing cells. The blot was cut around 70 kDa; the top part was incubated with either anti–VEGFR-2 or anti-NRP2 and the bottom part with anti-Erk antibodies. (B-C) Phosphorylation of VEGFR-2 induced by VEGF-C in cells coexpressing NRP2 (C) or not (B). Cells were stimulated with the indicated concentration of VEGF-C for 10 minutes at 37°C. Cell lysates were immunoprecipitated with anti–VEGFR-2 antibodies. Samples from PAEC VEGFR-2 and PAEC VEGFR-2/NRP2 were run on 2 separate gels, but membranes were exposed together on the same film for the same amount of time. Proteins were immunoblotted with anti-phosphotyrosine (PY), anti–VEGFR-2, or anti-NRP2 (C). The values below the anti-NRP2 indicate the fold increase in the association between NRP2 and VEGFR-2 in response to VEGF-C. (D-E) Phosphorylation of VEGFR-2 was quantified by ELISA, and specific signal was normalized to the total amount of VEGFR-2 which was also measured by ELISA. Cells were stimulated with the indicated concentration of either VEGF-A or VEGF-C as described in panels B and C. Results are representative of 2 independent experiments, and data are shown as mean ± SEM.
We then analyzed the effect of NRP2 expression on the threshold of VEGFR-2 phosphorylation. As shown in Figure 2B, in PAEC–VEGFR-2 the detection of VEGFR-2 phosphorylation required stimulation with at least 30 ng/mL VEGF-C. This result was systematically obtained in all repeat experiments. The level of VEGFR-2 autophosphorylation increased therefore in parallel with the concentration of VEGF-C. These results indicate that VEGFR-2 responds in a dose-dependent manner to VEGF-C stimulation. These experiments were confirmed by using an ELISA test to quantify VEGFR-2 phosphorylation on stimulation by VEGF-C (Figure 2D). As expected, similar results were also obtained after stimulation with VEGF-A (Figure 2D). In the presence of NRP2, the phosphorylation threshold of VEGFR-2 was lowered. Indeed, under these conditions, phosphorylation already appeared with 10 ng/mL VEGF-C (Figure 2C-D). In addition, for higher concentrations of VEGF-C, the level of phosphorylation was greatly enhanced in VEGFR-2/NRP2 PAECs compared with VEGFR-2 transfectants. Similar results were obtained when VEGFR-2 phosphorylation was quantified by ELISA (Figure 2D). Furthermore, a similar pattern of phosphorylation was also observed after stimulation with VEGF-A (Figure 2E). These findings show that the interaction of NRP2 with VEGFR-2 enhances VEGFR-2 phosphorylation induced by VEGF-Aand VEGF-C in porcine aortic endothelial cells.
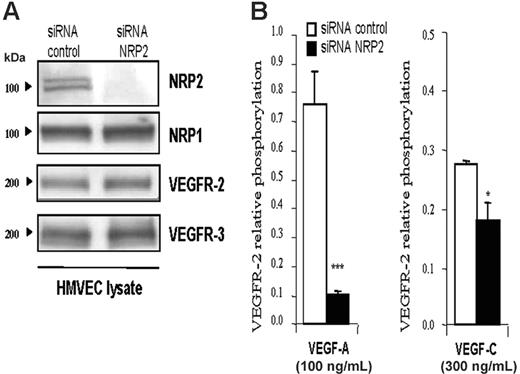
Neuropilin 2 knock-down inhibits VEGFR-2 phosphorylation induced by VEGF-A and VEGF-C in primary human endothelial cells. To determine whether NRP2 is also able to act on VEGFR-2 phosphorylation in primary human endothelial cells, we used HMVECs. Usually, HMVECs represent a mixture of endothelial and lymphatic cells. Phenotypic analysis by Western blot (Figure 3A) and flow cytometry (Figure S2) indicated that the majority of these cells have a lymphatic phenotype because they express VEGFR-2, VEGFR-3, and neuropilin-2. They also express both lymphatic-specific markers Prox1 and Lyve1 (Figure S2). Moreover, these cells do not express VEGFR-1 and do not respond to PLGF stimulation (Figures S2 and S3). Thus, because NRP-2 has been involved in lymphatic vessel formation, these HMVECs constitute a relevant and useful tool to study the role of NRP2 in lymphatic function. Therefore, we first knocked-down NRP2 in HMVECs using the siRNA technique, and VEGFR-2 phosphorylation was quantified by ELISA.
The efficiency and the specificity of NRP2 siRNA were analyzed by Western blot. Figure 3A shows that NRP2 siRNA greatly inhibited NRP2 expression in HMVECs. This knock-down was specific to NRP2, because NRP-1, VEGFR-2, and VEGFR-3 expression was not affected (Figure 3A). siRNA control had no effect on any of these genes.
As shown in Figure 3B, stimulation with VEGF-A and VEGF-C induced phosphorylation of VEGFR-2 in HMVECs transfected with control siRNA. In contrast, knock-down of NRP2 inhibited the phosphorylation of VEGFR-2 induced by both ligands (Figure 3B). The inhibition of VEGFR-2 phosphorylation by blunting NRP2 was reproduced in 4 independent experiments with VEGF-C and in 2 experiments with VEGF-A (Figure S4). However, the level of VEGFR-2 phosphorylation in response to VEGF-A and VEGF-C and the extent of inhibition of this response by NRP2-specific siRNA varied between experiments. These variations were due to differences in the state of these primary cells and in siRNA transfection efficiency. Similar inhibition results were also obtained when SEMA3F was used to inhibit VEGFR-2 phosphorylation induced by VEGF-A and -C (data not shown). These findings indicate that the presence of NRP2 in HMVECs is required to reach optimal phosphorylation of VEGFR-2 induced by VEGF-A and VEGF-C, as is the case in PAECs.
NRP2 enhances the VEGFR-2 phosphorylation threshold induced by VEGF-A and VEGF-C in HMVECs. (A) Immunoblot analysis of HMVECs transfected with control or NRP2 siRNA (100 nM each). Thirty-six hours after transfection, cells were lysed, and 20 μg total protein extracts were submitted to Western blot analysis with the indicated antibody. (B) Level of VEGFR-2 phosphorylation induced by VEGF-A (100 ng/mL) or VEGF-C (300 ng/mL) in HMVECs transfected with control or NRP2 siRNA. Phosphorylation was quantified by ELISA as described in Figure 2. Data are plotted as mean ± SEM. The statistical analysis of differences in VEGF-induced responses between control siRNA and NRP2 siRNA-transfected cells was carried out using ANOVA (*P ≤ .05; ***P < .001). Similar results were obtained in 2 independent experiments with VEGF-A and 4 with VEGF-C.
NRP2 enhances the VEGFR-2 phosphorylation threshold induced by VEGF-A and VEGF-C in HMVECs. (A) Immunoblot analysis of HMVECs transfected with control or NRP2 siRNA (100 nM each). Thirty-six hours after transfection, cells were lysed, and 20 μg total protein extracts were submitted to Western blot analysis with the indicated antibody. (B) Level of VEGFR-2 phosphorylation induced by VEGF-A (100 ng/mL) or VEGF-C (300 ng/mL) in HMVECs transfected with control or NRP2 siRNA. Phosphorylation was quantified by ELISA as described in Figure 2. Data are plotted as mean ± SEM. The statistical analysis of differences in VEGF-induced responses between control siRNA and NRP2 siRNA-transfected cells was carried out using ANOVA (*P ≤ .05; ***P < .001). Similar results were obtained in 2 independent experiments with VEGF-A and 4 with VEGF-C.
Neuropilin-2 overexpression enhances VEGF-A– and VEGF-C–induced survival of human endothelial cells
Transgenic mice with targeted deletions in both Nrp1 and Nrp2 had a more severe abnormal vascular phenotype than either Nrp1 or Nrp2 single knockouts and died in utero at E8.5.33 Mice deficient for one Nrp gene but heterozygous for the other one died at E10 to E10.5.33 These results suggest that both NRPs are required and should be expressed at sufficient levels for normal vessel development. This is also confirmed by the fact that transgenic mice overexpressing neuropilin 1 show hypervascularization.29
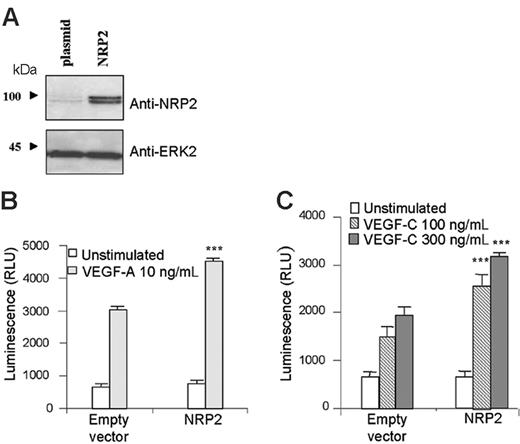
To determine the role of NRP2 in primary human endothelial cells, we overexpressed NRP2 in HMVECs (Figure 4A) and investigated its effect on human endothelial cell survival. HMVECs were transfected with NRP2 cDNA or its corresponding empty vector. Cells were then left unstimulated or stimulated with VEGF-A or VEGF-C. Five days later, the amount of surviving cells was quantified using the cell Titer-glo luminescent cell viability assay.
VEGF-A and VEGF-C induced survival of HMVECs transfected with empty vector (Figure 4B-C). Interestingly, NRP2 overexpression increased cell survival induced by VEGF-A and VEGF-C (Figure 4B-C). In agreement with previous experiments, these results indicate that the level of NRP2 expression is important in the survival of HMVECs in response to VEGF-A and VEGF-C.
NRP2 overexpression enhances human endothelial cell survival induced by VEGF-A and VEGF-C. HMVECs were transfected with either empty vector (plasmid) or vector containing NRP2. (A) The level of NRP2 expression was assessed by Western blot analysis. (B-C) For survival assay, cells were seeded in triplicate in gelatinized 96-well plates in RPMI containing 0.1% FCS and stimulated with either VEGF-A 10 ng/mL (B) or VEGF-C 100 ng/mL and 300 ng/mL (C). Data are plotted as mean ± SEM. The statistical analysis of differences in VEGF-stimulated responses between empty vector and NRP2-transfected cells was carried out by using ANOVA (***P < .001). Results are representative of 3 independent experiments.
NRP2 overexpression enhances human endothelial cell survival induced by VEGF-A and VEGF-C. HMVECs were transfected with either empty vector (plasmid) or vector containing NRP2. (A) The level of NRP2 expression was assessed by Western blot analysis. (B-C) For survival assay, cells were seeded in triplicate in gelatinized 96-well plates in RPMI containing 0.1% FCS and stimulated with either VEGF-A 10 ng/mL (B) or VEGF-C 100 ng/mL and 300 ng/mL (C). Data are plotted as mean ± SEM. The statistical analysis of differences in VEGF-stimulated responses between empty vector and NRP2-transfected cells was carried out by using ANOVA (***P < .001). Results are representative of 3 independent experiments.
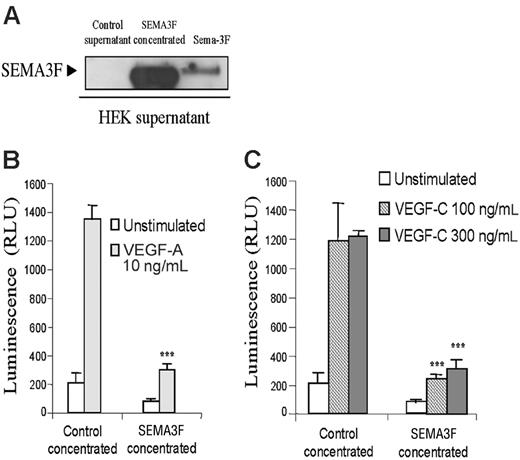
SEMA3F inhibits HMVEC survival induced by VEGF-A and VEGF-C. (A) Supernatants from HEK cells transfected with empty vector or SEMA3F were analyzed by Western blot analysis. Twenty-four hours after transfection, supernatants were harvested and replaced with serum-free media for additional 24 hours. Supernatants were then collected, centrifuged, and filtered prior to concentration. An aliquot containing 20 μg total proteins was analyzed by Western blot. (B-C) Survival assay of HMVECs stimulated in the presence of control or SEMA3F-concentrated medium. Cells were seeded in triplicate in gelatinized 96-well plates in medium containing 0.1% FCS. Cells were then left unstimulated or stimulated with VEGF-A 10 ng/mL (B) or VEGF-C 100 and 300 ng/mL (C) in the presence of control- or SEMA3F-conditioned media (***P < .001 versus the respective stimulation of cells incubated with the control medium). Data are shown as mean ± SEM. Results are representative of 2 independent experiments.
SEMA3F inhibits HMVEC survival induced by VEGF-A and VEGF-C. (A) Supernatants from HEK cells transfected with empty vector or SEMA3F were analyzed by Western blot analysis. Twenty-four hours after transfection, supernatants were harvested and replaced with serum-free media for additional 24 hours. Supernatants were then collected, centrifuged, and filtered prior to concentration. An aliquot containing 20 μg total proteins was analyzed by Western blot. (B-C) Survival assay of HMVECs stimulated in the presence of control or SEMA3F-concentrated medium. Cells were seeded in triplicate in gelatinized 96-well plates in medium containing 0.1% FCS. Cells were then left unstimulated or stimulated with VEGF-A 10 ng/mL (B) or VEGF-C 100 and 300 ng/mL (C) in the presence of control- or SEMA3F-conditioned media (***P < .001 versus the respective stimulation of cells incubated with the control medium). Data are shown as mean ± SEM. Results are representative of 2 independent experiments.
VEGF-A– and VEGF-C–induced human endothelial cell survival is inhibited by semaphorin-3F
Semaphorin-3F has been shown to inhibit HUVEC survival induced by VEGF-A.36 Because NRP2 binds VEGF-A but also VEGF-C, we tested whether SEMA3F would be able to inhibit endothelial cell survival induced by VEGF-C.
SEMA3F was produced in the supernatant after transient expression in HEK293T cells. The supernatant from empty vector (control supernatant) or SEMA3F-transfectant cells was cleared, concentrated, and submitted to Western blot analysis (Figure 5A), prior to use on endothelial cells. HMVECs were incubated with control medium or with concentrated SEMA3F-conditioned medium, containing either vehicle, VEGF-A, or VEGF-C. In agreement with results obtained by Kessler et al,36 Figure 5B shows that Sema-3F inhibits VEGF-A–induced cell survival. Moreover, Sema-3F was also able to inhibit HMVEC survival induced by VEGF-C (Figure 5C). These data indicate that binding of semaphorin-3F to NRP-2 is able to inhibit VEGF-A– and VEGF-C–induced endothelial cell survival, suggesting a major role of NRP-2 in endothelial cell physiology. However, the NRP2-specific siRNA failed to inhibit HMVEC proliferation. This may be due to technical problems resulting from the stability of the siRNA. Indeed, the proliferation assay requires 7 days of stimulation, and, by using FITC-conjugated siRNA, we observed that the higher intracellular level of siRNA is obtained between 24 and 48 hours (Figure S5). These times are compatible with the phosphorylation and the migration experiments but unfortunately not with the survival assay. Thus, this result cannot exclude a role of NRP2 on cell survival.
VEGF-A– and VEGF-C–induced HMVEC migration is inhibited by semaphorin 3F
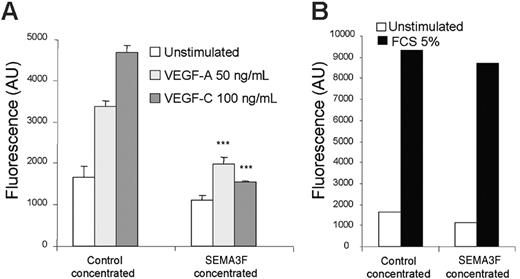
It is well established that the binding of semaphorin 3F on NRP2 induces neurite repulsion in neuronal cells.42,43 A similar phenomenon was also recently described in human endothelial cells.35 The molecular mechanism involved in this cellular response requires F-actin depolymerization, which is also involved in cell motility.23,44 Therefore, we tested the effect of semaphorin-3F on VEGF-A and VEGF-C–induced endothelial cell migration. In contrast to control medium, SEMA3F-containing medium clearly inhibited VEGF-A and VEGF-C–induced cell migration (Figure 6A). Interestingly, fetal calf serum–induced migration was not inhibited by SEMA3F, demonstrating that its action was directed specifically toward VEGF-A and VEGF-C signaling (Figure 6B). The fact that semaphorin-3F, a specific NRP ligand, inhibits endothelial cell survival and migration induced by VEGF-A and VEGF-C strongly suggests that NRP2 plays a pivotal role in VEGF signaling mediated through VEGFR-2 and VEGFR-3.
Neuropilin-2 knock-down inhibits VEGF-A– and VEGF-C–induced HMVEC migration
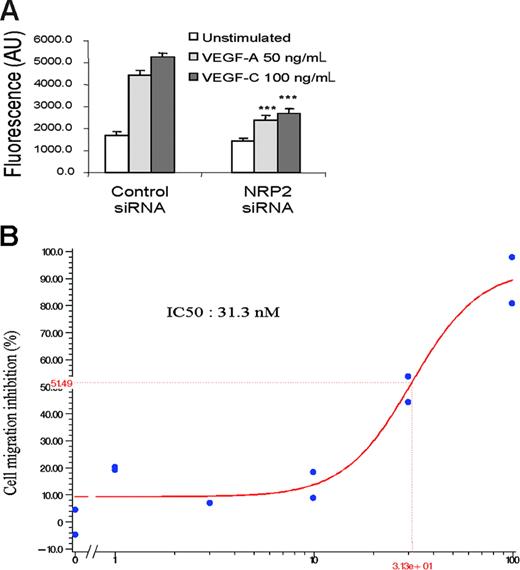
The results obtained using SEMA3F as a specific antagonist of VEGFs on NRP receptors suggest that SEMA3F may block VEGF-A and VEGF-C signaling by acting on NRP2. Another possibility could be that SEMA3F might interfere with the binding of these ligands to other receptors such as NRP1, which is also able to bind SEMA3F with a 10-fold lower affinity than NRP2.43 To distinguish between these 2 possibilities, a migration assay has been conducted on HMVECs after silencing of the NRP2 gene. HMVECs transfected with control siRNA showed migration in the presence of VEGF-A and VEGF-C, indicating that siRNA transfection does not affect migration of HMVECs (Figure 7A). In contrast, the knock-down of NRP2 resulted in strong inhibition of VEGF-A– and VEGF-C–induced migration of HMVECs, thus confirming the results obtained using Sema-3F (Figure 7A).
SEMA3F inhibits HMVEC migration induced by VEGF-A and VEGF-C. HMVECs were seeded in triplicate in the upper wells of fluoroblok chambers in the presence of either control or SEMA3F–concentrated media. RPMI alone or RPMI containing either VEGF-A (50 ng/mL) or VEGF-C (100 ng/mL) (A) was added in the bottom wells. Data are shown as mean ± SEM. ***P < .001 versus the respective stimulation of cells incubated with the control media. (B) The bottom wells were filled with RPMI containing 5% FCS. The day after, cells were labeled with calcein, and the fluorescence corresponding to migrating cells was quantified. Results are representative of 2 independent experiments.
SEMA3F inhibits HMVEC migration induced by VEGF-A and VEGF-C. HMVECs were seeded in triplicate in the upper wells of fluoroblok chambers in the presence of either control or SEMA3F–concentrated media. RPMI alone or RPMI containing either VEGF-A (50 ng/mL) or VEGF-C (100 ng/mL) (A) was added in the bottom wells. Data are shown as mean ± SEM. ***P < .001 versus the respective stimulation of cells incubated with the control media. (B) The bottom wells were filled with RPMI containing 5% FCS. The day after, cells were labeled with calcein, and the fluorescence corresponding to migrating cells was quantified. Results are representative of 2 independent experiments.
NRP2 knock-down inhibits HMVEC migration induced by VEGF-A and VEGF-C. (A) Migration assay of HMVECs transfected with control or NRP-2 siRNA. After 36 hours, transfected cells were seeded in triplicate in the upper wells of a fluoroblok chamber. RPMI alone or containing VEGF-A 50 ng/mL or VEGF-C 100 ng/mL was added in the bottom wells. The amount of migrating cells was quantified using calcein labeling. Data are shown as mean ± SEM. (B) HMVEC migration induced by VEGF-C 100 ng/mL in the presence of increasing doses (1-100 nM) of CEP5214 kinase inhibitor. Results are representative of 3 independent experiments.
NRP2 knock-down inhibits HMVEC migration induced by VEGF-A and VEGF-C. (A) Migration assay of HMVECs transfected with control or NRP-2 siRNA. After 36 hours, transfected cells were seeded in triplicate in the upper wells of a fluoroblok chamber. RPMI alone or containing VEGF-A 50 ng/mL or VEGF-C 100 ng/mL was added in the bottom wells. The amount of migrating cells was quantified using calcein labeling. Data are shown as mean ± SEM. (B) HMVEC migration induced by VEGF-C 100 ng/mL in the presence of increasing doses (1-100 nM) of CEP5214 kinase inhibitor. Results are representative of 3 independent experiments.
To further confirm the role of VEGFRs/NRP2 complexes in VEGF-A and VEGF-C signaling, we used CEP5214, a potent VEGFR tyrosine-kinase inhibitor.45 CEP5214 inhibited in a dose-dependent manner VEGF-A– and VEGF-C–induced migration (IC50 of about 30 nM) (Figure 7B). These results confirm that signaling through the complex formed by NRP2 and VEGFRs is required for an optimal endothelial cell migration induced by VEGF-A and VEGF-C.
Discussion
Although knock-out mice for NRP2 have deficiencies in small veins and lymphatic vessels,14 data reporting the biological function of neuropilins in adult endothelial cells concern almost exclusively NRP1. In this study, we have investigated the role of NRP2 in human primary endothelial cells. Our data show that NRP2 interacts with VEGFR-2 and VEGFR-3 in a ligand-dependent as well as ligand-independent manner. This interaction results in the lowering of the activation threshold of VEGFR-2, thus making endothelial cells more sensitive to VEGF-A and VEGF-C. Using different approaches to overexpress or inhibit NRP2 function, we show that NRP2 is critical for optimal VEGFR-2 phosphorylation, as well as endothelial cell survival and migration induced by VEGF-A and VEGF-C. In complement to previous work, our results demonstrate for the first time the crucial role of NRP2 in endothelial cell survival and migration and provide new insights on the biochemical events involved in these mechanisms.
Our results support the idea that like NRP1, NRP2 acts as a coreceptor for VEGFRs. Although NRP2 was previously shown to interact with VEGFR-1, neither the functional consequences nor the physiologic relevance of this interaction were documented.34 Moreover, nothing is known about its interaction with either VEGFR-2 or VEGFR-3. On the basis of the coexpression of NRP2 and VEGFR-3 in lymphatic cells and because both proteins bind VEGF-C, it has been speculated that NRP2 may interact with VEGFR-3.14 Because VEGFR-2 is always present on endothelial cells expressing VEGFR-3, we demonstrate for the first time that NRP2 interacts not only with VEGFR-3 but also with VEGFR-2. Interestingly, the interaction of neuropilin 2 with VEGFR-2 was increased by VEGF-A and VEGF-C, whereas its interaction with VEGFR-3 was only increased by VEGF-C. This observation indicates that the interaction between VEGFR and NRP2 is potentiated by a coligand, suggesting that NRP2 acts as a coreceptor of both VEGFRs. This conclusion is further strengthened by the fact that overexpression of NRP2 lowered the threshold concentrations of VEGF-A and VEGF-C, which were required to induce VEGFR-2 phosphorylation and conversely knocking-down NRP2 lowered the level of VEGFR-2 phosphorylation.
The role of NRP1 as coreceptor of VEGFR-2 in response to VEGF-A was initially demonstrated in PAECs stably transfected with NRP1 and VEGFR-2.21 In this system, it was shown that NRP1 could enhance migration induced by VEGF-A.21 Moreover, semaphorin-3A, a competitor of VEGF-A binding to NRP1, was also shown to inhibit migration of endothelial cells.23 However, the question of whether the interaction of NRP2 with VEGFRs may act in a similar way still remained open. We addressed this question using an NRP2 ligand: SEMA3F, a competitor which completely inhibited cell migration and confirmed that SEMA3F acts through inhibition of NRP2, because the same results were obtained by knocking-down NRP2. The fact that both NRP1 and NRP2 acted as modulators indicates that they could be either sequentially or together associated with VEGFR-2.
The effect of neuropilin overexpression on human primary endothelial cells has not been studied up to now. However, it was shown previously that mice embryos transgenic for NRP1 exhibit excess capillaries and blood vessels29 and that SEMA3F, which binds preferentially NRP2, inhibits HUVEC survival induced by VEGF-A.36 Here, we show that overexpression of NRP2 in HMVECs enhances cell survival induced by VEGF-A and VEGF-C. Moreover, survival induced not only by VEGF-A but also by VEGF-C was inhibited by SEMA3F. These data confirm that NRP2 has a pivotal role in VEGF-induced endothelial cell responses. In agreement with our results, Shen et al46 have recently shown that NRP2 plays a critical role in VEGF-A–mediated angiogenesis in vivo. In that report, the researchers showed that in NRP2-deficient mice, VEGF-A–induced retinal angiogenesis is strongly inhibited. These experiments suggest that NRP2 has an important role in VEGF-A–induced endothelial cell activation not only in vitro but also in vivo.46
Altogether, our results demonstrate that NRP2 acts as a coreceptor for VEGFRs in response to VEGF-A and VEGF-C. The association of NRP2 with VEGFR-2 may enhance the signaling pathways induced by VEGF-A and VEGF-C by different manners. NRP2 might lower the affinity of VEGFR-2 for VEGF-A and VEGF-C and thus amplify the ligand-induced response. The cytoplasmic tail of NRP2 may also be involved and would allow the recruitment of components essential for VEGFR-2 complex formation, stabilization, and signaling. However, the results presented here do not exclude that NRP2 and SEMA3F may also act through plexins or integrins to deliver a negative signal to the endothelial cell. A concept that recently has emerged in the area of signaling research is that the receptor/ligand interaction can initiate the formation of a multimolecular complex named signalosome.47 The formation of a complete signalosome would allow to control, enhance, and sustain a proper signaling. It cannot be excluded that in HMVECs, VEGF-A and VEGF-C may induce the formation of a multimolecular complex that includes VEGFR-2, neuropilin 2, phosphatases, integrins, plexins, and other proteins. In our case, by disturbing the integrity of neuropilin-2, we may have impaired the formation of the signalosome induced on VEGF-A and VEGF-C stimulation.
VEGFs and bFGF are well-established activators of endothelial integrin function both in vivo and in vitro.48-52 It has recently been demonstrated that SEMA3F inhibits the mitogenic effects of VEGF-A165 and bFGF on HUVECs.36 Inhibition of bFGF-induced proliferation did not seem to rely on the inhibition of the binding of bFGF to its receptors or on the up-regulation of VEGF or VEGFR-2 expression, as demonstrated previously.53,54 Moreover, SEMA3A inhibited both VEGF-A– and bFGF-enhanced adhesion of endothelial cells to fibronectin. On the contrary, disruption of SEMA3 function in endothelial cells stimulates integrin-mediated adhesion and migration to extracellular matrices,55 suggesting a physiologic role for Sema-3/NRP proteins in regulating integrin-mediated adhesion of endothelial cells.
In conclusion, these data demonstrate for the first time an important role of NRP2 in endothelial cell function in association with VEGF receptors and perhaps with plexins and integrins. How all these receptors work together is an interesting question to be clarified to better understand the mechanisms underlying angiogenesis and lymphangiogenesis and to design new drugs to better control these phenomena during autoimmune diseases and tumor development. In this regard, our results strongly suggest that NRP-2 represents an interesting pharmacologic target.
Prepublished online as Blood First Edition Paper, April 18, 2006; DOI 10.1182/blood-2005-11-4447.
B.F. and A.A. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Bruce Ruggeri (Cephalon) for providing us with CEP5214.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal