Abstract
The laminins are a group of extracellular matrix proteins with constitutive expression in all tissues, including bone marrow stroma. A functional role for the nonintegrin laminin receptor p67 has been described for cancer metastasis and lymphocyte trafficking. Expression of p67 was also reported for other subsets of mature leukocytes and for malignant hematopoietic or nonhematopoietic cells. We explored p67 expression on normal hematopoietic progenitor cells (HPCs) and its putative role in bone marrow retention of transplanted HPCs. We found p67 expression on a subset of primary human CD34+ cells coexpressing erythroid markers. Of importance, p67 recognizes early erythroid progenitors, since sorted p67+ cells were significantly enriched for burst-forming units–erythroid (BFU-Es) and depleted of colony-forming units–granulocyte/macrophage (CFU-GMs). Blockade of p67 binding of donor cells, using antifunctional antibody, reduced bone marrow homing of BFU-Es. These studies identify p67 as a novel phenotypic marker for erythroid HPCs of functional importance for lineage-specific homing/retention among adult transplanted HPCs.
Introduction
Homing of mature immunoregulatory cells to sites of inflammation follows a well-characterized sequence of rolling, firm adhesion, and transmigration. Although some of the same major players in this cascade are also involved in hematopoietic progenitor cell (HPC) homing to bone marrow (BM), differences in the hierarchy of molecular pathway usage exist, likely because of different tissue microenvironments and receptor repertoires.1,2 α4/β1-integrin/VCAM-1 and CXCR4/SDF-1 are dominant pathways of hematopoietic–stromal cell interactions, important for retention of transplanted HPCs in BM.3-5 In addition, an array of disparate dominant and ancillary molecules,1,2 which work within a complex, interactive network, is involved in BM homing.6,7 Within BM, lineage-committed HPCs and stem cells are likely partitioned in different functional/spatial “niches,”8,9 to encounter an environment accommodating their specific functional requirements. How these niches are defined and the specific molecular interactions between seeded HPCs and their supportive microenvironmental niches are incompletely understood.
Laminins are expressed in BM, and cognate receptors are expressed on HPCs, as demonstrated by HPC adhesion to laminin.10-12 Instrumental roles of the nonintegrin laminin receptor p67 were described in metastasis, embryo implantation, and lymphocyte trafficking.13,14 p67-mediated laminin binding was also reported for subsets of normal and malignant hematopoietic cells.15-18 Expression of p67 on HPCs and its role in hematopoiesis were not previously addressed. Our present study shows that p67 recognizes erythroid progenitors/precursors, and that its inhibition blocks BM homing of BFU-Es.
Study design
Animals and conditioning regimen
NOD/SCIDβ2microglobulin–/– mice (Jackson Laboratories, Bar Harbor, ME), housed and bred in the Helicobacter-free section of the Specific-Pathogen-Free vivarium at University of Washington, with water and chow ad libitum, served as recipients. Mice were lethally irradiated and received CD34+ cell transplants as described,7 in accordance with procedures approved by the University of Washington IACUC.
Cells
Cryopreserved cadaveric BM-derived or healthy volunteer-donor mobilized peripheral blood (MPB)–derived CD34+ cells were received from the NIH repository (Fred Hutchinson Cancer Research Center) with permission of the University of Washington IRB. Cells were thawed and incubated overnight with 25 ng/mL recombinant human stem cell factor (rhuSCF; Peprotech, Rocky Hill, NJ).7
Flow cytometry and sorting
Fluorescence-activated cell sorter (FACS) analysis was performed according to standard protocols, using anti-p67 antibody (anti-p67Ab) (MLuC5; NeoMarkers, Fremont, CA) with Alexa 488–labeled rat anti–mouse IgM secondary antibody (MolecularProbes, Eugene, OR) or secondary antibody alone, and PE- or APC-labeled anti–glycophorin A, anti-CD34, anti-CD45, anti-CD11a, anti-CD71, or isotype-control antibodies (BD Pharmingen, San Diego, CA). Antigen expression was quantified using the FACSCalibur (BD Immunocytometry Systems, San Jose, CA). p67+ and p67– cell populations were isolated on the FACSAria (BD Immunocytometry Systems). Flow data were analyzed with CellQuest software (BD Immunocytometry Systems) for OS10.
Colony-forming cells in culture (CFU-Cs) assay
Colony assays were performed as described and evaluated for CFU-GMs, CFU-mixed, and BFU-Es after 14 days.19 Methyl cellulose colony-assay medium was from StemCell Technologies (Vancouver, BC) or Miltenyi Biotech (Auburn, CA). Colonies were picked on days 7 and 14 to evaluate p67 expression in early and late erythroid and nonerythroid precursor cells.20 Plasma-clot assays were done to enumerate BFU-Es after benzidine staining, as described.21
Homing assay
Statistical analysis
Descriptive statistics and t tests were calculated using Excel (Microsoft, Redmond, WA). A P value less than .05 was considered statistically significant.
Results and discussion
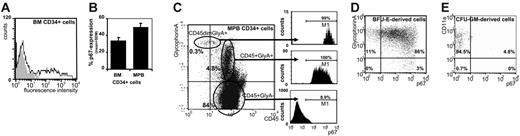
Using FACS, we found p67 expression on 34% ± 4% of primary CD34+ BM cells (mean ± SEM), with a distinct negative fraction, and a wide shoulder of p67+ cells (Figure 1A). The curve shape for p67 expression on MPB CD34+ cells was similar, but the percentage of p67+ cells was greater than in BM cells (Figure 1B). Further FACS analysis revealed that although the number of glycophorin A+ cells among the CD34+p67+ cells was small, p67 expression on this population (CD34+ glycophorin A+) reached almost 100% (Figure 1C). Similarly, almost all CD34+CD71high BM cells (93.9%) expressed p67 (not shown). Compared with MPB cells, the number of CD34+ glycophorin A+ cells was low in BM cell samples studied, which can explain the lower percentage of p67 expression found in the BM CD34+ population. To test whether p67 recognized determined progenitors of erythroid lineage, CD34+p67+ and CD34+p67– cells were isolated by FACS sorting and plated in colony assays. Most of the BFU-E–forming activity resided within the p67+ fraction. Whereas among total CD34+ cells only 1 in 25 cells was a BFU-E, BFU-E frequency was increased to almost 1 in 5 cells in the CD34+p67+ population. The CD34+p67– population was significantly depleted of BFU-Es (P < .005 for all differences) (Figure 2A-C). To study p67 expression at various stages of erythroid maturation, “white” (ie, nonhemoglobinized) day-7 BFU-E–derived cells were picked from methyl cellulose cultures. Early BFU-Es were identified by colony morphologic appearance. Most of the cells were proerythroblasts, as determined by cell morphology on Wright-Giemsa–stained cytospins, glycophorin A expression on FACS, and negative benzidine staining (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Similarly, erythroblasts were picked from day-14 methyl cellulose plates from mature, visibly hemoglobinized BFU-Es. Their identity was additionally confirmed by evaluation of the same parameters (ie, morphology, glycophorin A positivity, and benzidine staining). The day-7 population, which contained mostly proerythroblasts, overwhelmingly expressed p67 (Figure 1D), as did a subset (20%-30%) of late erythroblasts (Figure S1), suggesting some attenuation of p67 expression with differentiation. Myeloid cells derived from CFU-GMs were p67 negative (Figure 1E).
p67 expression defines erythroid progenitor cells. (A) p67 expression on CD34+ cells: p67 expression was detected on CD34+ cells from BM by staining with anti-p67Ab. (Representative FACS histogram, n = 8; grey area indicates FITC-labeled secondary Ab; black line, anti-p67+ FITC-labeled secondary Ab.) (B) Differential p67 expression on BM and MPB CD34+ cells: 34% ± 4% (mean ± SEM; range: 20%-48%) of BM CD34+ cells expressed p67. The frequency of p67-expressing cells among MPB CD34+ cells was 50% ± 5% (range: 38%-60%, P = .024, 8 donors for BM, 5 donors for MPB). (C) p67 expression by CD34+ cells is selective for erythroid cells: Among MPB CD34+ cells, glycophorin A+ cells were relatively infrequent (5.1%). However, 99% of the rare CD45dim glycophorin A+ cells (top) and 100% of the CD45+ glycophorin A+ cells (middle) expressed p67. p67 was also expressed on less than 10% of CD45+ glycophorin A– cells (bottom). Similar data were obtained from 2 samples of BM cells: Although in these BM samples CD34+ glycophorin A+ cells were even less frequent (0.2%), essentially all coexpressed p67 (not shown). (D) Coexpression of p67 and erythroid lineage markers: The majority of early BFU-E–derived cells coexpress glycophorin A and p67. By morphology and benzidine staining, these cells were identified as proerythroblasts (Figure S1) (representative FACS dot plot from 3 independent experiments). (E) Myeloid cells do not express p67: CFU-GM–derived cells, identified as myeloid cells by CD45 and CD11a expression and negativity for glycophorin A, did not express p67 (representative FACS dot plot from 2 independent experiments).
p67 expression defines erythroid progenitor cells. (A) p67 expression on CD34+ cells: p67 expression was detected on CD34+ cells from BM by staining with anti-p67Ab. (Representative FACS histogram, n = 8; grey area indicates FITC-labeled secondary Ab; black line, anti-p67+ FITC-labeled secondary Ab.) (B) Differential p67 expression on BM and MPB CD34+ cells: 34% ± 4% (mean ± SEM; range: 20%-48%) of BM CD34+ cells expressed p67. The frequency of p67-expressing cells among MPB CD34+ cells was 50% ± 5% (range: 38%-60%, P = .024, 8 donors for BM, 5 donors for MPB). (C) p67 expression by CD34+ cells is selective for erythroid cells: Among MPB CD34+ cells, glycophorin A+ cells were relatively infrequent (5.1%). However, 99% of the rare CD45dim glycophorin A+ cells (top) and 100% of the CD45+ glycophorin A+ cells (middle) expressed p67. p67 was also expressed on less than 10% of CD45+ glycophorin A– cells (bottom). Similar data were obtained from 2 samples of BM cells: Although in these BM samples CD34+ glycophorin A+ cells were even less frequent (0.2%), essentially all coexpressed p67 (not shown). (D) Coexpression of p67 and erythroid lineage markers: The majority of early BFU-E–derived cells coexpress glycophorin A and p67. By morphology and benzidine staining, these cells were identified as proerythroblasts (Figure S1) (representative FACS dot plot from 3 independent experiments). (E) Myeloid cells do not express p67: CFU-GM–derived cells, identified as myeloid cells by CD45 and CD11a expression and negativity for glycophorin A, did not express p67 (representative FACS dot plot from 2 independent experiments).
These studies thus identify p67 as a novel lineage marker among adult human erythroid HPCs. A transferrin receptor isoform was previously described to be selectively expressed in erythroid HPCs.23 However, unlike transferrin receptor, which is clearly related to erythroid-specific proliferative/metabolic functions, the functional role of p67 was not immediately apparent. The hypothesis was entertained that p67 might interact with BM laminins, to mediate homing/retention of BFU-Es. MLuC5 antibody was previously demonstrated to block p67-mediated adhesion, thereby attenuating laminin binding.24 Blockade of laminin binding by MLuC5 antibody was incomplete, seemingly due to HPC expression of additional laminin receptors, such as VLA-6.14,25 Therefore, to test the effect of blocking p67-mediated binding on BM homing, BM or MPB CD34+ cells were incubated with anti-p67Ab and injected into lethally irradiated NOD/SCIDβ2m–/– recipients. Control mice received untreated CD34+ cells from the same cell sample. Total colony number and frequency of erythroid or mixed colonies were the same for anti-p67Ab–incubated and control-incubated cells, indicating absence of in vitro toxicity or antiproliferative effects of the antibody (Figure 2D). The fraction of BM-derived CFU-Cs homed to BM was 50% reduced by anti-p67Ab, predominantly at the expense of BFU-Es (Figure 2E). The effect of anti-p67Ab treatment on BM homing of MPB CD34+ CFU-Cs was similar in pattern and magnitude to that of BM CD34+ CFU-Cs (see Figure S2 for comparison).
p67 is expressed on erythroid progenitors and guides their marrow homing. (A-D) BFU-Es reside in the CD34+p67+ fraction: CD34+p67+ or CD34+p67– BM cells were isolated by FACS sorting and cultured in colony assays, to test frequency and lineage distribution of CFU-Cs. (A) Methyl cellulose colony assays: Most of the CFU-Cs in the CD34+p67– fraction (left panel, insert) were CFU-GMs, whereas the CD34+p67+ sample contained mostly BFU-Es (right panel, insert). Total CFU-C frequency was much greater in the p67+ fraction. (1500 sorted cells/35-mm plate). Dark-field photography was performed with a DIX Nikon camera, and a 105AFMacroNikkor objective (Nikon, Melville, NY). Plates were photographed with 1:1 macro; the insets, each showing one representative colony, with 4:1 macro. Images were captured with Nikon Capture/View acquisition software and processed with Photoshop (Adobe Systems, San Jose, CA) for white balance adjustment. (B) Plasma-clot assays: Total BM cells were sorted based on p67 expression and plated in plasma-clot assays. BFU-Es were depleted in the p67– and enriched in the p67+ BM fraction. (Note that 10 000 cells/clot were plated in unsorted sample, compared with 5000 cells/clot in p67– and p67+ samples.) Images were captured with a benzidine stain, using a Leica MZ6 dissecting microscope (Leica, Heidelberg, Germany), 0.63 ×/1.0 NA objective, and a Nikon CoolPix 995 camera, 2 × zoom. Unadjusted color image gray-scaled in Powerpoint (Microsoft). For color image, see Figure S3). (C) CFU-C frequency and distribution: Among CD34+p67+ BM cells, the frequency of CFU-Cs was 2-fold higher than in unsorted CD34+ cells (224 ± 19 vs 114 ± 20 CFU-Cs/1000 CD34+ cells, P < .005). This increase of colony-forming activity in the CD34+p67+ population was restricted to BFU-Es, which were 5-fold enriched in absolute (218 ± 16 vs 43 ± 8 BFU-Es/1000 cells) and 2.5-fold enriched in relative (95% ± 4% vs 38% ± 1% of CFU-Cs) frequency compared with unsorted BM (P < .005). CFU-GMs were accordingly depleted by selecting for p67+ cells (P < .005). Inversely, BFU-E activity was reduced in the sorted CD34+p67– population, to 13% ± 5% of all CFU-Cs. Shown on the Y-axis are CFU-Cs (mean + SEM of 3 independent experiments)/1000 sorted CD34+ cells. Essentially similar data were observed with sorted MPB CD34+ cells (Figure S2). (D) Anti-p67Ab is nontoxic for HPCs: Incubation of human CD34+ cells with anti-p67Ab (without washing prior to culture) did not affect CFU-C frequency and lineage distribution compared with untreated CD34+ cells (ctrl), indicating that anti-p67Ab is nontoxic for human CFU-Cs in vitro (2 experiments for BM CD34+ cells [shown here]; 1 experiment for MPB CD34+ cells). The cells plated for these experiments are aliquots of the cell suspensions injected for the homing assays depicted in Figure 2E and Figure S2. (E) p67 mediates marrow homing of BFU-Es: BM CD34+ cells, incubated with blocking anti-p67Ab, had 49.7% ± 6.6% (P < .005) reduced BM homing compared with untreated control, almost exclusively at the expense of BFU-Es (BFU-Es reduced by 86.3% ± 3.3%, P < .005) and CFU-mixed (reduced by 90.5% ± 3.7%, P < .005) (CFU-GMs: reduced by 14.7% ± 11.6%, P = .38). Similar inhibition was seen when MPB CD34+ cells were transplanted (Figure S2). (n = 3 independent experiments, each with 5 recipients/group. Mean + SEM of percent of total injected CFU-Cs homed to marrow 20 hours after transplantation.)
p67 is expressed on erythroid progenitors and guides their marrow homing. (A-D) BFU-Es reside in the CD34+p67+ fraction: CD34+p67+ or CD34+p67– BM cells were isolated by FACS sorting and cultured in colony assays, to test frequency and lineage distribution of CFU-Cs. (A) Methyl cellulose colony assays: Most of the CFU-Cs in the CD34+p67– fraction (left panel, insert) were CFU-GMs, whereas the CD34+p67+ sample contained mostly BFU-Es (right panel, insert). Total CFU-C frequency was much greater in the p67+ fraction. (1500 sorted cells/35-mm plate). Dark-field photography was performed with a DIX Nikon camera, and a 105AFMacroNikkor objective (Nikon, Melville, NY). Plates were photographed with 1:1 macro; the insets, each showing one representative colony, with 4:1 macro. Images were captured with Nikon Capture/View acquisition software and processed with Photoshop (Adobe Systems, San Jose, CA) for white balance adjustment. (B) Plasma-clot assays: Total BM cells were sorted based on p67 expression and plated in plasma-clot assays. BFU-Es were depleted in the p67– and enriched in the p67+ BM fraction. (Note that 10 000 cells/clot were plated in unsorted sample, compared with 5000 cells/clot in p67– and p67+ samples.) Images were captured with a benzidine stain, using a Leica MZ6 dissecting microscope (Leica, Heidelberg, Germany), 0.63 ×/1.0 NA objective, and a Nikon CoolPix 995 camera, 2 × zoom. Unadjusted color image gray-scaled in Powerpoint (Microsoft). For color image, see Figure S3). (C) CFU-C frequency and distribution: Among CD34+p67+ BM cells, the frequency of CFU-Cs was 2-fold higher than in unsorted CD34+ cells (224 ± 19 vs 114 ± 20 CFU-Cs/1000 CD34+ cells, P < .005). This increase of colony-forming activity in the CD34+p67+ population was restricted to BFU-Es, which were 5-fold enriched in absolute (218 ± 16 vs 43 ± 8 BFU-Es/1000 cells) and 2.5-fold enriched in relative (95% ± 4% vs 38% ± 1% of CFU-Cs) frequency compared with unsorted BM (P < .005). CFU-GMs were accordingly depleted by selecting for p67+ cells (P < .005). Inversely, BFU-E activity was reduced in the sorted CD34+p67– population, to 13% ± 5% of all CFU-Cs. Shown on the Y-axis are CFU-Cs (mean + SEM of 3 independent experiments)/1000 sorted CD34+ cells. Essentially similar data were observed with sorted MPB CD34+ cells (Figure S2). (D) Anti-p67Ab is nontoxic for HPCs: Incubation of human CD34+ cells with anti-p67Ab (without washing prior to culture) did not affect CFU-C frequency and lineage distribution compared with untreated CD34+ cells (ctrl), indicating that anti-p67Ab is nontoxic for human CFU-Cs in vitro (2 experiments for BM CD34+ cells [shown here]; 1 experiment for MPB CD34+ cells). The cells plated for these experiments are aliquots of the cell suspensions injected for the homing assays depicted in Figure 2E and Figure S2. (E) p67 mediates marrow homing of BFU-Es: BM CD34+ cells, incubated with blocking anti-p67Ab, had 49.7% ± 6.6% (P < .005) reduced BM homing compared with untreated control, almost exclusively at the expense of BFU-Es (BFU-Es reduced by 86.3% ± 3.3%, P < .005) and CFU-mixed (reduced by 90.5% ± 3.7%, P < .005) (CFU-GMs: reduced by 14.7% ± 11.6%, P = .38). Similar inhibition was seen when MPB CD34+ cells were transplanted (Figure S2). (n = 3 independent experiments, each with 5 recipients/group. Mean + SEM of percent of total injected CFU-Cs homed to marrow 20 hours after transplantation.)
Inhibition of homing interactions between HPCs and BM stroma was previously reported.1,2,4,6,25 However, a lineage-specific effect was not previously observed. As shown, the laminin-binding receptor p67 is preferentially expressed on human erythroid progenitors and precursors, and its blockade preferentially restricts BM homing/retention of BFU-Es. This specificity is likely explained by low expression of p67 on nonerythroid CFU-Cs. The existence of lineage-specific homing requirements may not be unexpected, given the different microenvironmental needs for specialized lineage development, but they were not previously documented. These data, albeit associated with the known caveats of antifunctional antibodies, suggest that laminin may be a functional component of the erythroid niche. Although the p67 dependence was observed in a xenogeneic setting, we speculate that it may be also operative within human BM. These studies do not exclude the possibility that nonerythroid HPCs might interact with laminins through other receptors, such as VLA-6.25
Prepublished online as Blood First Edition Paper, April 11, 2006; DOI 10.1182/blood-2005-12-013508.
Supported by an American Society of Hematology (ASH) Fellow Scholar Award (H.B.) and National Institutes of Health (NIH) grant HL58734 (T.P.).
H.B. conceived of the studies, planned and performed experiments, analyzed the data, and cowrote the paper; K.-H.C. performed experiments (blinded scoring of CFU-C assays, FACS, cytospins, and staining); B.N. performed experiments (plasma clots, photography); and T.P. advised with planning of experiments and data analysis and cowrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The generous gift of primary cells by Shelly Heimfeld (FHCRC) and Joanna Reems (Puget Sound Blood Center) is gratefully acknowledged.

![Figure 2. p67 is expressed on erythroid progenitors and guides their marrow homing. (A-D) BFU-Es reside in the CD34+p67+ fraction: CD34+p67+ or CD34+p67– BM cells were isolated by FACS sorting and cultured in colony assays, to test frequency and lineage distribution of CFU-Cs. (A) Methyl cellulose colony assays: Most of the CFU-Cs in the CD34+p67– fraction (left panel, insert) were CFU-GMs, whereas the CD34+p67+ sample contained mostly BFU-Es (right panel, insert). Total CFU-C frequency was much greater in the p67+ fraction. (1500 sorted cells/35-mm plate). Dark-field photography was performed with a DIX Nikon camera, and a 105AFMacroNikkor objective (Nikon, Melville, NY). Plates were photographed with 1:1 macro; the insets, each showing one representative colony, with 4:1 macro. Images were captured with Nikon Capture/View acquisition software and processed with Photoshop (Adobe Systems, San Jose, CA) for white balance adjustment. (B) Plasma-clot assays: Total BM cells were sorted based on p67 expression and plated in plasma-clot assays. BFU-Es were depleted in the p67– and enriched in the p67+ BM fraction. (Note that 10 000 cells/clot were plated in unsorted sample, compared with 5000 cells/clot in p67– and p67+ samples.) Images were captured with a benzidine stain, using a Leica MZ6 dissecting microscope (Leica, Heidelberg, Germany), 0.63 ×/1.0 NA objective, and a Nikon CoolPix 995 camera, 2 × zoom. Unadjusted color image gray-scaled in Powerpoint (Microsoft). For color image, see Figure S3). (C) CFU-C frequency and distribution: Among CD34+p67+ BM cells, the frequency of CFU-Cs was 2-fold higher than in unsorted CD34+ cells (224 ± 19 vs 114 ± 20 CFU-Cs/1000 CD34+ cells, P < .005). This increase of colony-forming activity in the CD34+p67+ population was restricted to BFU-Es, which were 5-fold enriched in absolute (218 ± 16 vs 43 ± 8 BFU-Es/1000 cells) and 2.5-fold enriched in relative (95% ± 4% vs 38% ± 1% of CFU-Cs) frequency compared with unsorted BM (P < .005). CFU-GMs were accordingly depleted by selecting for p67+ cells (P < .005). Inversely, BFU-E activity was reduced in the sorted CD34+p67– population, to 13% ± 5% of all CFU-Cs. Shown on the Y-axis are CFU-Cs (mean + SEM of 3 independent experiments)/1000 sorted CD34+ cells. Essentially similar data were observed with sorted MPB CD34+ cells (Figure S2). (D) Anti-p67Ab is nontoxic for HPCs: Incubation of human CD34+ cells with anti-p67Ab (without washing prior to culture) did not affect CFU-C frequency and lineage distribution compared with untreated CD34+ cells (ctrl), indicating that anti-p67Ab is nontoxic for human CFU-Cs in vitro (2 experiments for BM CD34+ cells [shown here]; 1 experiment for MPB CD34+ cells). The cells plated for these experiments are aliquots of the cell suspensions injected for the homing assays depicted in Figure 2E and Figure S2. (E) p67 mediates marrow homing of BFU-Es: BM CD34+ cells, incubated with blocking anti-p67Ab, had 49.7% ± 6.6% (P < .005) reduced BM homing compared with untreated control, almost exclusively at the expense of BFU-Es (BFU-Es reduced by 86.3% ± 3.3%, P < .005) and CFU-mixed (reduced by 90.5% ± 3.7%, P < .005) (CFU-GMs: reduced by 14.7% ± 11.6%, P = .38). Similar inhibition was seen when MPB CD34+ cells were transplanted (Figure S2). (n = 3 independent experiments, each with 5 recipients/group. Mean + SEM of percent of total injected CFU-Cs homed to marrow 20 hours after transplantation.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/4/10.1182_blood-2005-12-013508/3/m_zh80160699630002.jpeg?Expires=1769286345&Signature=tvSw-1hcBcTUch6xcqZjiTO6wXTWDeMYyZQOXoV1X-mEBfASiJydlg-VFw9D3O8QeXmjDFQ9iWr88M98460k~dO~0OcBMCKRn1LeimBmLlvHC5Z6q2IAskD3BOgb2S1pLtohol-E8Ids3ZjoiyIYtN4Y3A5EMnry~7clLuLMtZcokPxf~8O6NB0Zt7g91zKzGNBXLCNSdi3uwjb~xPTdWt4lSLJQATopJeF01JlpQ1dpqF5UAUhxEpq3yNTRI0t32LwCY5GJ2eWiqmSyWV1FiOc0ozxNaKMAoqr4gLHdyzsqZwP6ULNPFphFWiOowhTV9z3oD7RcJrWtBo3-pSb2eQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal