Abstract
Regulatory dendritic cells (DCs) have been reported recently, but their origin is poorly understood. Our previous study demonstrated that splenic stroma can drive mature DCs to proliferate and differentiate into regulatory DCs, and their natural counterpart with similar regulatory function in normal spleens has been identified. Considering that the spleen microenvironment supports hematopoiesis and that hematopoietic stem cells (HSCs) are found in spleens of adult mice, we wondered whether splenic microenvironment could differentiate HSCs into regulatory DCs. In this report, we demonstrate that endothelial splenic stroma induce HSCs to differentiate into a distinct regulatory DC subset with high expression of CD11b but low expression of Ia. CD11bhiIalo DCs secreting high levels of TGF-β, IL-10, and NO can suppress T-cell proliferation both in vitro and in vivo. Furthermore, CD11bhiIalo DCs have the ability to potently suppress allo-DTH in vivo, indicating their preventive or therapeutic perspectives for some immunologic disorders. The inhibitory function of CD11bhiIalo DCs is mediated through NO but not through induction of regulatory T (Treg) cells or T-cell anergy. IL-10, which is secreted by endothelial splenic stroma, plays a critical role in the differentiation of the regulatory CD11bhiIalo DCs from HSCs. These results suggest that splenic microenvironment may physiologically induce regulatory DC differentiation in situ.
Introduction
Dendritic cells (DCs) play crucial roles in the initiation and regulation of immune responses.1,2 The ability of DCs to initiate immune responses or induce tolerance is strictly dependent on their maturation state or subsets. It has been reported that immature DCs that are deficient of costimulatory molecules can induce T-cell anergy, generate regulatory T (Treg) cells, and promote alloantigen-specific tolerance. Several types of DCs with negative regulatory functions have been reported.3 Most regulatory DCs are prepared in vitro using immunosuppressive cytokines, such as IL-10 and TGF-β.4-6 However, this may not reflect the real differentiation of regulatory DCs in the immune microenvironment in vivo.
The development of hematopoietic cells in vivo occurs in the context of microenvironment or niche,7 which consists of many types of stromal cells, such as fibroblasts, macrophages, endothelium cells, and adipose cells. The microenvironment provides various signals for hematopoietic cell development.7 Different microenvironments support different types of cell differentiation. The spleen is an important lymphoid organ, and the mouse spleen maintains hematopoietic function throughout life.8 Furthermore, hematopoietic stem cells (HSCs) are found in spleens of adult mice.8 Therefore, it is conceivable that HSCs in the spleen may differentiate into different immune cells in situ.
Splenic stromal cells cultured in vitro could mimic the splenic microenvironment in vivo to some extent, despite their differences in some constituents. There is evidence that long-term cultured splenic stromal cells can support the development of dendritic-like cells in the absence of exogenous cytokines, and the dendritic-like cells have the phenotype and function of DCs,9-13 strongly suggesting that splenic microenvironment could induce hematopoietic progenitors to differentiate directly into DCs. Stromal cells cultured in vitro consist of multiple components. Purification of the various components will help to study the role of specific cell type in the induction of DCs. We established the method of preparing endothelial splenic stroma cells (ESSCs) and identified their characteristics, finding that ESSCs can drive mature DCs (mDCs) to proliferate and differentiate into regulatory DCs, whose natural counterpart exists in normal spleens.14 Therefore, we wondered whether its other origin in addition to mDCs; that is, could the splenic microenvironment differentiate hematopoietic progenitors to regulatory DCs? If so, what are its characteristics, and what is the mechanism? In this study, we show that ESSCs can differentiate HSCs into a distinct regulatory DC subset, CD11bhiIalo DCs, which secret TGF-β, IL-10, and NO and have regulatory functions in vitro and in vivo. IL-10, secreted by ESSCs, plays a critical role in their differentiation. Its inhibitory function is mediated through NO but not through induction of Treg cells or T-cell anergy. These results suggest that splenic microenvironment may physiologically induce regulatory DC differentiation in situ.
Materials and methods
Mice
Homozygous DO11.10 mice, heterozygous C57BL/6-TgN(ACTbEGFP)1Osb mice (GFP mouse), and IL-10 gene knock-out mice (H-2kb) were purchased from the Jackson Laboratory (Bar Harbor, ME). Because of the low expression of Sca-1 on HSCs from Balb/c mice,15 (C57BL/6 × DO11.10) F1 mice were prepared by crossing C57BL/6 with DO11.10 mice. (GFP × DO11.10) F1 mice were obtained by crossing DO11.10 mice with C57BL/6-TgN (ACTbEGFP)1Osb mice.
Reagents
RPMI-1640 medium (PAA Laboratories, Linz, Austria) was supplemented with 10% FCS (PAA Laboratories), 2 mM L-glutamine, 1% sodium pyruvate, and 2 × 10–5 M β2 mercaptoethanol, all from Sigma-Aldrich (St Louis, MO). Recombinant mouse GM-CSF and IL-4 were purchased from PeproTech (London, United Kingdom). Fluorescein-conjugated mAbs to CD3e, CD4, CD8α, CD11b, CD11c, CD14, CD25, CD40, B220, Gr-1, CD69, CD80, CD86, CD117, Sca-1, I-A, KJ1-26, biotinylated Flt3 mAb, and isotype control mAbs were purchased from BD Pharmingen (San Diego, CA). Microbead-conjugated mAbs to CD4, CD8α, CD11b, CD11c, B220, CD117, FITC, and PE and the Lineage Cell Depletion Kit were from Miltenyi Biotec (Bergisch Gladbach, Germany). Fluorescein-conjugated mAbs to IL-2 and IFN-γ were obtained from eBioscience (San Diego, CA). Neutralization Abs to IL-10, TGF-β, M-CSF, and isotype control mAbs were purchased from R&D Systems (Minneapolis, MN). 2.4G2 for blocking mouse FcR I and II was purchased from BD Pharmingen. 7-AAD, lipopolysaccharide (LPS), saponin, brefeldin A, bovine serum albumin (BSA), 1-methyltryptophan, indomethacin, 1,4 PBIT, and CFSE were from Sigma (St Louis, MO). Rat sera were purchased from Gibco (Grand Island, NY) for blocking use.
Cell preparation
ESSCs derived from newborn mice were prepared and maintained in long-term culture in RPMI1640/20% FCS by passaging to new plates each week.14
The strategy for HSC preparation was described by Christensen and Weissman.16 Briefly, cells from bone marrow were labeled with biotinylated mAbs against lineage antigens (CD5, B220, Gr-1, 7-4, and Ter-119) and Flt3 and were then incubated with streptavidin-PerCP–labeled antibody or antibiotin microbeads (for GFP+ mice to deplete Lin+Flt3+ cells by magnetic cell sorting [MACS]). These cells were further labeled with PE-conjugated anti-CD117 mAb and FITC or PE-Cy5 (for GFP+ mice) conjugated anti–Sca-1 mAb. Lin–Flt3–CD117+Sca-1+ cells were sorted as HSCs by fluorescence-activated cell sorting (FACS) Diva (Becton Dickinson, Mountain View, CA).
In clonal assay experiments, single Lin–Flt3–CD117+Sca-1+ HSCs from normal or GFP+ mice were directly seeded onto ESSC monolayers in 96-well flat plates by FACSDiva. Lin–Flt3–CD117+Sca-1+ cells (1 × 103/mL) were added onto ESSC monolayers or polyformaldehyde-fixed ESSC monolayers in 24-well plates. After 10 days of culture, nonadherent cells were purified using anti-CD11b microbeads and collected as CD11bhiIalo DCs. The purity was greater than 97%. Transwell experiments were performed in 24-well plates: 1 × 103 Lin–Flt3–CD117+Sca-1+ HSCs in 300 μL culture medium were plated in the upper insert, separated from a coculture of ESSC monolayer by a 0.4-μm permeable membrane (a total culture volume of 1 mL). In some experiments, large numbers of CD11bhiIalo regulatory DCs were needed, so Lin–CD117+ bone marrow progenitors, prepared with the Lineage Cell Depletion Kit and anti-CD117 microbeads, were cocultured with ESSC monolayer.
Bone marrow–derived DCs (BMDCs) were generated from bone marrow cells in the presence of GM-CSF and IL-4 using established protocols.17-20 For immature DCs (imDCs), on day 4, the dendritic proliferation clusters were collected and purified by anti-CD11c micobeads.21 Purified imDCs under the stimulation of LPS (0.01 μg/mL, for 4 days) were used as mDCs. The purity was greater than 95%.
CD4 T cells were obtained by negative selection using anti-CD11b and anti-CD11c microbeads and then positive selection using anti-CD4 microbeads. The purity was greater than 96%.
To isolate the in vivo natural counterpart of CD11bhiIalo DCs, CD4–CD8–B220– splenocytes were collected by negative selection with MACS. The negative cells were further labeled with PE-Cy5–conjugated anti-CD11c mAb, PE-conjugated anti-CD11b mAb, and FITC-conjugated anti-Ia mAb. Next, the cells with CD11c low expression were gated, and then CD11cloCD11bhiIalo cells were sorted by FACSDiva.
Flow cytometry
Cell surface marker and intracellular cytokine detection, proliferating T-cell counts, and analysis of phagocytosis ability were carried out as we described before.14 To test T-cell proliferation in 96-well plates after 5 days of culture, 120 μL/well culture supernatants were collected for cytokine assay by enzyme-linked immunosorbent assay (ELISA), then FITC-conjugated anti-CD4 mAb and 7AAD in 10 μL PBS were added. Alternatively, in some experiments, PE-conjugated anti–KJ1-26 mAb was also added and incubated for 15 minutes at 4°C. Before being collected for analysis by FACSCalibur (Becton Dickinson), 160 μL PBS per well was added to bring the final volume of each well up to 250 μL. Finally, cells were collected at high speed for 52 seconds, and the numbers of CD4+7AAD– or CD4+KJ1-26+7AAD– cells were counted.
Mixed lymphocyte reaction
Naive or activated (by mDCs for 24 hours in the presence of OVA323-339 peptide) CD4 T cells (1 × 105) were cultured in 96-well round-bottom plates with 1 × 104 APCs (imDCs, mDCs, or CD11bhiIalo DCs) in the presence of or absence of CD4 T cells (1 × 105) primed by CD11bhiIalo DCs; after 5 days of culture, viable cell numbers were counted by FACS. In some experiments, 1-methyltryptophan, indomethacin, 1,4 PBIT, neutralizing anti–IL-10 mAbs or anti–TGF-β1, -β2, and -β3 mAbs were added at the beginning of the culture.
Assay for cytokines and NO
Cytokines produced in culture supernatants at day 5 from mixed lymphocyte reaction (MLR) were detected by using ELISA kits for IL-2 and IFN-γ. For detection of IL-12p70, IL-10, and TGF-β produced by APCs, cells were cultured for 24 hours with or without LPS stimuli (0.5 μg/mL). NO production was measured as the nitrite concentration using the Griess assay.14
Assay for the suppressive function of CD11bhiIalo DCs in vivo
Naive DO11.10 T cells (6 × 106) were transferred intraperitoneally into BALB/c mice. APCs (6 × 106) were incubated with 1 μM OVA323-339 peptide at 37°C for 6 hours, washed, and injected intraperitoneally into DO11.10 T-cell–transferred mice. After 5 days of immunization, the single cells from mesenteric lymph nodes and spleens were double stained with FITC-conjugated anti-CD4 mAb, PE-conjugated anti-OVA322-339 peptide–specific T-cell receptor (TCR) mAb KJ1-26, and were counted by FACS. The cells were stained with both F27C-CD4 and PE-KJI-26.
Assay for allo-DTH in vivo
The in vivo suppression of allo-DTH by CD11bhiIalo DCs was performed as reported.22 Briefly, B6 mice were immunized on the dorsal flank by subcutaneous inoculation of BALB/c spleen cells (1 × 07/mouse) on day 0 and challenged on day 7 and day 14 at the hind footpad by injecting the same antigens (1 × 107/mouse). APCs, derived from B6 mice, were injected intraperitoneally (3 × 106/mouse) on days –6, –4, 0, 3, and 6. Footpad thickness was then measured on day 8 and day 15, respectively, with a calipers-type engineers' micrometer by a third experimenter who did not know the sample identity. The extent of swelling was calculated as the thickness of the right footpad (receiving BALB/c spleen cells) minus the baseline thickness of the left footpad (receiving B6 spleen cells).
Statistical analysis
All experiments were performed at least 3 times. All data analysis was performed using a 2-tailed Student t test. P less than .05 was considered statistically significant.
Our study protocol was approved by the review board of Second Military Medical University.
Results
Endothelial splenic stroma induces Lin–Flt3–CD117+Sca-1+ HSCs into a distinct DC subset
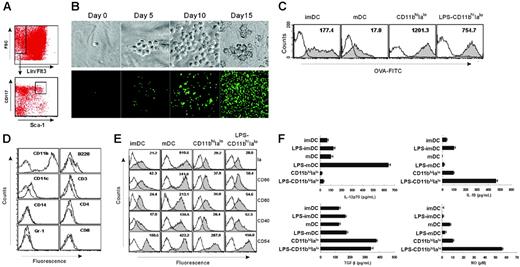
ESSCs, as described previously, express endothelial cell marker CD106 and have endothelial cell morphology.14 Lin–Flt3–CD117+Sca-1+ HSCs were sorted out from mouse bone marrow by FACSDiva (Figure 1A) and cultured with ESSC monolayer for at least 10 days before analysis. During the culture, the small round HSCs proliferated and gradually became larger nonadherent dendritic cell-like (DC-like) cells with many long dendrites (Figure 1B), displaying high phagocytic capacity (Figure 1C) like imDCs. These DC-like cells express high levels of the myeloid lineage marker CD11b and low levels of CD11c without expressing any other lineage markers, including CD3, CD4, CD8, B220, Gr-1, and CD14 (Figure 1D). Instead, these DC-like cells express all of the functional markers of DCs, such as Ia, CD86, CD80, and CD40 at low levels, similar to imDCs (Figure 1E). However, in contrast to imDCs, such expression of these markers at low levels was not increased when the cells were stimulated by LPS (Figure 1E), indicating a stable immature-like phenotype for these cells. On the basis of the phenotypic profiles, we termed these DC-like cells CD11bhiIalo DCs. Analysis of cytokine profiles revealed that CD11bhiIalo DCs spontaneously secreted TGF-β at a higher levels than imDCs and mDCs. IL-10 and NO were also secreted by CD11bhiIalo populations and were significantly enhanced by LPS stimulation (Figure 1F), suggesting that CD11bhiIalo DCs maybe be involved in immune regulation.
ESSCs induce BM HSC differentiation into CD11bhiIalo DCs. (A) HSCs were purified by FACSDiva according to the phenotype of HSCs. Flt3–Lin– bone marrow cells were first gated and further gated by using the marker of CD117 and Sca-1, and, finally, Lin–Flt3–CD117+Sca-1+cells were sorted as HSCs. (B) The morphology of HSCs were differentiated in clonal formation assay from small round to large DC-like cells. Photos were taken by a digital imaging system on the indicated days. Top panel (×400), HSC from normal mice. Bottom panel (×100), HSCs from GFP transgenic mice. Photos were taken with a Leica DMZRB (Leica, Wetzlar, Germany) using Meta Imaging Series 5.0 (Molecular Devices, Downingtown, PA). Images in the top panel were taken with a 40×/0.55 NA objective; those in the lower panel, with a 10×/0.25 objective. (C) The phagocytic ability of imDCs, mDCs, DC-like cells (CD11bhiIalo), and LPS-treated DC-like cells (LPS-CD11bhiIalo) as tested by measuring OVA-FITC phagocytosis using FACS. Filled histograms represent imDCs, mDCs, DC-like cells (CD11bhiIalo), or LPS-treated DC-like cells (LPS-CD11bhiIalo) cultured with 100 μg/mL OVA-FITC at 37°C for 2 hours and were marked with geometric mean fluorescence. Open histograms represent cells cultured with 100 μg/mL OVA-FITC at 4°C for 2 hours as a control. One of at least 3 independent experiments with similar results is shown. (D) The expression of lineage markers on the surface of DC-like cells. After HSCs grown on ESSC monolayers for 10 days, nonadherent DC-like cells were collected and stained with the indicated fluorescein-conjugated antibodies. Dotted lines represent cells stained with isotype-matched control mAbs. (E) The expression of functional molecules on DC-like cells. DC-like cells purified with microbead-conjugated anti-CD11b mAbs, further stimulated with LPS (10 ng/mL for 4 days) or not, were labeled with fluorescein-conjugated mAbs, comparing with imDCs and mDCs. Open histogram lines represent cells stained with isotype-matched control mAbs. Filled histograms are labeled with the geometric mean fluorescence of each DC population. (F) The cytokine profiles and NO secretion of CD11bhiIaloDCs, imDCs, and mDCs (stimulated with or without 0.5 μg/mL LPS for 24 hours) as measured by ELISA and Griess assay, respectively. Data are mean + SD of triplicate wells.
ESSCs induce BM HSC differentiation into CD11bhiIalo DCs. (A) HSCs were purified by FACSDiva according to the phenotype of HSCs. Flt3–Lin– bone marrow cells were first gated and further gated by using the marker of CD117 and Sca-1, and, finally, Lin–Flt3–CD117+Sca-1+cells were sorted as HSCs. (B) The morphology of HSCs were differentiated in clonal formation assay from small round to large DC-like cells. Photos were taken by a digital imaging system on the indicated days. Top panel (×400), HSC from normal mice. Bottom panel (×100), HSCs from GFP transgenic mice. Photos were taken with a Leica DMZRB (Leica, Wetzlar, Germany) using Meta Imaging Series 5.0 (Molecular Devices, Downingtown, PA). Images in the top panel were taken with a 40×/0.55 NA objective; those in the lower panel, with a 10×/0.25 objective. (C) The phagocytic ability of imDCs, mDCs, DC-like cells (CD11bhiIalo), and LPS-treated DC-like cells (LPS-CD11bhiIalo) as tested by measuring OVA-FITC phagocytosis using FACS. Filled histograms represent imDCs, mDCs, DC-like cells (CD11bhiIalo), or LPS-treated DC-like cells (LPS-CD11bhiIalo) cultured with 100 μg/mL OVA-FITC at 37°C for 2 hours and were marked with geometric mean fluorescence. Open histograms represent cells cultured with 100 μg/mL OVA-FITC at 4°C for 2 hours as a control. One of at least 3 independent experiments with similar results is shown. (D) The expression of lineage markers on the surface of DC-like cells. After HSCs grown on ESSC monolayers for 10 days, nonadherent DC-like cells were collected and stained with the indicated fluorescein-conjugated antibodies. Dotted lines represent cells stained with isotype-matched control mAbs. (E) The expression of functional molecules on DC-like cells. DC-like cells purified with microbead-conjugated anti-CD11b mAbs, further stimulated with LPS (10 ng/mL for 4 days) or not, were labeled with fluorescein-conjugated mAbs, comparing with imDCs and mDCs. Open histogram lines represent cells stained with isotype-matched control mAbs. Filled histograms are labeled with the geometric mean fluorescence of each DC population. (F) The cytokine profiles and NO secretion of CD11bhiIaloDCs, imDCs, and mDCs (stimulated with or without 0.5 μg/mL LPS for 24 hours) as measured by ELISA and Griess assay, respectively. Data are mean + SD of triplicate wells.
CD11bhiIalo DCs alone fail to promote proliferation of CD4 T cells
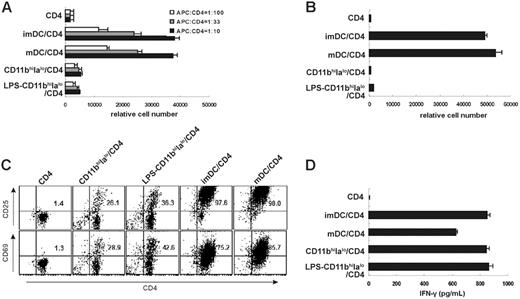
Although it is reported that splenic stromal cell–induced DCs are potent stimulators for allogeneic and syngeneic MLR and for antigen-specific T-cell proliferation,11 CD11bhiIalo DCs had a very limited (in allo-MLR) or no (in auto-MLR) capacity to stimulate T-cell proliferation even when CD11bhiIalo DCs were stimulated by 10 ng/mL LPS for 4 days (Figure 2A-B). Interestingly, imDCs had a similar capacity to stimulate T-cell proliferation in allo-MLR and auto-MLR as mDCs, because live imDCs in this system may be induced to mature by T cells (Figure 2A-B). These results demonstrate that CD11bhiIalo DCs were a distinct subset of DCs with stable immature-like phenotype, different from imDCs.
We further characterized the capacity of CD11bhiIalo DCs in antigen presentation to T cells. As shown in Figure 2C, compared with mDCs and imDCs, CD11bhiIalo DCs could only make 30% to 40% of T cells express the early activated markers of T cells. Furthermore, we found that CD11bhiIalo DCs could promote peptide-specific CD4 T cells to secrete high levels of IFN-γ, similar to mDCs (Figure 2D). We postulated that this distinct DC subset, CD11bhiIalo DCs, which had a stable immature-like phenotype, could spontaneously secret TGF-β, and also had a powerful capacity to stimulate T cells to secrete high levels of IFN-γ, may have special functions in immune regulation, because it has been shown that IFN-γ plus NO can inhibit T-cell proliferation.23
CD11bhiIalo DCs exhibit a limited or no capacity of stimulating CD4 T-cell proliferation in allo-MLR and auto-MLR. (A) CD4+ T cells (H-2d) were cultured with imDCs (H-2b), mDCs (H-2b), CD11bhiIalo DCs (H-2b), or LPS-treated CD11bhiIalo DCs (H-2b) at various APC/T cell ratios, and the relative cell number of viable CD4+ T cells in each well was measured on day 5 by FACS. (B-D) CD11bhiIalo DCs, LPS-treated CD11bhiIalo DCs, imDCs, or mDCs were cocultured with OVA323-339 peptide–specific congenetic CD4 T cells in the presence of OVA323-339 peptides. After 5 days, the relative number of viable CD4+ T cells in each well (B) and the activation-related markers CD25 and CD69 expression on CD4 T cells (C) were detected by FACS, and the levels of IFN-γ in the supernatant (D) were assayed by ELISA. Data in panels A, B, and D represent mean ± SD. Plots in panel C are labeled with the percentage of double-positive cells.
CD11bhiIalo DCs exhibit a limited or no capacity of stimulating CD4 T-cell proliferation in allo-MLR and auto-MLR. (A) CD4+ T cells (H-2d) were cultured with imDCs (H-2b), mDCs (H-2b), CD11bhiIalo DCs (H-2b), or LPS-treated CD11bhiIalo DCs (H-2b) at various APC/T cell ratios, and the relative cell number of viable CD4+ T cells in each well was measured on day 5 by FACS. (B-D) CD11bhiIalo DCs, LPS-treated CD11bhiIalo DCs, imDCs, or mDCs were cocultured with OVA323-339 peptide–specific congenetic CD4 T cells in the presence of OVA323-339 peptides. After 5 days, the relative number of viable CD4+ T cells in each well (B) and the activation-related markers CD25 and CD69 expression on CD4 T cells (C) were detected by FACS, and the levels of IFN-γ in the supernatant (D) were assayed by ELISA. Data in panels A, B, and D represent mean ± SD. Plots in panel C are labeled with the percentage of double-positive cells.
CD11bhiIalo DCs suppress mature DC-induced T-cell proliferation both in vitro and in vivo
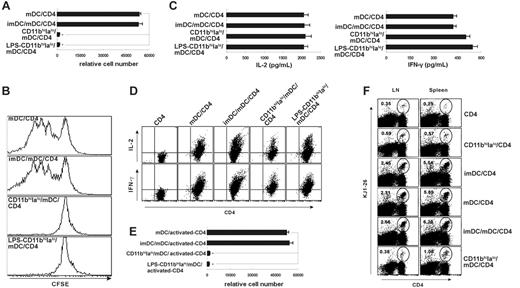
As shown in Figure 1, CD11bhiIalo DCs had a stable immature-like phenotype and secrete TGF-β, an important cytokine with negative regulation of immune response.23,24 We postulated that CD11bhiIalo DCs may have regulatory functions. To determine this, we added CD11bhiIalo DCs into CD4/mDCs coculture systems to see whether CD11bhiIalo DCs could inhibit T-cell proliferation. As shown in Figure 3A, CD11bhiIalo DCs, not imDCs, significantly suppressed the peptide-specific proliferation of naive peptide-specific CD4 T cells stimulated by mDCs. Similar results were observed in CFSE-labeling experiments (Figure 3B). However, the concentrations of IL-2 and IFN-γ in culture supernatant and levels of intracellular expression of IL-2 and IFN-γ were not reduced in the presence of CD11bhiIalo DCs (Figure 3C-D).
Then we wondered whether CD11bhiIalo DCs could suppress the proliferation of activated T cells that had been primed by mDCs. To determine this, naive peptide-specific CD4 T cells were stimulated by mDCs for 24 hours, and then CD11bhiIalo DCs were added into the cultures. As shown in Figure 3E, the proliferation of the activated T cells was also completely suppressed by CD11bhiIalo DCs, suggesting that the suppressive function of CD11bhiIalo DCs may not be in the primary phase of T-cell activation.
To determine whether CD11bhiIalo DCs had the ability to suppress the proliferation of T cells in vivo, purified naive peptide-specific CD4 T cells, together with various APCs that had been pulsed with OVA323-339 peptide for 6 hours in vitro, were transferred into the peritoneal cavities of BALB/c mice. After 5 days, KJ1-26–positive cells from draining lymph nodes and spleens of mice that had received the transfers were detected with FACS. As shown in Figure 3F, the proliferation of DO11.10 T cells was significantly suppressed by CD11bhiIalo DCs.
Taken together, the results showed that CD11bhiIalo DCs have a powerful ability to suppress T-cell proliferation both in vitro and in vivo, demonstrating that CD11bhiIalo DCs are a distinct subset of DCs with regulatory function.
In vivo infusion with CD11bhiIalo DCs suppress allo-DTH reaction
Because CD11bhiIalo DCs were potent in inhibiting the proliferation of CD4 T cells, which would suppress the immune response, we wondered whether CD11bhiIalo DCs could suppress allospecific immune reactions in vivo. Allo-DTH experiments were performed for the allo-DTH experiment's simplicity and reliability to study allospecific immune reactions.25 As shown in Figure 4, the footpad swelling of B6 mice receiving the alloantigen (BALB/c splenocytes) was suppressed more significantly by infusion with CD11bhiIalo DCs derived from B6 mice. These results suggested that CD11bhiIalo DCs could be used as a kind of negative regulator for immune response, thus outlining one approach to prevent or treat transplantation rejection or autoimmune diseases.
CD11bhiIalo DCs do not induce Treg cells or T-cell anergy
There is evidence showing that suppressive effects of DCs on T-cell proliferation were indirectly through the induction of Treg cells,4-6 with an immunosuppressive function and cytokine profiles distinct from either Th1 and Th2 T cells,26 or T-cell anergy, characterized by growth arrest state following an antigen encounter.27 To test whether CD11bhiIalo DCs induce Treg cells or cause T-cell anergy, we cocultured naive (DO-11.10 × GFP) F1GFP+ CD4 (GFP+CD4) T cells with CD11bhiIalo DCs (from F1 mice) in the presence of OVA322-339 peptide. On day 7, these CD11bhiIalo DC-induced GFP+CD4 T cells purified from the coculture system by microbeads conjugated with anti-CD4 mAbs or naive (DO-11.10 × GFP) F1GFP–CD4 (GFP–CD4) T cells were stimulated by mDCs at the same time. As Figure 5A shows, CD11bhiIalo DC-induced GFP+CD4 T cells displayed a proliferation ability similar to that of naive GFP–CD4 T cells, showing that CD11bhiIalo DC-induced GFP+CD4 T cells were not anergic. Similarly, if the purified CD11bhiIalo DC-induced GFP+CD4 T cells were added into the mDC/GFP–CD4 coculture system, the proliferation of naive GFP–CD4 T cells was not suppressed in the presence of CD11bhiIalo DC-induced GFP+CD4 T cells (Figure 5B), demonstrating that CD11bhiIalo DCs did not induce Treg cells, which had the capacity to suppress other T-cell proliferation. These results suggested that there may be other mechanisms involved in the inhibitory function of CD11bhiIalo DCs.
CD11bhiIalo DCs suppress mature DC-induced proliferation of peptide-specific CD4 T cells. (A,C,D) The influence of CD11bhiIalo DCs on the antigen-presentation capacity of mDCs in vitro was assayed. CD11bhiIalo DCs or LPS-treated CD11bhiIalo DCs were added into CD4/mDC coculture system in the presence of OVA323-339 peptide (CD11bhiIalo DCs/mDCs = 1:1), compared with imDCs. After 5 days, the relative number of live CD4+ T cells in each well was counted by FACS (A), the levels of IFN-γ and IL-2 in the supernatant (C) and in the CD4 T cells (D) were assayed by ELISA and FACS, respectively. *P < .001. (B) The influence of CD11bhiIalo DCs on T-cell division following antigen presentation was measured by CFSE-labeled peptide-specific CD4 T cells, as compared with imDCs. (E) The influence of CD11bhiIalo DCs on the mDC-primed proliferation of activated peptide-specific CD4 T cells was assayed. Peptide-specific CD4 T cells were activated by mDCs for 24 hours in the presence of OVA323-339 peptide, and then CD11bhiIalo DCs, LPS-treated CD11bhiIalo DCs, or imDCs were added. After 5 days, the relative cell number of viable peptide-specific CD4 T cells was measured by FACS. (F) The influence of CD11bhiIalo DCs on the proliferation of peptide-specific CD4 T cells stimulated by mDCs in vivo was assayed. OVA323-339 peptide–loaded CD11bhiIalo DCs, imDCs, or mDCs were cotransferred with peptide-specific CD4 T cells into recipient mice. The ratio of the combination of APCs was 1:1. After 5 days, lymphocytes in spleen and mesenteric lymph nodes were double-stained with anti–CD4-FITC and KJ1-26-PE for FACS analysis. The percentage of peptide-specific CD4 T cells (KJ1-26+) among total CD4 T cells was calculated. One of at least 3 independent experiments with similar results was shown.
CD11bhiIalo DCs suppress mature DC-induced proliferation of peptide-specific CD4 T cells. (A,C,D) The influence of CD11bhiIalo DCs on the antigen-presentation capacity of mDCs in vitro was assayed. CD11bhiIalo DCs or LPS-treated CD11bhiIalo DCs were added into CD4/mDC coculture system in the presence of OVA323-339 peptide (CD11bhiIalo DCs/mDCs = 1:1), compared with imDCs. After 5 days, the relative number of live CD4+ T cells in each well was counted by FACS (A), the levels of IFN-γ and IL-2 in the supernatant (C) and in the CD4 T cells (D) were assayed by ELISA and FACS, respectively. *P < .001. (B) The influence of CD11bhiIalo DCs on T-cell division following antigen presentation was measured by CFSE-labeled peptide-specific CD4 T cells, as compared with imDCs. (E) The influence of CD11bhiIalo DCs on the mDC-primed proliferation of activated peptide-specific CD4 T cells was assayed. Peptide-specific CD4 T cells were activated by mDCs for 24 hours in the presence of OVA323-339 peptide, and then CD11bhiIalo DCs, LPS-treated CD11bhiIalo DCs, or imDCs were added. After 5 days, the relative cell number of viable peptide-specific CD4 T cells was measured by FACS. (F) The influence of CD11bhiIalo DCs on the proliferation of peptide-specific CD4 T cells stimulated by mDCs in vivo was assayed. OVA323-339 peptide–loaded CD11bhiIalo DCs, imDCs, or mDCs were cotransferred with peptide-specific CD4 T cells into recipient mice. The ratio of the combination of APCs was 1:1. After 5 days, lymphocytes in spleen and mesenteric lymph nodes were double-stained with anti–CD4-FITC and KJ1-26-PE for FACS analysis. The percentage of peptide-specific CD4 T cells (KJ1-26+) among total CD4 T cells was calculated. One of at least 3 independent experiments with similar results was shown.
CD11bhiIalo DCs inhibit allo-DTH in vivo. B6 mice were immunized on the dorsal flank by subcutaneous inoculation of BALB/c spleen cells (1 × 107/mouse) on day 0, and challenged on day 7 and day 14 at the hind footpad by injecting the same antigens (1 × 107/mouse). APCs were injected intraperitoneally (3 × 106/mouse) on days –6, –4, 0, 3, and 6. Footpad thickness was then measured on day 8 and day 15 with a calipers-type engineer's micrometer by a third experimenter who did not know the sample identity. Data are representative of 2 independent experiments, showing the means ± SDs of footpad swelling on indicated day. *P < .05, **P < .01.
CD11bhiIalo DCs inhibit allo-DTH in vivo. B6 mice were immunized on the dorsal flank by subcutaneous inoculation of BALB/c spleen cells (1 × 107/mouse) on day 0, and challenged on day 7 and day 14 at the hind footpad by injecting the same antigens (1 × 107/mouse). APCs were injected intraperitoneally (3 × 106/mouse) on days –6, –4, 0, 3, and 6. Footpad thickness was then measured on day 8 and day 15 with a calipers-type engineer's micrometer by a third experimenter who did not know the sample identity. Data are representative of 2 independent experiments, showing the means ± SDs of footpad swelling on indicated day. *P < .05, **P < .01.
NO is involved in the regulatory function of CD11bhiIalo DCs
Because CD11bhiIalo DCs did not induce the production of Treg cells or make T-cell anergy, we postulated that maybe other factors were involved in this process. First, to determine whether this inhibitory effect was mediated by direct contact or by soluble factors, the effects of fixed CD11bhiIalo DCs or CD11bhiIalo DC supernatant were detected. As shown in Figure 5C, the proliferation of CD4 T cells were significantly suppressed by CD11bhiIalo DC supernatant, not by fixed CD11bhiIalo DCs, suggesting that the inhibitory effect of CD11bhiIalo DCs was dependent on the autocrine soluble factors.
Next, we tried to find out what factors in the supernatant were involved in this process. IL-10 and TGF-β produced by CD11bhiIalo DCs are 2 cytokines that are well known for inhibiting the proliferation of CD4 T cells.28,29 However, addition of blocking anti–IL-10 and anti–TGF-β antibody did not reverse the inhibitory effects of CD11bhiIalo DCs (Figure 5D).
Mediators involved in the inhibitory function of CD11bhiIalo DCs. (A-B) CD11bhiIalo did not induce T-cell anergy (A) and the generation of Treg cells (B). GFP+ OVA323-339 peptide–specific CD4 T cells from (DO11.10 × GFP) F1 GFP+mice, stimulated by CD11bhiIalo for 7 days in the presence of OVA323-339 peptide (GFP+CD4), and naive GFP– OVA323-339 peptide–specific TCR CD4 T cells from (DO11.10 × GFP) F1 GFP– mice (GFP–CD4) were primed with mDCs in the presence of OVA323-339 peptide. On day 5, the proliferation of GFP–CD4 T cells and GFP+CD4 T cells (A) and the effect of GFP+CD4 T cells pretreated by CD11bhiIalo DCs on mDC-induced GFP–CD4 T-cell proliferation (B) were assayed by FACS. *P > .05. (C) CD4/mDC system was cocultured either with paraformaldehyde-fixed CD11bhiIalo DCs (CD11bhiIalo-fixed) or with 50% CD11bhiIalo DCs culture supernatant (CD11bhiIalo-SN). On day 5, viable peptide-specific CD4 T cells were tested by FACS. *P < .01, **P > .005. (D-E) Anti–IL-10 (5 μg/mL), and anti–TGF-β (5 μg/mL) neutralizing mAbs (D) and 1-methyltryptophan (1 mM), IDO inhibitor, and indomethacin (40 μM), PGE2 inhibitor (E) were added at the beginning of CD11bhiIalo/mDC/CD4 coculture. On day 5, viable peptide-specific CD4 T cells were tested by FACS. *P > .05. (F-G) The inhibitory function of CD11bhiIalo DCs was NO involved. Serious concentrations of NO donor NOC-18 (F) or serious concentrations of NOS inhibitor PBIT (G) were added into coculture system as indicated. On day 5, viable peptide-specific CD4 T cells were assayed by FACS. *P < .01. Data in panels represent mean ± SD of triplicate samples.
Mediators involved in the inhibitory function of CD11bhiIalo DCs. (A-B) CD11bhiIalo did not induce T-cell anergy (A) and the generation of Treg cells (B). GFP+ OVA323-339 peptide–specific CD4 T cells from (DO11.10 × GFP) F1 GFP+mice, stimulated by CD11bhiIalo for 7 days in the presence of OVA323-339 peptide (GFP+CD4), and naive GFP– OVA323-339 peptide–specific TCR CD4 T cells from (DO11.10 × GFP) F1 GFP– mice (GFP–CD4) were primed with mDCs in the presence of OVA323-339 peptide. On day 5, the proliferation of GFP–CD4 T cells and GFP+CD4 T cells (A) and the effect of GFP+CD4 T cells pretreated by CD11bhiIalo DCs on mDC-induced GFP–CD4 T-cell proliferation (B) were assayed by FACS. *P > .05. (C) CD4/mDC system was cocultured either with paraformaldehyde-fixed CD11bhiIalo DCs (CD11bhiIalo-fixed) or with 50% CD11bhiIalo DCs culture supernatant (CD11bhiIalo-SN). On day 5, viable peptide-specific CD4 T cells were tested by FACS. *P < .01, **P > .005. (D-E) Anti–IL-10 (5 μg/mL), and anti–TGF-β (5 μg/mL) neutralizing mAbs (D) and 1-methyltryptophan (1 mM), IDO inhibitor, and indomethacin (40 μM), PGE2 inhibitor (E) were added at the beginning of CD11bhiIalo/mDC/CD4 coculture. On day 5, viable peptide-specific CD4 T cells were tested by FACS. *P > .05. (F-G) The inhibitory function of CD11bhiIalo DCs was NO involved. Serious concentrations of NO donor NOC-18 (F) or serious concentrations of NOS inhibitor PBIT (G) were added into coculture system as indicated. On day 5, viable peptide-specific CD4 T cells were assayed by FACS. *P < .01. Data in panels represent mean ± SD of triplicate samples.
It has been reported that PGE230 and indoleamine 2,3-dioxygenase (IDO; a tryptophan-catabolizing enzyme)31 can inhibit T-cell proliferation, so we added indomethacin, an inhibitor of PGE2 biosynthesis or 1-methyltryptophan, a specific IDO inhibitor, into a CD4/mDC/CD11bhiIalo DC coculture system. We found that the inhibitory effects of CD11bhiIalo DCs were not substantially reversed (Figure 5E).
It has become increasingly evident that NO is involved in inhibition of T-cell proliferation.32 The high levels of NO secreted by CD11bhiIalo DCs (Figure 1F) suggest that NO may be involved. We added series of concentrations of NOC-18, an NO donor, into a CD4/mDC coculture system at the beginning of culture, and found that the proliferation of CD4 T cells was inhibited in a dose-dependent manner. However, similar inhibitory effects were achieved by a much higher concentration of NO than that of NO in CD4/mDC/CD11bhiIalo DC coculture systems (Figure 5F). Furthermore, as shown in Figure 5G, the CD4 T-cell proliferation suppressed by CD11bhiIalo DCs could be significantly restored by 5 μg/mL 1,4 PBIT, an inhibitor of iNOS, which reduces the concentration of NO to levels found in CD4/mDC groups. Increasing the concentration of 1,4 PBIT to 10 μg/mL did decrease the NO significantly but failed to further restore the proliferation of CD4 T cells, and increasing the concentration to 20 μg/mL resulted in toxicity to CD4 T cells. These results demonstrated that NO was indeed involved in suppressive effects of CD11bhiIalo DCs but not in a dependent manner, because completely inhibiting NO production only partly restored the suppressive effects, suggesting there may be novel factors that we do not yet know in the supernatants.
IL-10 from ESSCs is involved in the differentiation of CD11bhiIalo DCs from HSCs
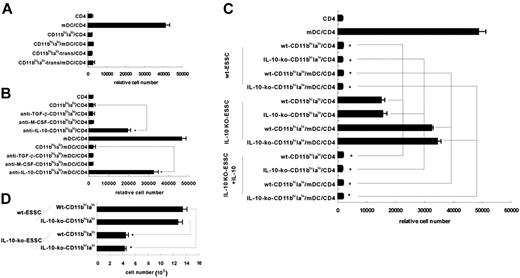
What factors determine the differentiation of Lin–Flt3– CD117+Sca-1+ bone marrow HSCs into regulatory CD11bhiIalo DCs? First, to test whether this differentiation process depends on direct contact with ESSCs or soluble factors, Lin–Flt3– CD117+Sca-1+ HSCs were cultured with fixed ESSCs or in the upper insert of a transwell system, separated from ESSCs by permeable membrane. As shown in Figure 6A, only CD11bhiIalo DCs derived from Lin–Flt3–CD117+Sca-1+ HSCs cultured in the transwell system (CD11bhiIalo-trans) had the capacity to inhibit the proliferation of CD4 T cells. These results demonstrated that CD11bhiIalo DC differentiation was dependent on soluble factors from ESSCs.
To further understand which factor was involved in CD11bhiIalo DC differentiation, TGF-β, IL-10, and M-CSF, secreted by ESSCs,14 were blocked by their respective neutralizing antibody. After 10 days of culture, different differentiated DCs were detected by their inhibitory functions. As shown in Figure 6B, blocking IL-10, instead of TGF-β or M-CSF, in the coculture system led to partial loss of inhibitory functions of CD11bhiIalo DCs, indicating IL-10 was involved in the process. To confirm this, ESSCs from IL-10 gene knockout mice were used. Although the morphology (data not shown) and phenotype (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article) of the cells induced by IL-10–KO-ESSCs were similar to those of the cells induced by wild-type ESSCs, their inhibitory ability was significantly reduced. However, when exogenous IL-10 was added into the IL-10–KO-ESSC coculture system, the lost inhibitory ability was recalled (Figure 6C). These results demonstrate that IL-10 is indeed important in the differentiation of CD11bhiIalo DCs from HSCs. This conclusion was further confirmed by another experiment, which showed that the yield of CD11bhiIalo DCs induced by IL-10–KO-ESSCs was significantly lower than that of CD11bhiIalo DCs induced by normal ESSCs (Figure 6D).
Identification of natural counterpart of CD11bhiIalo DCs in vivo and their reduction in the spleens of IL-10 KO mice
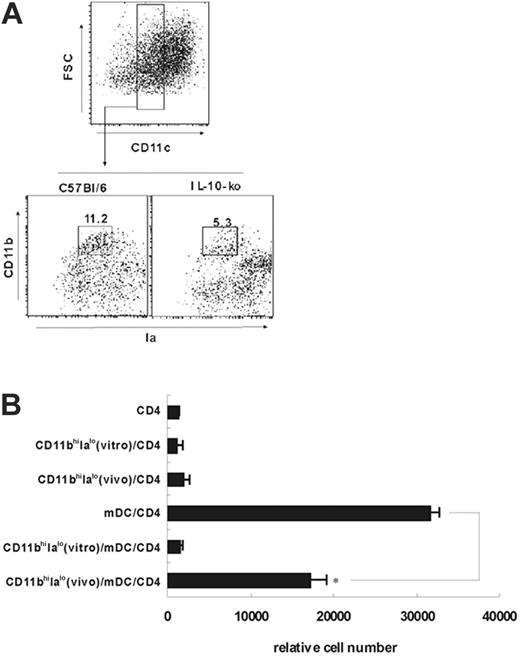
To determine whether the natural counterparts of CD11bhiIalo DCs exist in vivo, we first analyzed the splenocytes with anti-CD11b, anti-Ia, and anti-CD11c mAbs on the basis of the phenotype of CD11bhiIalo DCs and found that the CD4–CD8–B220–CD11cloCD11bhiIalo populations do exist in the spleen, and were about 11.7% of all CD4–CD8–B220–CD11c+ DCs in spleens of normal mice. Then these cells were sorted to test their functions (Figure 7A). The peptide-specific CD4 T-cell proliferation was significantly suppressed by CD11bhiIalo DCs that were directly sorted from the mouse spleen, although the suppressive capacity of this natural counterpart could not be comparable with that of CD11bhiIalo DCs induced in vitro. This might contribute to the difference between cultured cells in vitro and freshly isolated cells in vivo. Figure 7B also showed that neither the freshly sorted CD11bhiIalo DCs (in vivo) nor CD11bhiIalo DCs (in vitro prepared) could stimulate CD4 T-cell proliferation.
Endothelial stromal cell–derived IL-10 is involved in the differentiation of CD11bhiIalo DCs from HSCs. (A) HSCs were cocultured with ESSCs either directly or separated from ESSCs by permeable membrane of transwell insert in an indirect way. After 10 days of culture, CD11bhiIalo DCs in both culture systems were collected and added into CD4/mDC coculture to compare their inhibitory function. On day 5, the relative cell number of viable peptide-specific CD4 T cells was assayed by FACS. (B) At the beginning of the culture, indicated antibodies (5 μg/mL) were added into the transwell culture system. After 10 days, cells in the upper insert were collected to test their inhibitory functions. *P < .01. (C) HSCs were cocultured with wt-ESSCs or IL-10–KO-ESSCs or IL-10–KO-ESSCs plus exogenous IL-10 (5 ng/mL). After 10 days of culture, nonadherent cells from the coculture system were collected to compare their inhibitory function. On day 5, the relative cell number of viable peptide-specific CD4 T cells was assayed by FACS. *P < .01. (D) The nonadherent cell number from wt-ESSCs or IL-10–KO-ESSC coculture system were tested by FACS. *P < .01.
Endothelial stromal cell–derived IL-10 is involved in the differentiation of CD11bhiIalo DCs from HSCs. (A) HSCs were cocultured with ESSCs either directly or separated from ESSCs by permeable membrane of transwell insert in an indirect way. After 10 days of culture, CD11bhiIalo DCs in both culture systems were collected and added into CD4/mDC coculture to compare their inhibitory function. On day 5, the relative cell number of viable peptide-specific CD4 T cells was assayed by FACS. (B) At the beginning of the culture, indicated antibodies (5 μg/mL) were added into the transwell culture system. After 10 days, cells in the upper insert were collected to test their inhibitory functions. *P < .01. (C) HSCs were cocultured with wt-ESSCs or IL-10–KO-ESSCs or IL-10–KO-ESSCs plus exogenous IL-10 (5 ng/mL). After 10 days of culture, nonadherent cells from the coculture system were collected to compare their inhibitory function. On day 5, the relative cell number of viable peptide-specific CD4 T cells was assayed by FACS. *P < .01. (D) The nonadherent cell number from wt-ESSCs or IL-10–KO-ESSC coculture system were tested by FACS. *P < .01.
We also found that the in vivo quantity of CD11bhiIalo DCs in IL-10 KO mice was significantly lower than that of CD11bhiIalo DCs in normal mice (Figure 7A), convincingly demonstrating that IL-10 contributes to the development of CD11bhiIalo DCs in vivo. Because CD11bhiIalo DCs (in vivo) in IL-10–KO mice were too few to be isolated, we cannot compare their function with CD11bhiIalo DCs sorted from normal mice.
Discussion
In this work, we demonstrate that splenic stroma induces bone marrow HSCs to differentiate into regulatory DCs that are characterized by high expression of CD11b and low expression of CD11c and MHC II molecules, so these DCs were designated CD11bhiIalo DCs. CD80, CD86, and CD40 are stably expressed on its surface at low levels. Because LPS stimulation could not increase their expression, CD11bhiIalo DCs are regarded as some kind of mDCs, instead of the common DCs in an immature state. CD11bhiIalo DCs display powerful phagocytic capacity, appearing to contradict the conventional view on DCs' maturation, but there are several works indicating that even mDCs retain some capacity to endocytosis and present antigens.33-35 Moreover, we found that CD11bhiIalo DCs secrete high levels of TGF-β and IL-10, characteristic of tolerogenic/regulatory DCs.4-6 Indeed, our results show that CD11bhiIalo DCs have the capacity to inhibit the peptide-specific proliferation of CD4 T cells stimulated by mDCs both in vitro and in vivo, demonstrating that, functionally, CD11bhiIalo DCs are a subset of regulatory DCs.
Identification of the natural counterpart of CD11bhiIalo DCs in vivo. (A-B) Isolation of the natural counterpart of CD11bhiIalo DCs. (A) CD4+CD8+B220+ splenocyte cells from normal mice or from IL-10–KO mice were first depleted by negative selection by using MACS system, and CD4–CD8–B220– splenocytes were stained by Ia-FITC, CD11b-PE, and PE-Cy5 conjugated anti-CD11c mAbs. To sort CD11cloCD11bhiIalo cells, CD11clo cells were first gated and further sorted using CD11b and Ia marker by FACSDiva (wt-mice), and the percentage of CD11bhiIalo DCs among all CD4–CD8–B220–CD11c+ DCs was analyzed (wt-mice and IL-10–KO mice). (B) CD11bhiIalo cells sorted from wt-mice in vivo or induced by ESSCs in vitro were added into the CD4/mDC coculture system, respectively, to compare their inhibitory functions. After 3 days of culture, the relative cell number of viable CD4 T cells was assayed by FACS. *P < .001.
Identification of the natural counterpart of CD11bhiIalo DCs in vivo. (A-B) Isolation of the natural counterpart of CD11bhiIalo DCs. (A) CD4+CD8+B220+ splenocyte cells from normal mice or from IL-10–KO mice were first depleted by negative selection by using MACS system, and CD4–CD8–B220– splenocytes were stained by Ia-FITC, CD11b-PE, and PE-Cy5 conjugated anti-CD11c mAbs. To sort CD11cloCD11bhiIalo cells, CD11clo cells were first gated and further sorted using CD11b and Ia marker by FACSDiva (wt-mice), and the percentage of CD11bhiIalo DCs among all CD4–CD8–B220–CD11c+ DCs was analyzed (wt-mice and IL-10–KO mice). (B) CD11bhiIalo cells sorted from wt-mice in vivo or induced by ESSCs in vitro were added into the CD4/mDC coculture system, respectively, to compare their inhibitory functions. After 3 days of culture, the relative cell number of viable CD4 T cells was assayed by FACS. *P < .001.
There are several kinds of regulatory DCs reported, most of them induced by cytokine cocktail in vitro. Although these are very simple and easy methods to study the origin of regulatory DCs, they cannot reflect the actual differentiation of regulatory DCs in vivo. The regulatory DCs reported until now have similar functions but different phenotypes. The CD11c and Ia low expression of CD11bhiIalo DCs was different from that of CD11chiIahi regulatory DCs, induced by combination of mGM-CSF, mIL-10, and hTGF-β.4 Because CD80 and CD86 are expressed at low levels by CD11bhiIalo DCs, it is clearly distinguished from the CD80hiCD86hiCD40+Iaint DCs reported by Akbari et al.36 Although CD11bhiIalo DCs express CD45RB (data not shown), they do not express CD4, as expressed by CD11cloCD45RBhi DCs.6 Because CD11bhiIalo DCs express low levels of CD80 and CD40 and are differentiated from HSCs, they are also different from the diffDCs that we have described that express high levels of CD80 and CD40 and are derived from mDCs.14 These results show that, phenotypically, CD11bhiIalo DCs are a novel subset of regulatory DCs.
Functionally, inhibiting T-cell proliferation is the hallmark of regulatory DCs, but the molecular mechanisms of regulatory DCs are still poorly defined so far. There are at least 3 mechanisms that may account for their tolerogenic/regulatory properties, including absent costimulating signal, deletion of antigen-reactive cells by Fas/FasL system or tryptophan metabolites, and induction of Treg cells.37 However, we demonstrate that the inhibitory function of CD11bhiIalo DCs is not mediated by inducing the production of Treg cells or T-cell anergy, which was reported as the main inhibitory mechanism of regulatory DCs.4-6 Although high levels of TGF-β and IL-10, which were shown to be important in the induction of tolerance38-41 and Treg cells,42,43 were secreted by CD11bhiIalo DCs in our experiments, they did not promote the production of Treg cells. We thought this might contribute to the distinct functional features of CD11bhiIalo DCs, which are induced by splenic stroma, a very complex and physiologic condition, not simply under the conditions of single or cocktail cytokines. Also, FasL was not detected on the surface of CD11bhiIalo DCs (data not shown). Interestingly, we found that CD11bhiIalo DCs could effectively present antigen to peptide-specific CD4 T cells and vigorously activate them to secrete high levels of IFN-γ without proliferation, potently inhibiting the proliferation of peptide-specific CD4 T cells stimulated by mDCs, without depressing IL-2 or IFN-γ secretion. These results showed that regulatory functions of CD11bhiIalo DCs for T-cell response are multiple. Our further results suggest that NO was involved in this process, instead of IL-10, TGF-β, PGE2, or IDO.31 NO inhibits T-cell proliferation by acting on multiple members of the IL-2 signaling pathway, particularly JAK3 and JAK1.44 These results clearly showed that CD11bhiIalo DCs display their inhibitory function in a novel way: by directly secreting soluble inhibitory factors, including NO, instead of indirectly, such as by inducing Treg cells, confirming that, functionally, CD11bhiIalo DCs are a distinct regulatory DCs subset.
Interestingly, a regulatory DC, CD11cloCD45RB+, also induced by splenic stroma, was recently reported by Svensson et al45 ; there are several differences between those cells and the CD11bhiIalo DCs described here. First, CD11cloCD45RB+ DCs were derived from lineage-negative c-kit+ progenitor cells, whereas CD11bhiIalo DCs were from HSCs. Second, CD11bhiIalo DCs were induced by endothelial stroma instead of by stroma comprising principally fibroblasts and macrophages, as reported by Svensson et al.45 The most important difference is the inhibitory effect of CD11bhiIalo DCs was NO involved, instead of inducing Treg cells. All the differences showed that CD11cloCD45RB+ DCs and CD11hiIa DCs are not the same subsets of regulatory DCs.
In one study, Kamath et al34 found a rapid turnover of DC subsets in mouse spleen within 3 to 4 days, suggesting that in adult mice, the differentiation of DCs from progenitors in situ in the spleen might exist. But it is unclear how splenic microenvironment induces DC differentiation. Our results demonstrate that, in vitro, the ESSC-derived soluble factors are essential for the differentiation of CD11bhiIalo DCs, and we further demonstrate that IL-10 secreted by ESSCs is important in this process by using IL-10–blocking antibody and IL-10–KO-mice. Although it is very difficult to directly demonstrate HSC differentiation into DCs in the spleen in situ from the beginning of development in vivo, we cannot exclude this possibility, because the spleen has been the hematopoietic organ in new neonatal phase and HSCs can be found even in adult spleens.8 Maybe our system mimicked this development process.
Moreover, natural counterparts of CD11bhiIalo DCs that do not express CD4 and CD8 were found in vivo. Although CD4–CD8– DCs in spleens were reported,46 it is unclear whether subpopulations in these double-negative populations exist, and how they function is unclear. In this report, we clearly showed that CD11c-low expression population in CD4–CD8– DCs can be further classified into several subsets according to the extent of CD11b and Ia expression. Interestingly, CD11bhiIalo DCs had inhibitory function similar to that of CD11bhiIalo DCs induced by splenic stroma in vitro, indicating that these CD11cloCD11bhiIalo DCs in normal mouse spleens were the natural counterparts of CD11bhiIalo DCs in vitro and were a functionally specialized DC subset in the spleen. Our work, together with the findings of Wakkach et al,6 demonstrated that there are specialized regulatory DCs in the spleen that might play important roles in maintenance of immune balance.
We also demonstrate that in vitro–cultured CD11bhiIalo DCs have the ability to suppress the proliferation of the peptide-specific CD4 T cells in vivo and to suppress alloantigen-specific responses in vivo, opening new preventive or therapeutic perspectives for the use of CD11bhiIalo DCs in autoimmune disease, inflammatory disease, or allogeneic transplantation.
In conclusion, we demonstrate that the spleen microenvironment could self-regulate the immune response by driving HSCs in spleen to differentiate into CD11bhiIalo DCs, a novel regulatory DC subset in an IL-10–dependent way. Such DCs secret TGF-β, IL-10, and NO and exhibit regulatory functions both in vitro and in vivo. The inhibitory function is NO involved, instead of inducing Treg cells or anergy. The ability of CD11bhiIalo DCs to suppress allo-DTH in vivo indicates that these cells may have preventive or therapeutic prospects for some immunologic disorders.
Prepublished online as Blood First Edition Paper, April 20, 2006; DOI 10.1182/blood-2006-01-007187.
Supported by grants from the National Key Basic Research Program of China (2003CB515503 and 2001CB510002) and the National Natural Science Foundation of China (30490240 and 30121002).
X.C. and H.T. designed the research project, analyzed the data, and wrote the paper. H.T., Z.G., M.Z., G.C., and J.W. performed the experiments.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Qian Li, Dr Sheng Xia, Ms Rui Zhang, and Ms Chunfang Luo for their excellent technical assistance. We thank Prof You-Wen He from Duke University for helpful discussion.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal