Abstract
We studied the cellular and molecular effects of the combination of an anthracycline with 2 different histone deacetylase inhibitors (HDACIs): vorinostat (suberoylanilide hydroxamic acid) and valproic acid (VPA). The 10% inhibitory concentration (IC10) of idarubicin was 0.5 nM in MOLT4 and 1.5 nM in HL60 cells. Concentrations above 0.675 μM of vorinostat resulted in at least 80% loss of cell viability in both cell lines. Concentrations of 1.5 to 3 mM of VPA induced 50% to 60% loss in viability in HL60 and 80% in MOLT4 cells. The combination of idarubicin with vorinostat at 0.075 μM or VPA at 0.25 mM resulted in at least an additive loss of cell viability in both lines. Vorinostat (0.35 μM) and VPA (0.25 mM) in combination with idarubicin (0.5 nM) resulted in a significant increase in apoptotic cells in MOLT4 cells. The combination resulted in an increase in histone H3 and H4 acetylation at 24 hours, phosphorylated H2AX, as well as in the induction of p21CIP1 mRNA. No effect on cell cycle transition was observed. Of importance, the cellular and molecular effects observed were independent of the sequence used. In summary, the combination of an anthracycline with an HDACI should have significant clinical activity in patients with leukemia.
Introduction
In this manuscript, we describe the cellular and molecular effects of the combination of an anthracycline with a histone deacetylase inhibitor (HDACI) in leukemia cell lines.
DNA topoisomerase II inhibitors, such as anthracyclines, are potent antineoplastic agents commonly used in the treatment of patients with leukemia, lymphoma, and diverse solid tumor malignancies. They exert their function by inducing the formation of a stable complex between the enzyme DNA topoisomerase II and the DNA. This results in the introduction of double-strand DNA breaks and alterations in replication and transcription that trigger apoptosis and cell death.1
HDACIs are a relatively new class of antineoplastic agents.2 Both in vitro and in vivo, these agents have the capacity to inhibit histone deacetylase activity. This effect results in the acetylation of key lysine residues in core histones, a phenomenon associated with a permissive gene expression state. Several structurally diverse HDACIs are currently being studied in clinical trials in humans.3 In vitro, this class of drugs has been shown to have the capacity to induce apoptosis, cell differentiation, and cell cycle arrest.3 Two HDACIs, depsipeptide4,5 and the hydroxamic acid derivative vorinostat (suberoylanilide hydroxamic acid [SAHA]),6 have been shown to have activity in cutaneous T-cell lymphoma (CTCL). The activity in myelodysplastic syndrome (MDS) and leukemia has been more modest but not negligible,7,8 whereas durable complete responses in patients with solid tumors are rare.
Based on their mechanism of action, it is likely that HDACIs will have their broadest activity in combination with other agents. To model this concept in vitro and as the preclinical basis for future clinical studies, we have studied the effects of the combination of a frequently used DNA topoisomerase II inhibitor in leukemia, idarubicin, with 2 different and structurally unrelated HDACIs: vorinostat and valproic acid (VPA). We hypothesized that the combination of both classes of agents will result in synergistic antileukemia activity in vitro based on their chromatin effects. In particular, HDACIs induce histone acetylation, a phenomenon associated with an open chromatin configuration,3 whereas DNA topoisomerase II inhibitors induce DNA breaks. Of importance, DNA topoisomerase II consensus sequences have been reported to be present in genomic regions known as matrix attachment regions (MARs).9 MARs are known to flank polynuclesomes and to be involved in the temporal control of gene expression regulation. Based on this information, we predicted that the effect of the combination will be sequence dependent and that exposure first to an anthracycline would result in disruption of MARs, opening polynuclesomes and thus facilitating access to the HDACI.
To explore the cellular and molecular effects of the combination of anthracycline with an HDACI and as the preclinical bases for clinical trials, we have studied the combination of idarubicin, a potent anthracycline, and the HDACIs vorinostat and VPA in leukemia cell lines.
Patients, materials, and methods
Cell lines and drugs
The leukemia cell lines MOLT4, of lymphoid origin, and HL60, of myeloid origin, were obtained from the American Type Culture Collection (Manassas, VA) and were grown following standard conditions. Cells were plated 4 to 6 hours prior to drug exposure. Drugs used included idarubicin (Pharmacia Italia, Milan, Italy), valproic acid (VPA; Sigma, St Louis, MO), and vorinostat (Aton Pharma, Tarrytown, NY, a wholly owned subsidiary of Merck & Co, Whitehouse Station, NJ). Cell culture medium containing drug or the same volume of vehicle was changed every 24 hours. All experiments were performed at least in triplicate.
Cell viability assays and analysis of apoptosis
Cell viability was assessed using trypan blue staining (Sigma) following manufacturer instructions. Induction of apoptosis was quantitated measuring annexin V–positive cells by flow cytometry using the TACS Annexin V–FITC Kit (Trevigen, Gaithersburg, MD). Briefly, cells were washed with PBS and incubated with 4 μL annexin V incubation reagent in the dark for 15 minutes at room temperature. Subsequently, 400 μL1 × binding buffer was added before analysis using a FACSort flow cytometer (Becton Dickinson Systems, San Jose, CA) equipped with the Cell Quest Pro software. Data were analyzed using the ModFit LT v 3.1 software (Verity Software House, Topsham, ME).
Ex vivo analysis of fresh leukemic cells
Peripheral blood samples were obtained from patients with acute leukemia following institutional guidelines. Mononuclear cells were separated using Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) and were subsequently cultured in RPMI 1640 supplemented with 10% fetal bovine serum. After 24 hours in culture, cells were then treated with vorinostat (0.45 μM), idarubicin (1 to 1.5 nM), or the combination of both drugs at the same concentrations. Cells were exposed to the combination of idarubicin and vorinostat concomitantly or in sequence: vorinostat followed 4 hours later by idarubicin, or the reverse. Untreated cells from the same patient served as controls. The effects of vorinostat, idarubicin, or the combination on cell viability were assessed using trypan blue staining as described under “Cell viability assays and analysis of apoptosis” and with an MTS assay (CellTiter 96 AQueous One Solution Reagent; Promega Corporation, Madison, WI) following manufacturer's recommendations. The predicted versus observed effect on cell viability was measured using the method of Webb described below under “Statistical analysis.”10 Percentages were normalized to the number of viable cells detected in the untreated control experiments.
Cell cycle analysis
Cell cycle analysis was performed using propidium iodide (PI) staining. Cells (100 × 106) were washed with PBS and fixed by incubation in 70% ethanol at room temperature for at least 30 minutes, then stored at –20°C overnight. After washing twice, cells were stained with PI (50 μg/mL). RNase A (10 mg/mL; Bachem California, Torrance, CA) was added 30 minutes before flow cytometry analysis. Red fluorescence was measured with a FACScan (Becton Dickinson). Data were analyzed using ModFIT LT v 3.1 software (Verity Software House).
Measurement of histone H3 and H4 acetylation
Histone H3 and H4 acetylation was analyzed by Western blot using antibodies directed against acetylated histone H3 or H4. Proteins were extracted using a lysis buffer (50 mM Tris HCl, pH 8.0; 150 mM NaCl; 0.02% Na Azid; 0.2% SDS; 2% Nonidet P-40 [NP-40]; 0.5% Na deoxycholate). Thirty micrograms of protein from each cell lysate was separated in 12% SDS–polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to Immobilion-P Transfer Membranes (Millipore, Billerica, MA). Membranes were blocked in 3% nonfat milk in PBS containing 0.1% Tween 20 (PBS-T) probed overnight at 4°C with 1:2000 dilution of acetylated histone H3 or H4 antibodies (Upstate Biotechnology, Waltham, MA). Membranes were washed 3 times with PBS-T and were incubated with an antirabbit peroxidase-conjugated secondary antibody in all experiments (1:2000; Amersham Biosciences, Little Chalfont, United Kingdom) for 1 hour at room temperature. Membranes were then washed 3 times in PBS-T and the film was developed using enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). β-Actin (1:5000; Sigma) was used as internal control.
Analysis of γ H2AX induction
We have analyzed the induction of γH2AX as a marker of formation of double-strand DNA breaks. Two methods were used to detect γH2AX. First, we performed immunohistochemistry. Fixed paraffin-embedded cells were stained using a phospho-histone H2AX antibody (1:800; Becton Dickinson) followed by avidin-biotin–peroxidase labeling with an automated immunostainer (Autostainer Plus; DakoCytomation, Carpinteria, CA). All cell sections underwent heat-induced antigen retrieval. To confirm the immunohistochemistry results, Western blot analysis was performed using a polyclonal antiphospho-histone H2AX antibody (1:500; Upstate Biotechnology) following the same protocol as for acetylated histone H3 or H4 described under “Measurement of histone H3 and H4 acetylation,” except that the RIPA lysis buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EGTA; 1 mM PMSF; 1 μg/mL each aprotinin, leupeptin, pepstatin; 1 mM Na3VO4; 1 mM NaF) was used. Cell lysates from Jurkat cells treated with 0.5 μM of stausporine (Sigma) for 6 hours were used as a positive control of γH2AX induction.
Real-time PCR analysis of DNA topoisomerase IIα and p21CIP1 gene expression
We have used real-time polymerase chain reaction (PCR) assays to study the effects of the combination/sequences analyzed on the mRNA expression of known target genes for the drugs studied here. DNA topoisomerase II is the target enzyme of idarubicin. The cyclin-dependent kinase inhibitor p21CIP1 is reactivated by vorinostat and VPA.11,12 Total cellular RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Five micrograms total RNA was used for reverse transcription (RT) reactions using the Moloney murine leukemia virus RT enzyme (Invitrogen) following the manufacturer's protocol.
Levels of p21CIP1 and DNA topoisomerase IIα mRNA expression were analyzed using real-time PCR. Primers and probes were purchased from Applied Biosystems (Hs00 355 782_m1 and Hs00 172 214_m1) and analyzed using an Applied Biosystems Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). PCR reactions were performed using 20 × Assays-On-Demand Gene Expression Assay Mix (containing unlabeled PCR primers and TaqMan probes) and TaqMan Universal PCR Master mix (Applied Biosystems) according to the manufacturer's protocol. PCR conditions were 95°C for 10 minutes and then 95°C for 15 seconds and 60°C for 1 minute; the last 2 steps were repeated for 40 cycles. Experiments were performed in triplicate. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. Quantitative values were obtained from the cycle number (CT value) at which an increase in fluorescent signal associated with an exponential accumulation of PCR products was detected. The amount of target gene was normalized to the endogenous reference GAPDH to obtain the relative threshold cycle (ΔCT) and then related to the CT of the control to obtain the relative expression level (2–ΔΔCT) of target gene.
Statistical analysis
The fractional product of Webb was used to determine whether the effect on cell viability and apoptosis induced by the combinations was additive/synergistic or antagonistic.10 This method is appropriate to do this calculation, especially if nonexclusive drugs (agents with independent mechanisms of action) are studied. The method uses the following formula: 1–i1,2 = (1–i1)(1–i2); where i1,2 is the inhibitory fraction of the combination, i1 of one drug, i2 of the other drug. For instance if i1 = .5 and i2 = .5, then i1,2 = 0.25 and the fractional product F = (1–.25) = 0.75. A combination is considered unlikely to be antagonistic if the calculated i1,2,orthe F or (1–i1,2), is below the observed i1,2.
Results
Effects of idarubicin in combination with valproic acid or vorinostat on cell viability and apoptosis
MOLT4 and HL60 cells were exposed to increasing concentrations of idarubicin (0.5 to 20 nM), VPA (0.25 to 3 mM), or vorinostat (0.3 to 3 μM) daily for 3 days. In these experiments, medium containing drug or the same volume of vehicle was changed daily for 3 days. Using trypan blue cell viability assays, the 10% inhibitory concentration (IC10) of idarubicin was 0.5 nM for MOLT4 cells and 1.5 nM for HL60 cells (Figure 1A-B). Concentrations in excess of 0.675 μM of vorinostat resulted in more than 80% decrease in cell viability of both cell lines (Figure 1C-D). In contrast, concentrations of 1.5 mM to 3 mM of VPA were needed to induce 50% to 60% loss of viability in HL60 and 80% to 90% in MOLT4 cells (Figure 1E-F).
Subsequently, combination studies using idarubicin at its cell-specific IC10, as a fixed concentration, with vorinostat at increasing concentrations (0.075 to 1 μM), or VPA (0.25 to 3 mM) were performed. In these experiments both drugs were introduced simultaneously. Medium containing the combination of drugs, or vehicle, was replaced daily for 3 days. As shown in Figure 2, the combination of vorinostat or VPA with idarubicin resulted in a dose-dependent decrease in the number of viable cells in both cell lines. To predict whether the combinations studied could potentially result in antagonism, the fractional product method of Webb was used.10 At each dose level, the observed effect on cell viability was greater than the predicted (Table S1, available on the Blood website; see the Supplemental Tables link at the top of the online article). This indicates that that the effects of the combinations on cell viability are unlikely to be antagonistic and are most likely additive/synergistic.
Effects of idarubicin, vorinostat, or VPA in HL60 and MOLT4 cell viability. Cell viability was studied using trypan blue assays. Numbers on the y-axis represent the total number of viable cells. (A-B) HL60 and MOLT4 cells were treated with increasing concentrations of idarubicin (ida; 0.5-20 nM) daily for 3 days. Subsequently, both cell lines were treated with increasing concentrations of (C-D) vorinostat (Vor; 0.3-3 μM) or (E-F) VPA (0.25-3 mM) both daily for 3 days. Bars indicate the standard deviation.
Effects of idarubicin, vorinostat, or VPA in HL60 and MOLT4 cell viability. Cell viability was studied using trypan blue assays. Numbers on the y-axis represent the total number of viable cells. (A-B) HL60 and MOLT4 cells were treated with increasing concentrations of idarubicin (ida; 0.5-20 nM) daily for 3 days. Subsequently, both cell lines were treated with increasing concentrations of (C-D) vorinostat (Vor; 0.3-3 μM) or (E-F) VPA (0.25-3 mM) both daily for 3 days. Bars indicate the standard deviation.
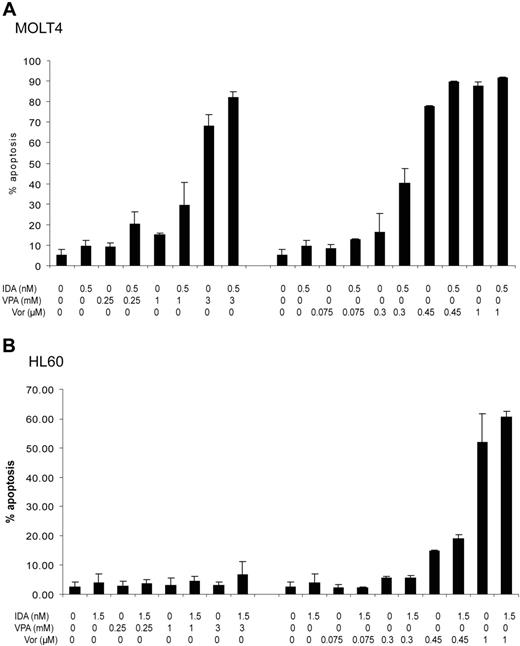
To study if the increment in nonviable cells was secondary to the induction of apoptosis, we performed annexin V flow cytometry assays (Figure 3). In MOLT4 cells, the combination of idarubicin with VPA at concentrations of at least 0.25 mM, or vorinostat of at least 0.3 μM, resulted in an increased fraction of apoptotic cells (Figure 3A). Using the Webb formula, this effect is unlikely to be antagonistic (Table S1). In contrast, HL60 cells were more resistant to the induction of apoptosis (Figure 3B). VPA alone or in combination was unable to induce a significant increase in annexin V–positive cells. Concentrations of at least 0.45 μM of vorinostat were needed to induce a significant fraction of apoptotic cells and concentrations of at least 1 μM to observe an additive/synergistic effect (Table S1).
Effect of the sequence of treatment on cell viability and apoptosis
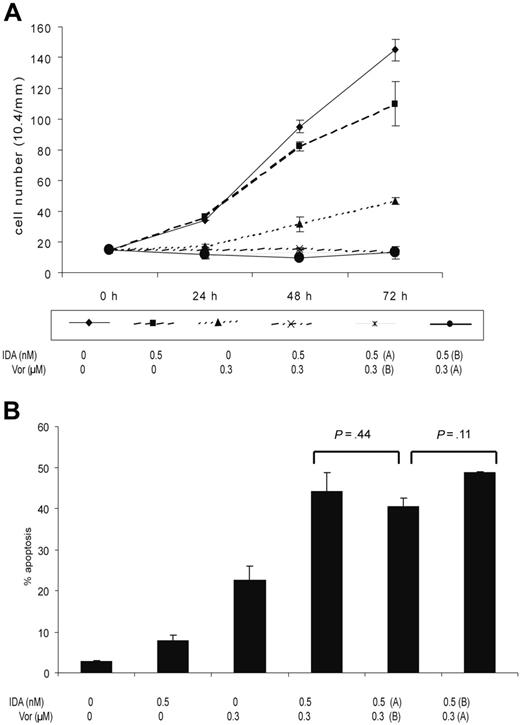
To study the effects on cell viability and apoptosis of the treatment sequence, MOLT4 cells were treated with vorinostat at 0.3 μM and idarubicin at 0.5 nM. Cells were exposed to both drugs concomitantly, or vorinostat was added 4 hours before or after idarubicin, daily for 3 days. In these experiments, medium was changed daily and the sequence of the combination of drugs, or vehicle, was added accordingly. As shown in Figure 4, cell viability and apoptosis were independent of the sequence used. The effect of the sequence was also studied with other concentrations of vorinostat (0.075-1 μM) and using different sequences, such as adding vorinostat 24 hours before or after idarubicin. No significant differences between the sequences used were observed using these other schedules (data not shown).
Effects of vorinostat or VPA in combination with idarubicin on cell viability. Cell lines were treated with increasing doses of VPA (0.25-3 mM) or vorinostat (0.075-1 μM) concomitantly with idarubicin at 0.5 nM in MOLT4 or 1.5 nM in HL60 cells daily for 3 days. Numbers on the y-axis represent the percentage of viable cells. (A) MOLT4 cells. (B) HL60 cells. IDA indicates idarubicin; VPA, valproic acid; and Vor, vorinostat. Error bars indicate standard deviation.
Effects of vorinostat or VPA in combination with idarubicin on cell viability. Cell lines were treated with increasing doses of VPA (0.25-3 mM) or vorinostat (0.075-1 μM) concomitantly with idarubicin at 0.5 nM in MOLT4 or 1.5 nM in HL60 cells daily for 3 days. Numbers on the y-axis represent the percentage of viable cells. (A) MOLT4 cells. (B) HL60 cells. IDA indicates idarubicin; VPA, valproic acid; and Vor, vorinostat. Error bars indicate standard deviation.
Effect of vorinostat, idarubicin, or the combination on the viability of fresh leukemic cells
The effects of vorinostat, idarubicin, or the combination (administered concomitantly or in sequence) on cell viability were analyzed in fresh leukemic cells. To perform these studies, cells from 3 patients with acute leukemia with excess of blasts in peripheral blood were collected. The characteristics of the patients are summarized in Table S2. The ex vivo effects of the different combinations are shown in Table 1. These values were normalized to the number/percentage of viable cells counted on the untreated control cells. Concentrations of vorinostat and idarubicin were selected from the initial experience with leukemic cell lines.
Observed versus predicted effect on cell viability and in fresh leukemic cells
. | Patient no. 1 . | . | Patient no. 2 . | . | Patient no. 3 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Combination . | Observed . | Predicted . | Observed . | Predicted . | Observed . | Predicted . | |||
| Ida + SAHA | 58 | 44 | 73 | 48 | 73 | 72 | |||
| Ida→SAHA | 61 | 44 | 70 | 48 | 72 | 72 | |||
| SAHA→Ida | 57 | 44 | 64 | 48 | 74 | 72 | |||
. | Patient no. 1 . | . | Patient no. 2 . | . | Patient no. 3 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Combination . | Observed . | Predicted . | Observed . | Predicted . | Observed . | Predicted . | |||
| Ida + SAHA | 58 | 44 | 73 | 48 | 73 | 72 | |||
| Ida→SAHA | 61 | 44 | 70 | 48 | 72 | 72 | |||
| SAHA→Ida | 57 | 44 | 64 | 48 | 74 | 72 | |||
Ida indicates idarubicin; SAHA, vorinostat; Ida + SAHA, concomitant administration; Ida→SAHA, idarubicin followed by vorinostat; SAHA→Ida, vorinostat followed by idarubicin.
As in the cell line experiments, treatment of fresh leukemic cells with the combination resulted in a greater reduction of cell viability compared with the predicted effects based on the Webb formula,10 and this effect was independent of sequence. These results were confirmed using an MTS assay (data not shown).
Changes in histone H3 and H4 in acetylation with VPA or vorinostat in combination with idarubicin
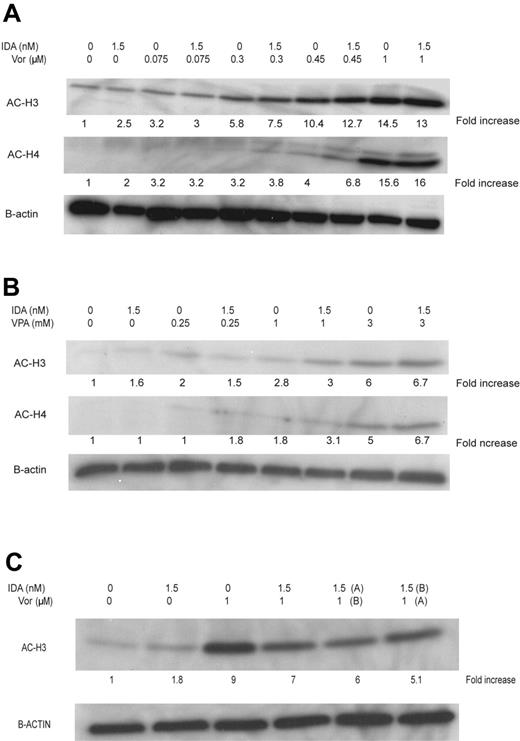
Global histone H3 and H4 acetylation was analyzed by Western blot using the combination sequences and doses described under “Effects of idarubicin in combination with valproic acid or vorinostat on cell viability and apoptosis” and “Effect of the sequence of treatment on cell viability and apoptosis.” An increase in H3 and H4 acetylation was observed at doses of vorinostat between 0.3 μM and 0.45 μM and VPA at 1 to 3 mM in combination with idarubicin at 24 hours (Figure 5A-B). This effect was independent of the sequence used.
Induction of γ H2AX
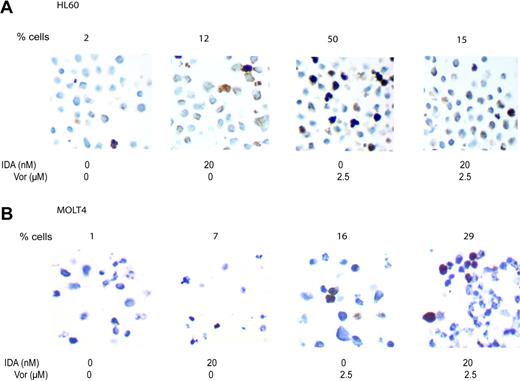
To evaluate the induction of γH2AX, idarubicin was used at a concentration of 20 nM and vorinostat at 2.5 μM. Using immunocytochemistry (Figure 6), γH2AX expression was detected in 1% of HL60 and MOLT4 cells before treatment. Upon exposure to idarubicin, 12% (HL60) and 7% (MOLT4) of cells expressed γH2AX. Using vorinostat alone, 50% of HL60 cells and 16% of MOLT4 cells expressed detectable γH2AX. With the combination of both drugs the results differed between the cell lines. In HL60 cells, a decreased percentage of positive cells was observed (19%), whereas an increase (29%) in MOLT4 cells was observed. These effects were independent of the sequence used (combining both drugs together or reversing the order of administration). These data were confirmed using Western blot (data not shown).
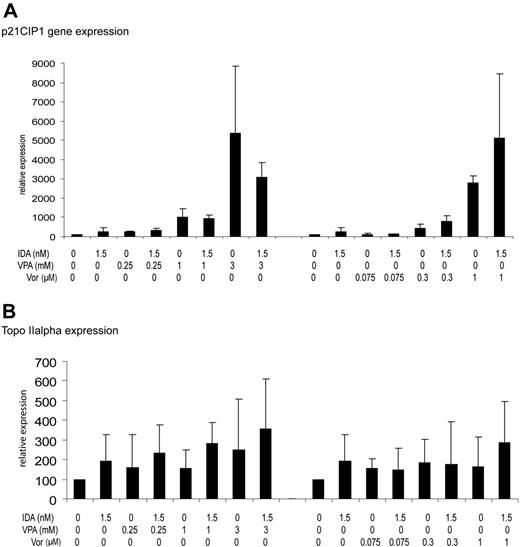
Induction of p21CIP1 and DNA topoisomerase IIα mRNA gene expression
Expression of p21CIP mRNA was restored by single-agent VPA, vorinostat, or idarubicin and was dose dependent (Figure 7A). The combination of idarubicin with either VPA or vorinostat did not result in any significant change in the induction of p21 compared with each drug alone.
Induction of apoptosis. The percentage of apoptotic cells was determined using annexin V flow cytometry assays. Cell lines were treated with increasing doses of VPA (0.25-3 mM) or vorinostat (0.075-1 μM) in combination with idarubicin at 0.5 nM in MOLT4 or 1.5 nM in HL60 cells daily for 3 days. Numbers on the y-axis represent percentage of apoptotic cells. (A) MOLT4 cells. (B) HL60 cells. IDA indicates idarubicin; VPA, valproic acid; and Vor, vorinostat (suberoylanilide hydroxamic acid). Error bars indicate standard deviation.
Induction of apoptosis. The percentage of apoptotic cells was determined using annexin V flow cytometry assays. Cell lines were treated with increasing doses of VPA (0.25-3 mM) or vorinostat (0.075-1 μM) in combination with idarubicin at 0.5 nM in MOLT4 or 1.5 nM in HL60 cells daily for 3 days. Numbers on the y-axis represent percentage of apoptotic cells. (A) MOLT4 cells. (B) HL60 cells. IDA indicates idarubicin; VPA, valproic acid; and Vor, vorinostat (suberoylanilide hydroxamic acid). Error bars indicate standard deviation.
In contrast, no significant effect was observed for DNA topoisomerase IIα mRNA expression. Only at doses of more than 1 mM of VPA, alone or in combination with idarubicin, was a modest increase on DNA topoisomerase IIα mRNA observed (Figure 7B). Of note, no decrease in DNA topoisomerase IIα mRNA was observed. This was not affected by the use of the drugs in combination (Figure 7B).
Effect of the combination of idarubicin with vorinostat or VPA on cell cycle
The induction of p21CIP1 by all 3 drugs and in combination raised the possibility that this activity may result in cell cycle arrest. To study this, we analyzed cell cycle status in both cell lines exposed to each drug alone or in combination for 3 days. As shown in Table S3, no effect was observed in cell cycle transition when using these combinations.
Discussion
Vorinostat is a hydroxamic acid derivative that has been extensively studied both in vitro and in vivo.13 Clinically, vorinostat has demonstrated activity in patients with CTCL, mesothelioma, and other solid tumors.14,15 In a phase 1 study in leukemia, vorinostat has demonstrated single-agent activity in patients with advanced acute myelogenous leukemia.8
VPA is a commonly used antiepileptic agent that has been shown to have HDAC inhibitory activity in vivo and in vitro,16,17 antileukemia activity in vitro,12 and modest activity as a single agent in MDS.18 Because of their effects on chromatin, by inducing histone acetylation, and their capacity to restore epigenetically suppressed genes, HDACIs have attracted significant clinical interest.19 Despite their well-characterized molecular and cellular effects, the single-agent activity of this class of drugs has been modest, and therefore there is a need for developing rationally designed combination strategies.
In this study, we have analyzed the cellular and molecular effects of the combination of an anthracycline and an HDACI in leukemia cell lines. The rationale for the combination of an HDAC inhibitor and anthracycline can be approached from a mechanistic or a functional perspective. Mechanistically, it has been proposed that exposure to either the HDACI or the anthracycline will result in a more permissive chromatin configuration, allowing better access to the respective drug. For instance, we based our initial hypothesis on the fact that polynucleosmes are flanked by MARs. These regions are crucial for the maintenance of chromatin integrity and are rich in DNA topoisomerase II consensus sequences.9 Exposure to the DNA topoisomerase II inhibitor first would allow better access to the nucleosome by the HDACI. Other groups have hypothesized that exposure first to the HDACI followed by the DNA topoisomerase II inhibitor would allow for a relaxed chromatin configuration that would enhance the effect of the anthracycline.20,21 Finally, HDACIs have been reported to enhance the mRNA expression of DNA topoisomerase II and therefore facilitate the activity of the anthracycline.22 The predictions from these different models have clinical implications, as they indicate that a specific sequence may be the most effective way to administer this combination.
Effect of the sequence on cell viability and apoptosis. MOLT4 cells were treated with vorinostat at 0.3 μM and idarubicin at 0.5 nM concomitantly or IDA was added 4 hours before vorinostat (Vor) (IDA, A; vorinostat, B) or after vorinostat (IDA, B; vorinostat, A) daily for 3 days. (A) Numbers on the y-axis represent the number of viable cells. (B) Numbers on the y-axis represent the percentage of apoptotic cells. Error bars indicate standard deviation.
Effect of the sequence on cell viability and apoptosis. MOLT4 cells were treated with vorinostat at 0.3 μM and idarubicin at 0.5 nM concomitantly or IDA was added 4 hours before vorinostat (Vor) (IDA, A; vorinostat, B) or after vorinostat (IDA, B; vorinostat, A) daily for 3 days. (A) Numbers on the y-axis represent the number of viable cells. (B) Numbers on the y-axis represent the percentage of apoptotic cells. Error bars indicate standard deviation.
As preclinical bases for a phase 1 study of the combination of idarubicin with vorinostat in leukemia, we performed a series of cellular studies to determine the cytotoxic characteristics of the combination, the effects of sequence, and the analysis of potential biomarkers that could then be used in clinical trials. Using a similar system, we have successfully completed a clinical trial combining a hypomethylating agent and VPA,12 and therefore we anticipate that the information derived from this study will have significant clinical potential.
Induction of histone H3 and H4 acetylation. HL60 cells were treated with (A) vorinostat (Vor) at increasing concentrations or (B) VPA. The effect on the sequence of IDA with vorinostat using concentrations of 1 μM of vorinostat and IDA at 1.5 nM administered concomitantly, or with IDA preceding vorinostat (IDA, A; vorinostat, A-B) or the reverse (IDA, B; vorinostat, A), were studied (C).
Induction of histone H3 and H4 acetylation. HL60 cells were treated with (A) vorinostat (Vor) at increasing concentrations or (B) VPA. The effect on the sequence of IDA with vorinostat using concentrations of 1 μM of vorinostat and IDA at 1.5 nM administered concomitantly, or with IDA preceding vorinostat (IDA, A; vorinostat, A-B) or the reverse (IDA, B; vorinostat, A), were studied (C).
To perform these studies, first we determined the IC10 of idarubicin in 2 leukemia cell lines and confirmed the antileukemia activity of both vorinostat and VPA in these cells. As expected, vorinostat at clinically achievable concentrations was a more potent inducer of loss of cell viability than VPA. The effect on cell viability of idarubicin was potentiated by the addition of either vorinostat or VPA, an effect that is unlikely to be antagonistic and likely additive/synergistic. These results were confirmed when fresh leukemic cells obtained from patients were treated ex vivo with idarubicin and vorinostat. This effect was concentration dependent but it was observed at low concentrations, clearly achievable in humans, of both VPA and vorinostat. Despite the fact that all combinations resulted in a significant loss of cell viability, the induction of apoptosis was cell specific. In MOLT4 cells, the loss of cell viability was directly related to the induction of annexin V–positive cells. In contrast, HL60 cells were more resistant to the induction of apoptosis, especially to VPA. Lack of induction of apoptosis, despite the loss of cell viability, has been observed in other cell models with VP-1620 and in combination with irradiation.23 It is possible that cell death in these models involves other mechanisms, such as necrosis.20 Recently autophagy has been proposed to be a mechanism involved in vorinostat-induced cell death.24
H2AX phosphorylation. HL60 cells (A) and MOLT 4 cells (B) were treated for 8 hours with vorinostat (Vor) 2.5 μM or IDA 20 nM or the combination. H2AX phosphorylation was analyzed using immunohistochemistry in paraffin-embedded cell suspensions. Numbers at the top of each image represent the percentage of cells staining for γH2AX. Images were acquired using a Zeiss Axiovert S100 inverted microscope equipped with a 40×/0.65 objective lens (Zeiss, Thornwood, NY) and a Hamamatsu cooled CCD camera (Hamamatsu, Hamamatsu City, Japan) and were digitally stored with WebSlide Browser (Bacus Laboratory, Lombard, IL). Images were obtained using 100× magnification.
H2AX phosphorylation. HL60 cells (A) and MOLT 4 cells (B) were treated for 8 hours with vorinostat (Vor) 2.5 μM or IDA 20 nM or the combination. H2AX phosphorylation was analyzed using immunohistochemistry in paraffin-embedded cell suspensions. Numbers at the top of each image represent the percentage of cells staining for γH2AX. Images were acquired using a Zeiss Axiovert S100 inverted microscope equipped with a 40×/0.65 objective lens (Zeiss, Thornwood, NY) and a Hamamatsu cooled CCD camera (Hamamatsu, Hamamatsu City, Japan) and were digitally stored with WebSlide Browser (Bacus Laboratory, Lombard, IL). Images were obtained using 100× magnification.
Several prior reports have provided information regarding the importance of sequence of administration of the combination of these drugs for maximal antineoplastic effect. Indeed, we had predicted that the administration of idarubicin followed by the HDACI will be the optimal sequence of this combination. In 3 other studies, pretreatment with the HDACI resulted in an increase in cell loss.20-22 In contrast, in another study, pretreatment with the HDACI resulted in suppression of apoptosis.25 Finally in a study using MS-275, another HDACI, with radiation therapy, cells exposed to the HDACI before and after irradiation were more susceptible to the combination.23 Because of the implications of these observations, we analyzed the cellular and molecular effects of different sequence schedules. Our results are in contrast with some of the prior observations, including our prediction, in that the effects on cell viability; apoptosis; induction of histone acetylation, γH2AX, and p21; and DNA topoisomerase II mRNA expression were independent of the sequence used. It should be noted that the majority of the prior models consisted of short exposures to both the HDACI and the DNA topoisomerase II inhibitor.20,21,25 In our studies, we incubated both cell lines using different sequences for 3 days, a model that is closer to current clinical schedules of anthracycline administration. Using our schedules, the concomitant use of both drugs resulted in optimal results in terms of cell kill and induction of apoptosis. The combination of both classes of drugs resulted in an increase in global histone acetylation at 24 hours. This was also independent of the sequence combination used.
DNA topoisomerase IIα gene expression and p21CIP1. The effect in HL60 cells of treatment for 3 days with vorinostat (Vor; 0.075-1 μM), VPA (0.25-3 mM), or IDA (1.5 nM) or the combination on p21CIP1 or DNA topoisomerase IIα mRNA expression was determined using real-time PCR assay. Numbers on the y-axis represent relative expression of delta CT. (A) p21CIP1. (B) DNA topoisomerase IIα. Error bars indicate standard deviation.
DNA topoisomerase IIα gene expression and p21CIP1. The effect in HL60 cells of treatment for 3 days with vorinostat (Vor; 0.075-1 μM), VPA (0.25-3 mM), or IDA (1.5 nM) or the combination on p21CIP1 or DNA topoisomerase IIα mRNA expression was determined using real-time PCR assay. Numbers on the y-axis represent relative expression of delta CT. (A) p21CIP1. (B) DNA topoisomerase IIα. Error bars indicate standard deviation.
The observation that the activity of the combination is independent of the sequence used is of importance. First, this suggests that clinically both drugs can be administered concomitantly. Second, this also indicates that we do not fully understand the mechanism of action of the drugs used here and that these drugs, in particular the HDACIs, may function by currently unknown mechanisms that need to be explored in future studies.
Induction of phosphorylation of the histone variant H2AX has been associated with the generation of double-strand DNA breaks.26 Our results indicate, using 2 different techniques, that both idarubicin and vorinostat are potent inducers of γH2AX when used as single agents. To our knowledge, this is one of the first reports indicating that single-agent HDACI has the capacity to induce H2AX phosphorylation. DNA topoisomerase II inhibitors are known to induce double DNA strand breaks. Of interest, although both agents had the capacity to induce γH2AX in both cell lines, the effects of the combination were cell specific. In MOLT4 cells the combination results in potent induction of apoptosis, and an increase in the number of γH2AX cells was observed with the concomitant use of both drugs. In contrast, in HL60 cells the combination resulted in a decrease in the fraction of γH2AX-positive cells. The significance of this observation is only speculative but it may suggest that induction of γH2AX may have a role in the capacity to induce apoptosis of these drugs. In a model of irradiation, loss of γH2AX resulted in clonogenic survival.27 These data are of importance, as they suggest that HDACIs may exert their cytotoxic effects by other mechanisms not directly linked to histone acetylation but perhaps DNA damage. Phosphorylation of H2AX could then be used as a surrogate marker of clinical activity.
HDACIs are known to downregulate the expression of a significant fraction of genes.28 If this was to be the case in our model, downregulation of DNA topoisomerase IIα, the target of idarubicin, could be detrimental from a therapeutic perspective. Using a real-time PCR assay, we have demonstrated that exposure to either idarubicin or the HDACI did not have an effect on mRNA expression of DNA topoisomerase IIα. Finally, we have documented that the use of these drugs has a potent effect on p21 activation, again not potentiated by the combination. Despite the potent activation of p21, no effect on cell cycle transition was observed. Similar results were observed when MS-275 was combined with irradiation.23
Finally, it should be noted that one of the limitations of the data presented here is the lack of information regarding the effects of the combination of these agents in nonneoplastic tissues such as normal hematopoietic stem cells.
In summary, our results indicate that the combination of an anthracycline with an HDACI results in synergistic antileukemia activity in vitro. Using these drugs for 3 days, these results are independent of the sequence used. Because anthracyclines are one of the most frequently used antineoplastic drugs in leukemia and other malignancies, these results have significant clinical implications and suggest that the combinations investigated here should be studied in human clinical trials.
Prepublished online as Blood First Edition Paper, May 4, 2006; DOI 10.1182/blood-2005-09-008086.
Supported by a Physician-Scientist Award from University of Texas MD Anderson Cancer Center funded by the Commonwealth Cancer Foundation for Research; an American Society of Clinical Oncology Career Development Award; and grants CA105771 and CA100067 from the National Institutes of Health (G.G.-M.).
V.M.R is an employee of Merck & Company, the manufacturer of vorinostat.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Vorinostat (suberoylanilide hydroxamic acid) was provided by Aton Pharma, Inc (now a Merck & Co subsidiary).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal