AMN107 (Novartis Pharmaceuticals, Basel, Switzerland) has potent in vitro and in vivo activity against the unmutated and most common mutant forms of Bcr-Abl. Treatment with the histone deacetylase inhibitor LBH589 (Novartis) depletes Bcr-Abl levels. We determined the effects of AMN107 and/or LBH589 in Bcr-Abl–expressing human K562 and LAMA-84 cells, as well as in primary chronic myelogenous leukemia (CML) cells. AMN107 was more potent than imatinib mesylate (IM) in inhibiting Bcr-Abl tyrosine kinase (TK) activity and attenuating p-STAT5, p-AKT, Bcl-xL, and c-Myc levels in K562 and LAMA-84 cells. Cotreatment with LBH589 and AMN107 exerted synergistic apoptotic effects with more attenuation of p-STAT5, p-ERK1/2, c-Myc, and Bcl-xL and increases in p27 and Bim levels. LBH589 attenuated Bcr-Abl levels and induced apoptosis of mouse pro-B BaF3 cells containing ectopic expression of Bcr-Abl or the IM-resistant, point-mutant Bcr-AblT315I and Bcr-AblE255K. Treatment with LBH589 also depleted Bcr-Abl levels and induced apoptosis of IM-resistant primary human CML cells, including those with expression of Bcr-AblT315I. As compared with either agent alone, cotreatment with AMN107 and LBH589 induced more loss of cell viability of primary IM-resistant CML cells. Thus, cotreatment with LBH589 and AMN107 is active against cultured or primary IM-resistant CML cells, including those with expression of Bcr-AblT315I.
Introduction
The deregulated activity of the Bcr-Abl tyrosine kinase (TK) encoded by the bcr-abl oncogene remains a therapeutic target in all phases of chronic myelogenous leukemia (CML).1-3 Bcr-Abl activates diverse progrowth and prosurvival mechanisms, which confer resistance to apoptosis.1,3 These include increased phosphorylation and transactivation by STAT-5 (signal transducer and activator of transcription), which leads to increased expression of the antiapoptotic Bcl-xL and Pim-2 protein,4-6 and increased Ras/Raf/MEK/ERK1/2, AKT, and NFκB activity.1,3,7,8 Bcr-Abl TK activity also leads to depletion of the cyclin-dependent kinase-2 inhibitor p27 and the BH3 domain-only–containing proapoptotic Bim protein.9-11 Collectively, these molecular perturbations promote cell proliferation and survival and contribute to Bcr-Abl–mediated leukemia transformation of normal bone marrow progenitor cells (NBMCs).1,3 Although highly active in inducing clinical and cytogenetic complete remissions, resistance to imatinib mesylate (IM; Gleevec) is a common clinical problem in CML, especially in the accelerated phase and blast crisis (BC) of CML.12 The mechanisms of resistance to IM include mutations in the kinase domain of BCR-ABL, amplification of the BCR-ABL gene, as well as Bcr-Abl–independent mechanisms of resistance.13-16 Approximately 40 different point mutations have been described in the kinase domain of Bcr-Abl.17 On the basis of the known crystal structure of Abl complexed with STI571, the mutations are of 2 broad categories: those that directly interfere with the ability of IM to bind to the kinase domain (eg, T315I) and those that impair the ability of Bcr-Abl from achieving the inactive conformation required for binding to IM, for example, those in the P loop (eg, E255K).13,14,18 Bcr-Abl mutations impart varying degrees of resistance to IM, including some that remain susceptible to higher concentrations of IM.13-15 Mutations that interfere directly with Bcr-Abl in binding IM are the most resistant to inhibition by IM.13-15 Polyclonal resistance in a single patient represented by 2 or more distinct mutations in Bcr-Abl has also been described in 35% of patients.13-15 These observations emphasize the need to develop and test novel anti–Bcr-Abl agents that are more potent than IM and/or are able to override the resistance to IM because of either mutations or amplifications of Bcr-Abl.15 AMN107 (Novartis Pharmaceuticals Inc, Basel, Switzerland) is a rationally designed Bcr-Abl TK inhibitor, which binds to the ATP-binding site of the kinase in its inactive conformation.19 AMN107 is approximately 10- to 20-fold more potent than IM in inhibiting the activity of the unmutated Bcr-Abl and induces growth inhibition and apoptosis of Bcr-Abl–transformed cells.20,21 AMN107 is also able to inhibit the activity of mutant Bcr-Abl, with mutations in the P loop and activation loop but not with those mutations that interfere directly with the binding of Bcr-Abl with IM, for example, T315I.20-22 Notably, in an early clinical trial, AMN107 has shown significant clinical activity against IM-resistant CML.23
Recent studies from our laboratory have also demonstrated that treatment with hydroxamic acid analog (HA) histone deacetylase inhibitors (HDIs) leads to increased levels of p21 and p27, as well as induces the levels of prodeath proteins (eg, Bax, Bak, and Bim) and down-regulates antiapoptotic proteins, (eg, Bcl-xL, XIAP, survivin, and AKT) in human leukemia cells.24-26 Collectively, this results in inhibition of cell growth and induces apoptosis of leukemia cells.27-29 Recent studies from our laboratory have also demonstrated that treatment with the HA-HDIs SAHA, LAQ824, and LBH589 alone depleted Bcr-Abl, as well as induced apoptosis and sensitized human leukemia cells to apoptosis induced by IM.27-29 By inhibiting HDAC6 and inducing acetylation of hsp90, LAQ824 and LBH589 attenuated the ATP-binding and chaperone function of hsp90.30 This led to polyubiquitylation, proteasomal degradation, and depletion of hsp90 client proteins, including Bcr-Abl, c-Raf, and AKT.28,29,31 Notably, our studies also showed that treatment with LAQ824 or LBH589 reduced the levels of the highly IM-refractory Bcr-AblT315I and induced apoptosis of primary IM-refractory CML-BC cells.28,29 Treatment with LAQ824 and LBH589 has also been shown to induce apoptosis of acute myelogenous leukemia (AML) cells, including those that contain FLT-3 mutations, while relatively sparing NBMCs.26,28,29 On the basis of the strong rationale generated by these observations taken together, we determined the combined effects of AMN107 and LBH589 against cultured and primary human CML cells. Additionally, we also determined the effect of this combination against mouse pro-B BaF3 cells with ectopic expression of the unmutated Bcr-Abl or with mutant Bcr-AblE255K and Bcr-AblT315I, as well as against IM-resistant primary CML cells, including those that harbor the point mutation Bcr-Abl T315I.
Materials and methods
Reagents and antibodies
LBH589 and AMN107 were provided by Novartis Pharmaceuticals (East Hanover, NJ). Polyclonal anti-PARP, anti–caspase-9, anti–caspase-3, and anti–p-ERK1/2 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Polyclonal anti–STAT-5 and goat polyclonal anti–Pim-2 antibody, as well as monoclonal anti–c-Myc and anti-Abl antibodies, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti–p-STAT5 antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Antibodies for the immunoblot analyses of p21, p27, p-CrkL, CrkL, p-AKT, AKT, Bim, Bcl-xL, and ERK1/2 were obtained as previously described.26-30
Cell lines and cell culture
Bcr-Abl–expressing, CML LAMA-84 and K562 cells were obtained from American Tissue Culture Collection (Manassas, VA) and maintained in culture in RPMI medium containing 10% fetal bovine serum and passaged twice a week, as previously described.27-30 Mouse pro-B BaF3 cells were cultured in complete RPMI-1640 media supplemented with 10% WEHI medium as the source of IL-3.32 For the studies described herein, logarithmically growing cells were exposed to the designated concentrations of AMN107 and/or LBH589. Following these treatments, cells or cell pellets were washed free of the drug(s) prior to the performance of the studies.
Site-directed mutagenesis and nucleofection
Three p210 Bcr-Abl constructs were used in the current studies. The p210 Bcr-Abl WT and p210 Bcr-Abl (T315I) constructs were generated as previously described.28,32 The p210 Bcr-Abl (E255K) mutant was created by site-directed mutagenesis of a Bcr-Abl containing pSVneo construct using a QuikChange II XL kit (Stratagene, Cedar Creek, TX) according to the manufacturer's recommendations, and the resulting clones were sequenced to confirm the point mutation.32,33 For nucleofection of the p210 Bcr-Abl constructs into BaF3 cells, 5 million BaF3 cells in 100 μL Nucleofector solution V (Amaxa, Gaithersburg, MD) were mixed with 5 μg p210 Bcr-Abl WT, p210 Bcr-Abl (T315I), or p210 Bcr-Abl (E255K) in a cuvette and nucleofected using program G-16.34 Following nucleofection, the cells were incubated at a concentration of 1 × 106 cells/mL in complete RPMI-1640 media supplemented with 10% WEHI medium as the source of IL-3, overnight, to recover. Stable transfectants of BaF3 cells expressing the WT or mutant form of Bcr-Abl (ie, T315I or E255K) were maintained in RPMI 1640 supplemented with 10% serum, 1.0 U/mL penicillin, 1 μg/mL streptomycin, and 0.75 mg/mL G418. Stably expressing cells were then further selected by removal of IL-3. After confirmation of Bcr-Abl expression by immunoblot analysis, cells were used for the studies described herein.
Primary CML-BC cells and NBMCs
Leukemia cells from the peripheral blood and/or bone marrow of 10 patients who had met the clinical criteria of IM-resistant Ph chromosome-positive CML-BC were harvested and purified, as previously described.27,28,35 Additionally, NBMCs were harvested and purified, as previously described.28 Informed consents were signed by all patients to allow use of their cells for these experiments, as part of a clinical protocol approved by the University of South Florida Institutional Review Board (IRB).
Sequencing of bcr-abl in CML-BC cells
Using the Trizol method (Invitrogen, Carlsbad, CA), total RNA was isolated from 10 to 15 million cells available from 2 patients, who were suspected to have Bcr-Abl T315I mutation as a result of their failure to respond to treatment with IM and AMN107. Total RNA (5 μg) was reverse transcribed with a first-strand cDNA synthesis kit (Invitrogen). Reverse transcribed cDNAs were used in polymerase chain reaction (PCR) amplifications to amplify a fragment of bcr-abl that included the bcr junction region and the c-abl kinase region as previously described.13,36 The amplified sequences were agarose gel-purified and cloned into pCR4-TOPO plasmid. The resulting plasmids were transformed into Escherichia coli Mach1 cells (Invitrogen) overnight at 37°C. Ten colonies for each sample were checked by colony PCR and subcultured for plasmid isolation. Isolated plasmids were sequence verified with T3 and T7 primers for c-abl kinase domain mutations.
Suspension culture or colony growth inhibition
Following treatment with the designated concentrations of AMN107 and/or LBH589 for 48 hours, untreated and drug-treated cells were washed in RPMI 1640 medium. Following this, cells were placed in suspension culture at a concentration of 200 000 cells/mL for 4 days. At the end of this incubation period, cell concentrations and percentage increase in cell numbers were determined. Alternatively, following treatment with the drugs, approximately 200 cells treated under each condition were resuspended in 100 μL RPMI 1640 media containing 10% FBS, then plated in duplicate wells in a 12-well plate containing 1.0 mL Methocult media (Stem Cell Technologies, Vancouver, Canada) per well, according to the manufacturer's protocol. The plates were placed in an incubator at 37°C with 5% CO2 for 10 days. Following this incubation, colonies consisting of 50 or more cells, in each well, were counted by an inverted microscope, and the percentage of colony growth inhibition compared with the untreated control cells was calculated.
Assessment of percentage of nonviable cells
Apoptosis assessment by annexin V staining
Untreated and drug-treated cells were stained with annexin V and PI, and the percentage of apoptotic cells was determined by flow cytometry, as described previously.37,38 Analysis of synergism between AMN107 and LBH589 in inducing apoptosis of K562 and LAMA-84 cells was performed by Median Dose-Effect analysis of Chou and Talalay39 using the commercially available software (Calcusyn; Biosoft, Ferguson, MO).
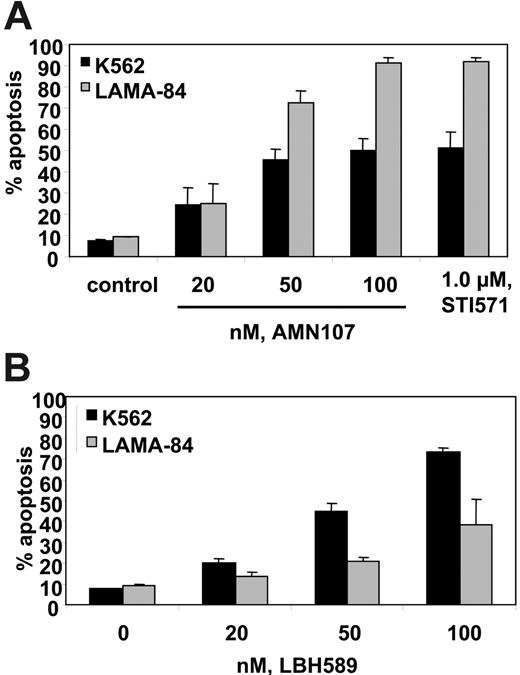
AMN107 and LBH589 induce apoptosis of K562 and LAMA-84 cells. Cells were treated with the indicated concentrations of AMN107 or IM (A) or LBH589 (B) for 48 hours. Following this, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. Values represented as bar graphs are mean of 3 experiments ± SEM.
AMN107 and LBH589 induce apoptosis of K562 and LAMA-84 cells. Cells were treated with the indicated concentrations of AMN107 or IM (A) or LBH589 (B) for 48 hours. Following this, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. Values represented as bar graphs are mean of 3 experiments ± SEM.
Western analyses of proteins
Immunoprecipitation of Bcr-Abl and immunoblot analyses
Following the designated drug treatments, cells were lysed in lysis buffer (20 mM Tris [pH 8], 150 nM sodium chloride, 1% NP40, 0.1 M sodium fluoride, 1 mM PMSF, 1 mM sodium orthovanadate, 2.5 μg/mL leupeptin, 5 μg/mL aprotinin) for 30 minutes on ice, and the nuclear and cellular debris was cleared by centrifugation.28,35 Cell lysates (200 μg) were incubated with the Abl-specific monoclonal antibody for 1 hour at 4°C. To this, washed Protein G agarose beads were added and incubated overnight at 4°C. The immunoprecipitates were washed 3 times in the lysis buffer, and proteins were eluted with the SDS sample loading buffer prior to the immunoblot analyses with specific antibodies against anti-Abl or antiphosphotyrosine antibody.28,35
Statistical analysis
Significant differences between values obtained in a population of leukemic cells treated with different experimental conditions were determined using the student t test. P values of less than .05 were assigned significance.
Results
AMN107 and LBH589 induce apoptosis of K562 and LAMA-84 cells
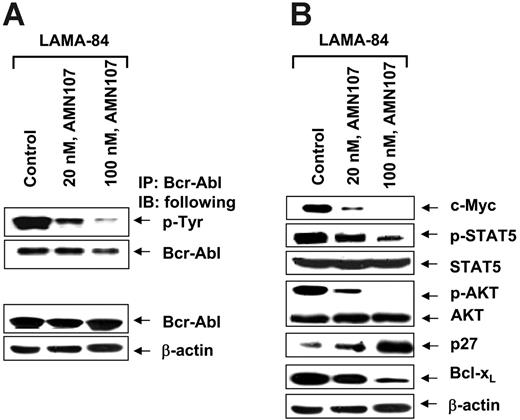
We determined the effects of AMN107 and/or LBH589 in cultured and primary CML-BC cells. We first determined the apoptotic effects of treatment with LBH589 or AMN107 alone on K562 and LAMA-84 cells. Figure 1A and 1B demonstrate that exposure to LBH589 or AMN107 alone induces apoptosis of K562 and LAMA-84 cells in a dose-dependent manner. Consistent with the previous reports, the data also show that AMN107 is approximately 10-fold more potent than IM in inducing apoptosis of K562 and LAMA-84 cells (Figure 1A). Treatment of LAMA-84 cells with AMN107 inhibited the levels of tyrosine phosphorylated Bcr-Abl in a dose-dependent manner, without affecting the levels of Bcr-Abl (Figure 2A). AMN107 treatment also inhibited the levels of p-CrkL (vide infra), suggesting that AMN107 inhibits the TK activity of Bcr-Abl. Treatment with AMN107 attenuated the levels of p-STAT5, as well as lowered the expressions of c-Myc and Bcl-xL, which are transactivated by STAT5 (Figure 2B). Treatment with AMN107 also inhibited the levels of p-AKT but not AKT, which was associated with induction of p27 levels (Figure 2B). This has also been observed following exposure to IM.40 Similar effects of AMN107 were also observed in K562 cells (data not shown).
Cotreatment with LBH589 and AMN107 exerts superior anti–Bcr-Abl activity and synergistically induces apoptosis of K562 and LAMA-84 cells
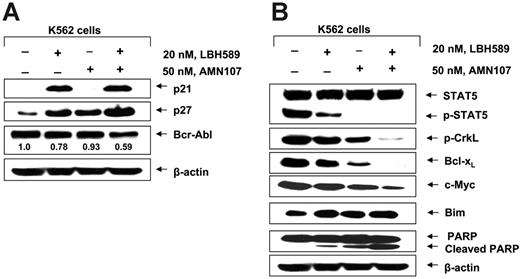
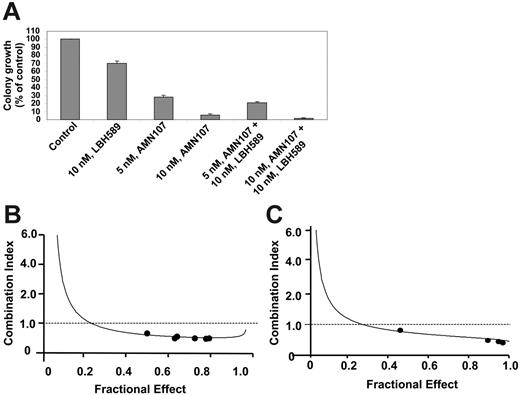
Next, we determined the effects of cotreatment with LBH589 and AMN107 on Bcr-Abl, as well as on the levels of the signaling proteins downstream of Bcr-Abl. Figure 3A demonstrates that, as compared with treatment with either agent alone, relatively low concentrations of LBH589 (20 nM) and AMN107 (50 nM) for 24 hours caused more depletion of Bcr-Abl and induced more p27 levels in K562 cells. In contrast, p21 levels were induced to a similar extent by combined treatment with AMN107 and LBH589, as compared with treatment with LBH589 alone. Combined treatment with LBH589 and AMN107 also caused more attenuation of the levels of p-CrkL, Bcl-xL, and c-Myc but induced more Bim (Figure 3B). Following cotreatment with AMN107 and LBH589, simultaneous induction of Bim and attenuation of Bcl-xL was associated with more PARP cleavage, which is due to increased activity of the effector caspases 3 and 7 during apoptosis.26,28 Similar effects of LBH589 and AMN107 were also observed against LAMA-84 cells (data not shown). Next, we determined the apoptotic effect of LBH589 and/or AMN107 on suspension culture and colony growth of K562 cells. Figure 4A demonstrates that cotreatment with AMN107 and LBH589 caused significantly more inhibition of colony growth than treatment with either drug alone (P < .05). Similar effect of the combination was also observed against suspension culture growth of K562 cells (data not shown). We also determined the apoptotic effect (increase in the percentage of annexin V–positive cells) of the combined treatment with AMN107 and LBH589 in K562 and LAMA-84 cells. Notably, exposure to the combination of AMN107 and LBH589 exerted synergistic apoptotic effect in K562 and LAMA-84 cells, as determined by the median dose-effect isobologram analysis described by Chou and Talalay.39 For AMN107 and LBH589, the combination index values were less than 1.0 in each cell type (Figure 4A-B). The CI values for K562 were 0.47, 0.36, 0.45, 0.45, and 0.45, respectively, and the CI values for LAMA84 were 0.85, 0.22, 0.21, and 0.16, respectively. The effect of AMN107 and/or LBH589 was also determined against NBMCs. Although AMN107 had no effect (up to 1.0 μM), exposure to 20 and 50 nM LBH589 for 48 hours induced loss of survival of 13.1% and 15.9% of NBMCs (mean of 2 samples with experiments performed in duplicate). Cotreatment with AMN107 did not significantly increase the loss of survival of NBMCs because of exposure to 50 nM LBH589 (P > .05).
AMN107 inhibits autophosphorylation of Bcr-Abl and attenuates p-STAT5, p-AKT, c-Myc, and Bcl-xL levels. (A) LAMA-84 cells were treated with the indicated concentrations of AMN107 for 24 hours. Following this, immunoprecipitates were obtained with protein G-agarose beads coated with anti-Abl antibody and immunoblotted with antiphosphotyrosine antibody or anti-Abl antibody. (B) Alternatively, cell lysates of LAMA-84 cells were used for Western blot analyses of p-STAT5, STAT5, c-Myc, Bcl-xL, p-AKT, AKT, and p27, using specific antibodies. The levels of β-actin served as the loading control.
AMN107 inhibits autophosphorylation of Bcr-Abl and attenuates p-STAT5, p-AKT, c-Myc, and Bcl-xL levels. (A) LAMA-84 cells were treated with the indicated concentrations of AMN107 for 24 hours. Following this, immunoprecipitates were obtained with protein G-agarose beads coated with anti-Abl antibody and immunoblotted with antiphosphotyrosine antibody or anti-Abl antibody. (B) Alternatively, cell lysates of LAMA-84 cells were used for Western blot analyses of p-STAT5, STAT5, c-Myc, Bcl-xL, p-AKT, AKT, and p27, using specific antibodies. The levels of β-actin served as the loading control.
Cotreatment with LBH589 and AMN107 causes greater attenuation of p-AKT, p-STAT5, p-CrkL, Bcl-xL, and c-Myc but induces more p27, Bim, and PARP cleavage in K562 cells. (A) Following treatment of K562 cells with 20 nM LBH589 and/or 50 nM AMN107 for 24 hours, Western blot analyses of Bcr-Abl, p21, and p27 was performed on the cell lysates. The levels of β-actin served as the loading control. (B) Following treatment of K562 or LAMA-84 cells with 20 nM LBH589 and/or 50 nM AMN107 for 24 hours, Western blot analyses of p-STAT5, STAT5, p-CrkL, Bcl-xL, c-Myc, Bim, and PARP was performed on the cell lysates. The levels of β-actin served as the loading control.
Cotreatment with LBH589 and AMN107 causes greater attenuation of p-AKT, p-STAT5, p-CrkL, Bcl-xL, and c-Myc but induces more p27, Bim, and PARP cleavage in K562 cells. (A) Following treatment of K562 cells with 20 nM LBH589 and/or 50 nM AMN107 for 24 hours, Western blot analyses of Bcr-Abl, p21, and p27 was performed on the cell lysates. The levels of β-actin served as the loading control. (B) Following treatment of K562 or LAMA-84 cells with 20 nM LBH589 and/or 50 nM AMN107 for 24 hours, Western blot analyses of p-STAT5, STAT5, p-CrkL, Bcl-xL, c-Myc, Bim, and PARP was performed on the cell lysates. The levels of β-actin served as the loading control.
Cotreatment with LBH589 and AMN107 inhibits more colony growth than either agent alone and induces synergistic apoptotic effects. (A) K562 cells were treated with the indicated concentrations of LBH589 and/or AMN107 for 48 hours. Following this, colony growth in semisolid medium was assessed after 7 days. The bar graphs represent the mean percentage values ± SEM of untreated control colony growth. K562 cells (B) and LAMA-84 cells (C) were treated with LBH589 and AMN107 at a fixed ratio of 1 to 2, respectively, with concentrations ranging between 10 and 100 nM, for 48 hours. Following this, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. Using Calcusyn software (Biosoft), the analysis of the dose-effect relationship for LBH589 and AMN107-induced apoptosis of K562 or LAMA-84 cells was performed according to the median effect method of Chou and Talalay.39 The combination index (CI) values were calculated for 3 independent experiments. CI < 1, CI = 1, and CI > 1 represent synergism, additivity, and antagonism of the 2 agents, respectively.
Cotreatment with LBH589 and AMN107 inhibits more colony growth than either agent alone and induces synergistic apoptotic effects. (A) K562 cells were treated with the indicated concentrations of LBH589 and/or AMN107 for 48 hours. Following this, colony growth in semisolid medium was assessed after 7 days. The bar graphs represent the mean percentage values ± SEM of untreated control colony growth. K562 cells (B) and LAMA-84 cells (C) were treated with LBH589 and AMN107 at a fixed ratio of 1 to 2, respectively, with concentrations ranging between 10 and 100 nM, for 48 hours. Following this, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. Using Calcusyn software (Biosoft), the analysis of the dose-effect relationship for LBH589 and AMN107-induced apoptosis of K562 or LAMA-84 cells was performed according to the median effect method of Chou and Talalay.39 The combination index (CI) values were calculated for 3 independent experiments. CI < 1, CI = 1, and CI > 1 represent synergism, additivity, and antagonism of the 2 agents, respectively.
LBH589 depletes mutant Bcr-Abl levels and induces apoptosis of IM-resistant BaF3 cells expressing Bcr-AblT315I or Bcr-AblE255K
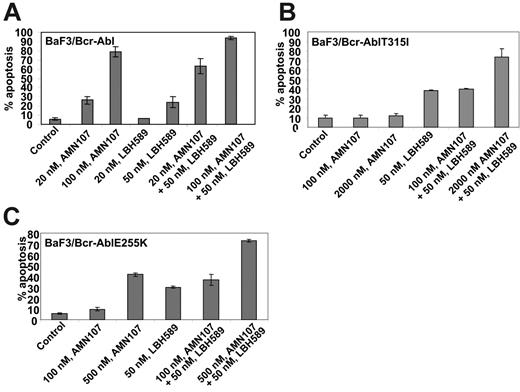
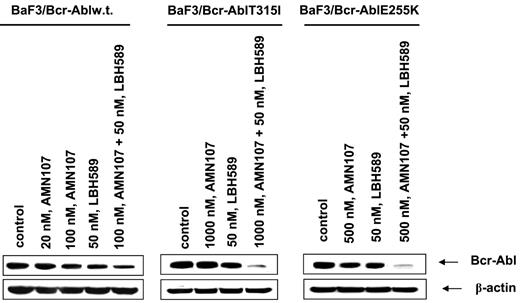
Next, we determined the effect of treatment with LBH589 and/or AMN107 on BaF3 cells with ectopic expression of either the unmutated Bcr-Abl or of the point mutant Bcr-AblE255K or Bcr-AblT315I. Similar to the effects seen in K562 and LAMA824 cells with endogenous expression of Bcr-Abl, AMN107 induced apoptosis of BaF3/Bcr-Abl cells in a dose-dependent manner (Figure 5A). Additionally, cotreatment with AMN107 and LBH589 induced significantly more apoptosis of BaF3/Bcr-Abl cells than either agent alone (Figure 5A; P < .05). Although exposure to IM induced dose-dependent apoptosis of BaF3/Bcr-Abl cells, BaF3/Bcr-AblT315I cells were resistant to IM up to levels as high as 10 μM (data not shown). In contrast, BaF3/Bcr-AblT315I cells were as sensitive as BaF3/Bcr-Abl cells to apoptosis induced by treatment with LBH589 alone (Figure 5A,B). Treatment with 50 nM LBH589 for 48 hours induced apoptosis in approximately 30% of BaF3/Bcr-Abl T315I cells (Figure 5B). Lower levels of LBH589 were less effective (data not shown). In contrast, BaF3/Bcr-AblT315I cells were resistant to AMN107 levels as high as 2000 nM (Figure 5B). Notably, cotreatment with 2000 nM but not 100 nM AMN107 significantly increased LBH589-induced apoptosis of BaF3/Bcr-AblT315I cells (P < .01; Figure 5B). Against BaF3/Bcr-AblE255K cells, although 100 nM AMN107 was ineffective, exposure to 200 (not shown) and 500 nM AMN107 induced apoptosis of 26.0% and 43.0% of cells, respectively (Figure 5C). Again, cotreatment with AMN107 (500 nM) and LBH589 (50 nM) induced significantly more apoptosis of BaF3/Bcr-AblE255K cells than treatment with either agent alone (P < .01), although cotreatment with 100 nM AMN107 was less effective (Figure 5C). Cotreatment with higher concentrations of AMN107 (1.0 or 2.0 μM) also enhanced LBH589-induced apoptosis of BaF3/Bcr-AblE255K (data not shown). Next, we also correlated the apoptotic effects of AMN107 and/or LBH589 with their effects on the levels of Bcr-Abl in BaF3/Bcr-Abl, BaF3/Bcr-AblE255K, and BaF3/Bcr-AblT315I cells. Treatment with any of the levels of AMN107 tested alone did not lower the levels of Bcr-Abl in any of the 3 cell types (Figure 6). Exposure to AMN107 also did not affect the levels of p-CrkL or CrkL (data not shown). In contrast, exposure to 50 nM LBH589 for 24 hours lowered Bcr-Abl levels in all 3 BaF3 transfectants. Notably, as compared with treatment with either agent alone, cotreatment with LBH589 and AMN107 induced more depletion of Bcr-Abl in BaF3/Bcr-Abl cells. Notably, combined treatment with LBH589 and AMN107 caused a more pronounced decline in the levels of Bcr-AblT315I and Bcr-Abl E255K levels in BaF3/Bcr-AblT315I and BaF3/Bcr-AblE255K cells, respectively (Figure 6). Similar effect was noted on p-CrkL but not CrkL levels (data not shown).
Cotreatment with LBH589 and AMN107 induces more apoptosis of BaF3/Bcr-Abl, BaF3/Bcr-AblT315I, and BaF3/Bcr-AblE255K cells. Cells were treated with the indicated concentrations of LBH589 and/or AMN107 for 48 hours, and the percentage of apoptotic cells was determined by annexin V staining followed by flow cytometry. Values (mean ± SEM of 3 experiments performed in duplicate) are depicted as bars.
Cotreatment with LBH589 and AMN107 induces more apoptosis of BaF3/Bcr-Abl, BaF3/Bcr-AblT315I, and BaF3/Bcr-AblE255K cells. Cells were treated with the indicated concentrations of LBH589 and/or AMN107 for 48 hours, and the percentage of apoptotic cells was determined by annexin V staining followed by flow cytometry. Values (mean ± SEM of 3 experiments performed in duplicate) are depicted as bars.
Cotreatment with LBH589 and AMN107 causes more depletion of Bcr-Abl in BaF3/Bcr-Abl, BaF3/Bcr-AblT315I, and BaF3/Bcr-AblE255K cells. Cells were treated with the indicated concentrations of LBH589 and/or AMN107 for 24 hours. Following this, the cell lysates were harvested and immunoblotted with the anti–Bcr-Abl antibody. The levels of β-actin served as the loading control.
Cotreatment with LBH589 and AMN107 causes more depletion of Bcr-Abl in BaF3/Bcr-Abl, BaF3/Bcr-AblT315I, and BaF3/Bcr-AblE255K cells. Cells were treated with the indicated concentrations of LBH589 and/or AMN107 for 24 hours. Following this, the cell lysates were harvested and immunoblotted with the anti–Bcr-Abl antibody. The levels of β-actin served as the loading control.
Cotreatment with AMN107 and LBH589 causes more attenuation of Bcr-Abl and loss of viability of primary, IM-resistant CML cells than either agent alone
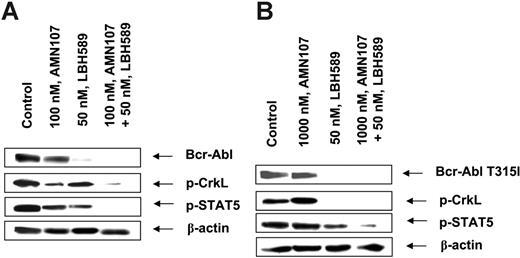
We next determined the antileukemia effects of LBH589 and/or AMN107 against primary CML cells isolated from the peripheral blood and/or bone marrow samples from 10 patients who had relapsed with IM-resistant CML-BC. Three of these samples were documented to have the expression of Bcr-AblT315I (samples 8, 9, and 10). In the remaining samples of IM-refractory primary CML cells (samples 1 to 7), the mutational status of Bcr-Abl could not be determined, because of inadequate sample size. Table 1 indicates that in the samples 1 to 7 both AMN107 and LBH589 induced loss of cell viability, which was dose dependent. Additionally, in these samples, cotreatment with LBH589 (20 or 50 nM) and AMN107 induced more loss of cell viability than treatment with either agent alone. Sample 7 was relatively resistant to lower concentrations of AMN107 but sensitive to LBH589 (Table 1). In the 3 samples with Bcr-AblT315I mutation (samples 8, 9, and 10), treatment with AMN107 did not augment loss of cell viability, whereas exposure to LBH589 alone for 48 hours markedly inhibited cell viability in a dose-dependent manner (Table 1). Notably, in these samples (8, 9, and 10), cotreatment with 50 or 100 nM AMN107 did not increase LBH589-induced loss of cell viability (Table 1). In one sample (no. 9), although exposure to even 2.0 μM AMN107 was ineffective, cotreatment of 50 nM LBH589 with 2.0 μM AMN107 induced apoptosis of 63.7% of cells, as compared with apoptosis of 42.0% of cells treated with 50 nM LBH589 alone (Table 1). Western blot analyses of the total cell lysates of sample 5 showed that cotreatment with 50 nM LBH589 and 100 nM AMN107 for 24 hours resulted in more attenuation of Bcr-Abl, p-CrkL, and p-STAT5 than treatment with either agent alone (Figure 7A). In contrast, in sample 9, treatment with even 1000 nM AMN107 alone had little effect on the levels of Bcr-Abl, p-CrkL, and p-STAT5, whereas cotreatment with 50 nM LBH589 and AMN107 markedly depleted the levels of Bcr-AblT315I, as well as of p-CrkL and p-STAT5 (Figure 7B). These findings were similar to those in BaF3 cells with the ectopic expression of Bcr-AblT315I.
Effect of LBH589 and/or AMN107 on percentage of nonviable primary CML cells
Patient sample . | Control . | AMN107, 50 nM . | AMN107, 100 nM . | LBH589, 20 nM . | LBH589, 50 nM . | 50 nM AMN107 + 50 nM LBH589 . | 100 nM AMN107 + 50 nM LBH589 . |
|---|---|---|---|---|---|---|---|
| 1 | 2.3 | 8.5 | 16.8 | 4.8 | 32.9 | 46.5 | 48.6 |
| 2 | 11.2 | 24.7 | 32.4 | 17.2 | 28.5 | 47.0 | ND |
| 3 | 7.1 | 29.0 | 44.0 | 39.0 | 56.0 | 62.0 | 63.0 |
| 4 | 9.6 | 30.7 | 35.8 | 16.5 | 28.6 | 42.6 | 49.2 |
| 5 | 2.2 | 18.6 | 29.0 | 22.8 | 38.6 | 58.9 | 67.6 |
| 6 | 5.0 | 10.8 | 23.0 | 34.6 | 60.1 | 69.4 | 68.8 |
| 7 | 8.0 | 10.6 | 22.4 | 24.6 | 51.0 | 58.1 | 62.9 |
| Mean ± SEM | 6.5 ± 1.2 | 19.0 ± 3.3 | 29.1 ± 3.2 | 22.8 ± 4.0 | 42.2 ± 4.7 | 54.9 ± 3.4* | 60.0 ± 3.1* |
| 8† | 9.5 | 12.1 | 10.5 | 23.5 | 37.8 | 38.1 | 38.0 |
| 9† | 16.0 | 17.5 | 16.4 | 28.8 | 42.0 | 42.9 | 43.0 |
| 10† | 15.0 | 13.8 | 14.7 | 35.9 | 47.4 | 47.6 | 46.2 |
| Mean ± SEM | 13.5 ± 1.7 | 14.5 ± 1.3 | 13.9 ± 1.4 | 29.4 ± 2.9 | 42.2 ± 2.3 | 42.9 ± 2.2‡ | 42.4 ± 1.9‡ |
Patient sample . | Control . | AMN107, 50 nM . | AMN107, 100 nM . | LBH589, 20 nM . | LBH589, 50 nM . | 50 nM AMN107 + 50 nM LBH589 . | 100 nM AMN107 + 50 nM LBH589 . |
|---|---|---|---|---|---|---|---|
| 1 | 2.3 | 8.5 | 16.8 | 4.8 | 32.9 | 46.5 | 48.6 |
| 2 | 11.2 | 24.7 | 32.4 | 17.2 | 28.5 | 47.0 | ND |
| 3 | 7.1 | 29.0 | 44.0 | 39.0 | 56.0 | 62.0 | 63.0 |
| 4 | 9.6 | 30.7 | 35.8 | 16.5 | 28.6 | 42.6 | 49.2 |
| 5 | 2.2 | 18.6 | 29.0 | 22.8 | 38.6 | 58.9 | 67.6 |
| 6 | 5.0 | 10.8 | 23.0 | 34.6 | 60.1 | 69.4 | 68.8 |
| 7 | 8.0 | 10.6 | 22.4 | 24.6 | 51.0 | 58.1 | 62.9 |
| Mean ± SEM | 6.5 ± 1.2 | 19.0 ± 3.3 | 29.1 ± 3.2 | 22.8 ± 4.0 | 42.2 ± 4.7 | 54.9 ± 3.4* | 60.0 ± 3.1* |
| 8† | 9.5 | 12.1 | 10.5 | 23.5 | 37.8 | 38.1 | 38.0 |
| 9† | 16.0 | 17.5 | 16.4 | 28.8 | 42.0 | 42.9 | 43.0 |
| 10† | 15.0 | 13.8 | 14.7 | 35.9 | 47.4 | 47.6 | 46.2 |
| Mean ± SEM | 13.5 ± 1.7 | 14.5 ± 1.3 | 13.9 ± 1.4 | 29.4 ± 2.9 | 42.2 ± 2.3 | 42.9 ± 2.2‡ | 42.4 ± 1.9‡ |
Samples of primary, IM refractory CML-BC cells, including 3 samples with expression of Bcr-AblT315I mutation (samples 8, 9, and 10), were treated with the indicated concentrations of LBH589 and/or AMN107 for 48 hours, and the percentage of nonviable cells was determined by the trypan blue exclusion method. The values represent mean of 2 experiments performed in duplicate.
ND represents treatment not studied.
Values significantly greater than those following treatment with either agent alone at the indicated concentrations (P < .05).
Samples with expression of Bcr-AblT315I.
Values not significantly different than those following treatment with 50 nM LBH589 alone (P > .05).
Cotreatment with LBH589 and AMN107 depletes the levels of Bcr-Abl, p-CrkL, and p-STAT5 in IM-resistant primary CML-BC cells. Two purified samples of IM-resistant CML-BC cells, sample 5 without a known mutation in Bcr-Abl (A) and sample 9 with expression Bcr-AblT315I (B), were exposed to LBH589 and/or AMN107 for 24 hours. Following this, the cell lysates were harvested and immunoblotted with the anti–Bcr-Abl, p-CrkL, or p-anti–p-STAT5 antibody. The levels of β-actin served as the loading control.
Cotreatment with LBH589 and AMN107 depletes the levels of Bcr-Abl, p-CrkL, and p-STAT5 in IM-resistant primary CML-BC cells. Two purified samples of IM-resistant CML-BC cells, sample 5 without a known mutation in Bcr-Abl (A) and sample 9 with expression Bcr-AblT315I (B), were exposed to LBH589 and/or AMN107 for 24 hours. Following this, the cell lysates were harvested and immunoblotted with the anti–Bcr-Abl, p-CrkL, or p-anti–p-STAT5 antibody. The levels of β-actin served as the loading control.
Discussion
In this report, we demonstrate for the first time that treatment with a combination of the pan-HDAC inhibitor LBH589 and the Bcr-Abl TK inhibitor AMN107 synergistically induced apoptosis of cultured mouse pro-B BaF3 and human CML cells with ectopic and endogenous expression of the unmutated Bcr-Abl, respectively. The combination is also more active than either drug alone against BaF3 cells with ectopic expression of the mutant Bcr-AblE255K or Bcr-AblT315I, as well as against IM-resistant primary CML cells. LBH589 also inhibits HDAC6, leading to the acetylation of hsp90 and inhibition of its ATP-binding and chaperone function.30 Not only does this deplete Bcr-Abl but also attenuates the levels of other progrowth and prosurvival signaling protein kinases (eg, c-Raf and AKT), which are known to be hsp90 client proteins.30,31,41,42 Additionally, treatment with an HA-HDI such as LBH589 results in attenuation of the levels of the Bcl-2 and IAP family of proteins. LBH589 also induced Bim, a protein induced by the forkhead family of transcription factors that are repressed by phosphorylation by AKT.43,44 Phosphorylation by extracellular signal regulated kinases is also known to diminish the association of Bim with Bax, which inhibits apoptosis.45 Collectively, these effects lower the threshold for apoptosis,46 which may explain why treatment with LBH589 also sensitized the cultured mouse BaF3/Bcr-Abl and human CML cells to apoptosis induced by AMN107. We have previously reported that the combination of a pan-HDAC inhibitor with a TK inhibitor, in which the TK is a client protein of hsp90 (eg, Her-2 and FLT-3) induces more apoptosis than either the HDI or the TK inhibitor alone.47,48 Present findings demonstrate a similar superior activity of the combination against Bcr-Abl–transformed cells.
Recent studies have tracked the response to IM by determining its effect on the bcr-abl mRNA transcript level through quantitative real-time polymerase chain reaction.17 These studies have shown that treatment with IM may not deplete CML stem cells, and a significant proportion of patients develop acquired resistance to IM because of mutations in the Bcr-Abl kinase domain.12-15,17 Amplification and increased expression of Bcr-Abl in CML progenitor cells may also confer IM resistance.49 A combination incorporating LBH589, which not only induces apoptosis through non–Bcr-Abl–dependent mechanisms but also lowers unmutated or mutant Bcr-Abl levels, and AMN107, which is more potent than IM in inhibiting Bcr-Abl TK activity, has the potential of overriding several of the IM-resistance mechanisms in CML progenitor cells. IM resistance may also be due to the dependence of CML cells for their growth and survival not on Bcr-Abl but on Lyn or the other signaling kinases.16,34,35,50 Again, on the basis of the multiple mechanisms of activity noted herein, LBH589 could potentially override non–Bcr-Abl and Bcr-Abl–dependent mechanisms of resistance in CML progenitor cells.30,35 Mutations in the kinase domain of Bcr-Abl conferring IM resistance fall into 2 main groups, that is, those inhibiting contact with IM and those that prevent Bcr-Abl from achieving the inactive conformation required for the binding of IM to Bcr-Abl. The point mutants Bcr-Abl E255K and Bcr-AblT315I have been recognized as the important and common examples of 2 groups of mutations that confer clinical resistance to IM.12-14 In the E255K mutation, which is located within the ATP-binding region (P loop) of the kinase domain of Bcr-Abl, glycine is replaced by lysine.12-14 This results in a significant decrease in the sensitivity of Bcr-AblE255K to IM in the kinase assay and in conferring IM resistance on BaF3/Bcr-AblE255K cells.12-15 In Bcr-Abl the threonine 315 makes a hydrogen bond contact with IM, and a single nucleotide C-to-T change that results in a threonine-to-isoleucine substitution at this residue has been shown to confer a high level of resistance to not only IM but also AMN107 and dasatinib.13,22,36 Recent preclinical studies have demonstrated that AMN107 and the dual Abl/Src kinase inhibitor dasatinib are not only more potent in inhibiting unmutated Bcr-Abl TK but also active against most of the mutant forms of Bcr-Abl, except Bcr-AblT315I.19-22,36 Note that treatment with hsp90 inhibitors has been reported to induce depletion of the mutant forms of Bcr-Abl and induce growth arrest and apoptosis of IM-resistant CML cells.33,35 Indeed, mutant forms of Bcr-Abl appear to be more susceptible than unmutated Bcr-Abl to depletion induced by the hsp90 inhibitors.29,33 A similar observation has also been reported with respect to the mutant versus unmutated forms of other hsp90 client proteins (eg, FLT-3).29,48 LBH589-mediated inhibition of the chaperone function of hsp90 may be responsible for the depletion of the Bcr-AblE255K and Bcr-AblT315I levels in LBH589-treated BaF3/Bcr-AblE255K and BaF3/Bcr-AblT315I cells, respectively.
In early clinical trials, both AMN107 and dasatinib have exhibited a promising level of clinical activity in IM-resistant CML.23,51 However, a substantial proportion of patients fail to achieve cytogenetic complete remission, especially in patients with more advanced phases of CML.23,51 Cells from these patients may harbor additional chromosomal abnormalities and genetic perturbations that often involve the recruitment of corepressors and HDAC activity. This can potentially repress genes involved in differentiation and apoptosis.1,24,52,53 Under these scenarios, cotreatment with LBH589 and AMN107 may override these mechanisms and induce growth arrest and apoptosis, as was reported in the K562 and the primary CML blast crisis cells.29 Additionally, cotreatment with LBH589 and AMN107 not only attenuates Bcr-Abl but also depletes the downstream, progrowth, and prosurvival signaling molecules, including p-AKT, p-ERK1/2, and p-STAT5. Thus, LBH589-mediated “longitudinal” 2-step inhibition of the signaling initiated by Bcr-Abl may be responsible for augmenting the growth inhibitory and apoptotic activity of LBH589 and AMN107 against CML-BC cells. A similar rationale has also been proposed to explain the synergistic effects of the combination of an mTOR inhibitor and IM against CML cells.54 A more pronounced inhibition of p-STAT5 resulting from cotreatment with LBH589 and AMN107 also correlated with greater attenuation of the STAT-5 target gene products Bcl-xL and c-Myc.4-6 Additionally, Bcl-2 family members have been shown to act in a complementary manner to promote Bcr-Abl–mediated induction of leukemia.55,56 Collectively, the more dramatic inhibitory effects on several progrowth and prosurvival signaling molecules may contribute to the synergistic apoptotic effects of the combination of LBH589 and AMN107 in the cultured and primary CML cells. However, it should be noted that in the absence of in vivo data documenting similar effects of the combination, the clinical significance of these observations should be cautiously interpreted.
Albeit preliminary, data presented here also indicate that cotreatment with LBH589 sensitizes BaF3/Bcr-AblE255K and BaF3/Bcr-AblT315I cells to higher but clinically achievable levels of AMN107. Because higher concentrations of AMN107 are able to inhibit the Bcr-Abl TK activity of the mutant Bcr-AblE255K, it is likely that cotreatment with LBH589 further enhances this activity of AMN107 by depleting the levels of Bcr-AblE255K and because of other downstream mechanisms cited. However, the structural basis for how cotreatment with LBH589 leads to increased activity of AMN107 (≥ 1.0 μM) against the contact inhibitory mutant Bcr-AblT315I is not entirely clear. It is possible that LBH589 may collaborate with AMN107 in significantly inhibiting the non–TK-dependent prosurvival mechanisms mediated by Bcr-Abl in CML cells. However, further studies are needed to characterize these mechanisms and determine how these would sensitize Bcr-AblT315I to the combination of LBH589 and AMN107. Alternatively, it is conceivable that LBH589-mediated inhibition of hsp90 chaperone function for Bcr-Abl affects its conformation in a manner that allows higher concentrations of AMN107 to interact with and inhibit Bcr-AblT315I.57 In a recent report, the non-ATP competitive Bcr-Abl kinase inhibitor ON012380 was shown to inhibit the activity of Bcr-AblT315I.58,59 Therefore, it would be important to determine whether cotreatment with LBH589 would further augment the activity of ON012380 against CML cells expressing Bcr-AblT315I.
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2005-11-4639.
Two of the authors (P.A. and P.W.M.) are employed by Novartis Pharmaceutical Inc, whose products, LBH589 and AMN107, were studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal