We tested the hypothesis that the ratio between the activating and inhibitory Fcγ receptor type II (FcγRII) in neutrophils determines their responsiveness to immune complexes. We measured mRNA levels of FcγRII isoforms and observed differences in the ratio of FcγRIIa to FcγRIIb2 mRNA in granulocytes of 50 white and 10 black healthy volunteers, and found 4 discrete groups of ratios (ie, 4:1; 3:1, 2:1, or 1:1). The response to either dimeric IgG or aggregated IgG (aIgG) was assessed. Up-regulation of CD11b on the surface as well as the elastase release was significantly more pronounced in neutrophils with a high FcγRIIa/FcγRIIb2 mRNA ratio of 4:1 compared with a 2:1 or 1:1 ratio. Individual ratios as well as the functional responsiveness of neutrophils were constant over time, as was tested over 12 months. Neutrophil stimulation with various agents in vitro did not alter the FcγRIIa/FcγRIIb2 mRNA ratio in the neutrophils of these donors, in clear contrast to the findings in their mononuclear cells. We found a strong association between the 2B.4 haplotype of the FCGR2B promoter with increased transcriptional activity in individuals with 1:1 ratios and the more common low-expression 2B.1 haplotype in individuals with FcγRIIa/FcγRIIb2 mRNA ratios of 2:1, 3:1, or 4:1.
Introduction
Fcγ receptors (FcγRs) are IgG binding molecules belonging to the immunoglobulin superfamily that links innate and adaptive immunity. Depending on their expression on effector cells, FcγRs exert different effects. For example, on phagocytes they induce phagocytosis, endocytosis, antibody-dependent cellular cytotoxicity (ADCC), and release of reactive oxygen species.1
Three types of FcγRs, types I, II, and III, are discriminated based on their affinity for monomeric IgG. Type I (Ka = 108-109 M–1) is a high-affinity receptor, whereas types II (Ka = 106 M–1) and III (Ka = 5.5 × 105 M–1)2,3 are low-affinity receptors. Of these receptors FcγRI and FcγRIII are dependent on the association with another molecule for signal transduction. In contrast, FcγRII contains, depending on the isoform, either an ITAM or an ITIM motif in its intracellular tail. FcγRIIa contains an immunereceptor tyrosine-based activation motif (ITAM motif) and is therefore an activating receptor, whereas FcγRIIb contains an immune-receptor inhibition motif (ITIM motif) and is therefore an inhibitory receptor.4,5
Under resting conditions, neutrophils express both FcγRIIa and FcγRIIIb (1-4 × 104 and 1-3 × 105 molecules per cell, respectively), but no FcγRI.6 In addition, neutrophils also express the splice variant FcγRIIb2,7 although quantification has not been substantiated. It has been suggested that the ratio between activating and inhibitory FcγR may determine the responsiveness of immune cells to immune complexes.7,8
In the present paper, we tested the hypothesis that the ratio between activating and inhibitory FcγRII on neutrophils may differ among individuals, resulting in a different sensitivity of these phagocytes to IgG agonists. Therefore, the mRNA coding for FcγRIIa and FcγRIIb2 was quantitated in highly purified neutrophils from various donors by specific polymerase chain reactions (PCRs), and related to the induction of CD11b expression from specific granules9,10 and to elastase release from the azurophil granules11,12 upon stimulation of the neutrophils with polymeric IgG.
Materials and methods
Selection of study population
Fifty white unrelated individuals aged between 20 to 55 years, with an equal distribution between males and females, were included. Ten black unrelated individuals, 2 males and 8 females, between the ages of 15 to 50 years were included. Of the white families included in this study, the age varied between 10 to 80 years. Blood was obtained after informed consent with written permission in line with the institutes' medical ethical committee regulations. Approval for these studies was obtained from Sanquin's institutional review board.
Immunoglobulin preparations
Intravenous immunoglobulin (IVIg, lot. no. 01H03H443A; Sanquin CLB, Amsterdam, The Netherlands) contains 60 g/L protein, of which at least 95% is IgG. Aggregated IgG (aIgG) was obtained by incubating IVIg at 10 mg/mL in phosphate-buffered saline, pH 7.4 (PBS), for 30 minutes at 63°C.13 Gel filtration chromatography on a Superdex 200 HR 16/30 column revealed that the preparation contained 43% aIgG, no dimeric IgG, and 57% monomeric IgG, as analyzed by a computer program (Unicorn version 4.5; Amersham Biosciences, Freiburg, Germany).
Intramuscular immunoglobulin (IMIg, lot. no. 01B26H403A; Sanquin CLB) contains 160 g/L protein, of which at least 90% is IgG. Dimeric IgG was isolated from IMIg by gel filtration on Hiload 16/60 Superdex 200 and subsequent pooling of the peaks containing dimeric IgG.
Monoclonal antibodies and reagents
The following monoclonal antibodies (mAbs) against human Fcγ receptors (FcγRs) were used: CD16 (anti-FcγRIII, clone 3G8, prepared as F(ab′)2 fragments; a generous gift from Dr Masja de Haas, Sanquin CLB); CD16-PE (anti-FcγRIII, IgG2a isotype, clone CLB-FcR-gran/1, 5D2; Sanquin CLB), CD32 (anti-FcγRII, Fab fragments of clone IV.3; also a generous gift from Dr Masja de Haas); anti–CD64-FITC (anti-FcγRI, IgG1 isotype, clone 10.1; InstruChemie, Delfzijl, The Netherlands); and CD11b-FITC (anti–LFA-1, IgM isotype, clone CLB-mon-gran/1, B2). Relevant isotype controls were obtained from Sanquin CLB: isotype control IgG2a-PE (clone 713) and IgG1-FITC (clone 203). Goat anti–mouse PE F(ab′)2 (DakoCytomation, Glostrup, Denmark) was used to visualize CD32 on the cell surface.
Isolation of neutrophils and PBMCs
Blood was obtained from healthy volunteers by venous puncture in heparin- or EDTA-containing tubes (Vacuette; Greiner Bio-one, Alphen a/d Rijn, The Netherlands).
EDTA blood was diluted 1:1 in PBS containing 10%, vol/vol, sodium citrate, layered on Percoll (δ= 1.078 g/mL; Amersham Biosciences) and centrifuged at 1000g for 25 minutes without break. The interface containing the peripheral blood mononuclear cells (PBMCs) and the pellet containing erythrocytes and neutrophils were collected, and erythrocytes were lysed in ice-cold NH4Cl-buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA at pH 7.4). Neutrophils and PBMCs were washed in PBS and counted. Purity and viability were more than 95%, as determined by flow cytometry and trypan blue exclusion, respectively. In the experiments, only neutrophil fractions that were negative for FcγRI (CD64) were used.
RNA isolation and reverse transcription (RT)
mRNA was isolated from 107 purified neutrophils by use of QiaAmp RNA blood mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Subsequently, first-strand complementary DNA (cDNA) was synthesized with the Superscript III first-strand synthesis system for RT-PCR (Invitrogen, Breda, The Netherlands). In short, RNA was primed with 2.5 μM oligo-dT for 5 minutes at 65°C. Reverse transcription was performed with 10 U/μL Superscript III in the presence of 5 mM MgCl2, 20 mM Tris-HCl, and 50 mM KCl (pH 8.4, RT-buffer), 0.5 mM dNTP, and 2 U/μL RNaseOUT (with no DTT, for reasons described by Lekanne Deprez et al14 ) for 50 minutes at 50°C. Thereafter, Superscript III was inactivated by incubation for 5 minutes at 85°C, followed by chilling on ice. Immediately thereafter, 2 U RNase H was added and incubated at 37°C for 20 minutes. Subsequently, cDNA was stored at –20°C until further use.
Primers
Intron-spanning primers were designed to specifically amplify cDNA and exclude amplification of genomic DNA, yielding products of 100 bp for β-glucuronidase (GUS), 244 bp for FcγRIIa, and 243 bp for FcγRIIb2.
The following primers were used: β-glucuronidase (GUS): forward primer, 5′-GAAAATATGTGGTTGGAGAGCTCATT-3′ and reverse primer, 5′-CCGAGTGAAGATCCCCTTTTTA-3′; FcγRIIa: forward primer, 5′-ATCATTGTGGCTGTGGTCATTGC-3′ and reverse primer, 5′-TCAGGTAGATGTTTTTATCATCG-3′; and FcγRIIb2: forward primer, 5′-GGAAAAAGCGCATTTGAGCCAATC-3′ and reverse primer, 5′-GGAAATACGAGATCTTCCCTCTCTG-3′.
Polymerase chain reaction
Amplification by PCR was performed on a LightCycler instrument (Roche, Almere, The Netherlands), with software version 3.5. The reaction was performed with LightCycler FastStart DNA MasterPLUS SYBR Green I (Roche), which has been optimized by the manufacturer such that MgCl2 optimalization is no longer needed. The annealing temperature used for all primers was 60°C. The reaction mixture consisted of 2 μL cDNA, 1 μMof each primer combination, and 4 μL LightCycler FastStart DNA MasterPLUS SYBR Green I mix (Roche) in a total volume of 20 μL. All cDNA amplified was compared with the standard within the same run and in every run the same standard was used, although there was very little variation in the standard between runs. For amplification, the following LightCycler protocol was used. The chemical cleft of the Taq polymerase was removed by preincubation for 10 minutes at 95°C; the template was amplified for 40 cycles of denaturation of 5 seconds at 95°C, annealing of the primers at 60°C for 30 seconds, followed by extension at 72°C for 15 seconds. The fluorescence was measured at the end of each cycle at 72°C. At the end of 40 cycles, a melting curve was generated to determine the unique features of the DNA amplified. To identify the product, it was submitted to a 1% (wt/vol) agarose gel to determine the size. Subsequently, the band obtained was purified by means of GFX PCR DNA and Gel Band purification kit (Amersham Biosciences) according to the manufacturer's instructions to remove excess dNTPs and primers. The product was sequenced by Big-dye Terminator Sequencing and ABI Prism software (Applied Biosystems, Foster City, CA). The sequence was verified with BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) to determine specificity. All products obtained were unique and had no overlap with other isoforms.
Standard curves and relative quantitation
As a source of cDNA for standard curves to which all samples were normalized, neutrophils were isolated from an apheresis buffy coat obtained from the blood bank North-West (Sanquin). Serial 10-fold dilutions from the cDNA obtained were made to which each sample was quantified with the method described in Technical Note No. LC 13/2001 (Roche Applied Science, Almere, The Netherlands). In short, the threshold cycle (CT) values, determined by the LightCycler software, were used to calculate and plot a linear regression curve, as performed by the software. From this regression, the quality of the standard curve can be evaluated by the slope and the correlation coefficient (r). The slope of the line was used to determine the efficiency of the reaction (E). From the CT's and the efficiencies obtained, the normalized ratio was calculated with the following formula: ETCpT(C) – CpT(S):ERCpR(C)–CpR(S), in which ET is the efficiency of the PCR of the target gene (FcγRIIa or FcγRIIb2); ER, the efficiency of the PCR of the reference gene (GUS); CpT(C), the measured CT of the target gene determined for standard or calibrator (FcγRIIa or FcγRIIb2 of one selected individual for all measurements); CpT(S), the measured CT of the target gene determined for the sample (donor of interest); CpR(C), the measured CT of the reference gene of the calibrator or standard; and CpR(S), the measured CT of the reference gene of the sample.
Validation of quantitative RT-PCR for FcγRII isoforms on the LightCycler
To study the expression levels of FcγRII isoforms on neutrophils, we set up a relative quantitative RT-PCR by means of the LightCycler instrument. This technique yielded a highly sensitive and specific method to determine FcγRIIa and FcγRIIb2 mRNA expression levels in neutrophils. The slopes of the standards for each PCR reaction were around –3.3, yielding an efficiency of about 2, indicating that during each cycle the specific product was doubled. Furthermore, each set of primers used resulted in a specific melting curve with its own melting temperature (Tm), whereas the nontemplate controls displayed a different melting curve or no product at all.
FCGR2B promoter haplotypes
A nonspecific FCGR2B/C PCR was performed to amplify a 629-bp fragment to screen for haplotypes at nucleotides (nts) –120 and –386 of the FCGR2B and FCGR2C promoters. The sense primer (5′-TGACATACCTCCTTGTCCTTGTT-3′) and the antisense primer (5′-GCAGTCAGCCCAGTCACTCTCAGT-3′) both anneal to FCGR2B and FCGR2C surrounding nts –120 and –386 as described by Blank et al.15 The PCR conditions were 94°C for 5 minutes, and then 35 cycles of 94°C for 1 second, 60°C for 1 second, and 72°C for 1 second, followed by 10 minutes at 72°C. The PCR product was purified by GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences) and sequenced with Big-dye terminator cycle sequencing on an ABI 3100 sequencer (Applied Biosystems). The same primers as used in the PCR were used for sequencing in both directions.
A long-range PCR was performed on those samples carrying the uncommon variants of either the –120 and/or –386 SNP. For this purpose, the Expand Long Template PCR System (Roche Applied Science) was used according to the manufacturer's instructions. In brief, a 15-kb fragment was amplified using a nonspecific FCGR2B/C sense primer (5′-GCCATCCTGACATACCTCCT-3′) annealing in the promoter region and a FCGR2B specific antisense primer (5′-CCCAACTTTGTCAGCCTCATC-3′) annealing in exon 7. The PCR conditions were 94°C for 2 minutes, and then 10 cycles of 94°C for 10 seconds, 60°C for 30 seconds, and 68°C for 12 seconds, followed by 20 cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 68°C for 12 seconds, with an elongation of each cycle for 20 seconds, followed by a final elongation at 68°C for 7 minutes. PCR products were purified by GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences) and sequenced with Big-dye terminator cycle sequencing with the sense primer used in the PCR reaction.
Ex vivo model to assess sensitivity of neutrophils for dimeric IgG and aIgG
A titration of dimeric IgG or aIgG (in 90 μL Iscove modified Dulbecco medium [IMDM]) was prepared in the wells of a 96-well round-bottom plate. Heparinized whole blood was then diluted 1:10 in the wells. The mixtures were incubated for 2 hours at 37°C in humidified air containing 5% CO2. Elastase release was estimated by assessing the amount of elastase released into the supernatant in relation to the total elastase content of the cells, which was determined in 1% (wt/vol) Triton X-100 lysates of the cells. Elastase concentrations were measured with sandwich enzyme-linked immunosorbent assay (ELISA) as described.16 After stimulation, the cells were also analyzed by flow cytometry on a FACScalibur for the expression of FcγR and CD11b. Sensitivity to stimulation with either dimeric IgG or aIgG was evaluated as fold increase in CD11b expression, which was calculated with the following formula: (MFI for CD11b of the stimulated cells – MFI for the isotype control of the stimulated cells)/(MFI for CD11b of the resting cells – MFI for the isotype control of the resting cells). As a positive control for degranulation, 1 μM N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP; Sigma-Aldrich, St Louis, MO) and 5 μg/mL cytochalasin B (Sigma-Aldrich) were added.
In vitro activation of neutrophils and PBMCs
Neutrophils and PBMCs were isolated as described in “Isolation of neutrophils and PBMCs” and cultured in 24-well plates at a density of 106 cells/mL. Wells contained either medium alone (control cells), 50 U/mL rhGM-CSF, 200 ng/mL rhIL-4 (generous gifts from Dr Lucien Aarden, Sanquin Research, Amsterdam, The Netherlands), 200 U/mL rhIFNγ (Boehringer Ingelheim, Germany), 5 ng/mL TNFα (PeproTech EC, London, United Kingdom), or 20 ng/mL LPS (Sigma-Aldrich) supplemented with rhLBP (LPS-binding protein; Boehringer Ingelheim). Each condition was as applied in triplicate. After 4 hours, samples were taken for analysis by flow cytometry. The remainder of cells was used for determination of the FcγRIIa/FcγRIIb2 mRNA ratio.
Statistical analysis
Results are depicted as mean ± SEM. Where applicable, Student t test or one-way ANOVA was used. For comparison of the various time points, a 2-way ANOVA was performed. For analysis of the FCGR2B promoter haplotypes, Fisher exact test was used. P values less than .05 were considered as a significant difference.
Results
Validation of quantitative RT-PCR for FcγRII isoforms on the LightCycler
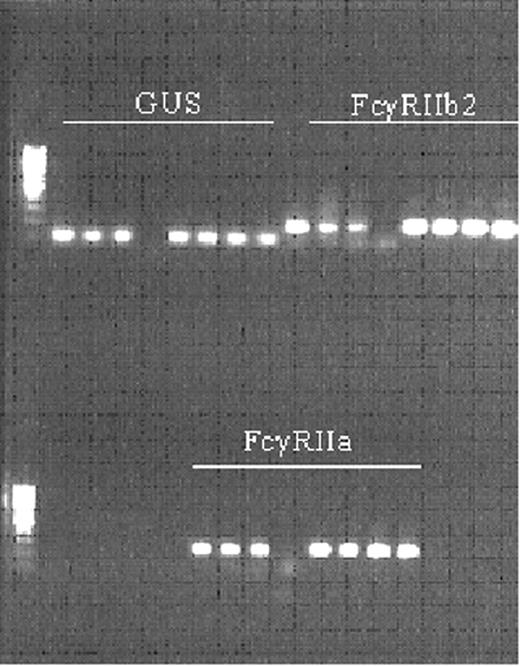
To study the expression levels of FcγRII isoforms on neutrophils, we set up a quantitative RT-PCR (as described in “Materials and methods”). When the PCR products were separated on a 1% agarose gel, each product showed one distinct band of the predicted size (Figure 1). Finally, the sequence of each band was determined and verified through BLAST. All products were fully specific for their target mRNA.
FcγRIIa/FcγRIIb2 mRNA ratio relates to increase in CD11b expression on neutrophils in response to IgG complexes
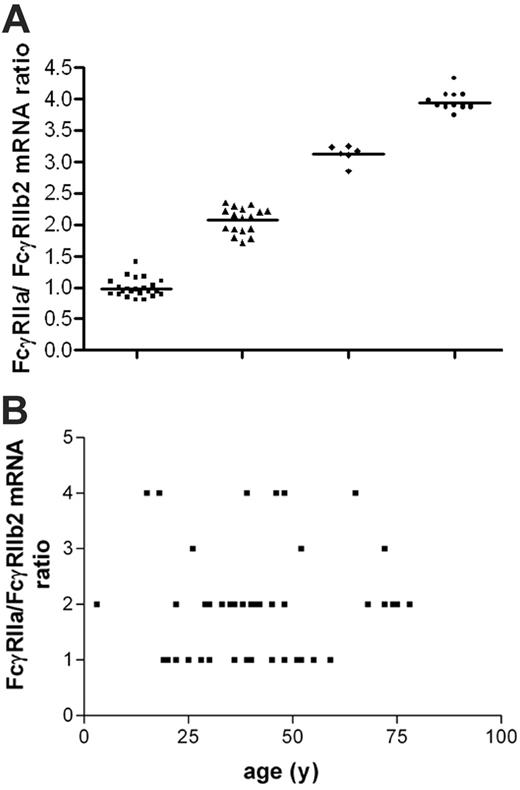
We explored the expression of FcγRII isoform mRNA in neutrophils of 50 white and 10 black healthy volunteers. Transcripts for FcγRIIa and FcγRIIb2, but not FcγRIIb1, were found (data not shown). Upon examination of the FcγRIIa/FcγRIIb2 mRNA ratio on neutrophils of white healthy volunteers as well as the black volunteers, we distinguished 4 categories of donors, with a ratio of 1:1, 2:1, 3:1, or 4:1 (Figure 2), independent of racial background.
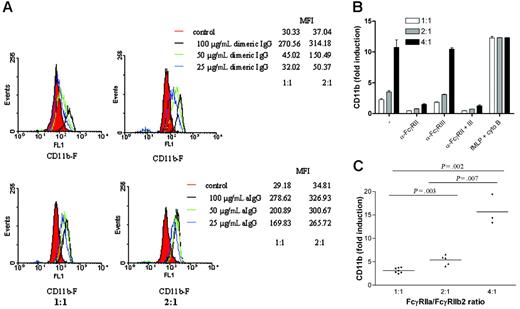
We investigated whether this ratio was linked to differences in sensitivity to activation by immune complexes. There was a highly significant difference in the up-regulation of CD11b expression on the cell surface of neutrophils in response to dimeric IgG (50 μg/mL) between individuals with a 2:1 ratio and a 1:1 ratio (P = .003) (Figure 3A,C). This was true for various concentrations of IgG dimers and aIgG tested (Figure 3A). Blockade of FcγRIII had no effect whatsoever on the expression of CD11b in response to 50 μg/mL dimeric IgG, whereas anti-FcγRII blocked this phenomenon completely. Moreover, the fMLP-induced CD11b up-regulation was identical in the individuals tested, excluding any inherent difference in neutrophil reactivity per se (Figure 3B). Furthermore, the induction of CD11b in response to dimeric IgG also differed significantly between individuals, with a ratio of 4:1 versus those with a ratio of 2:1 or 1:1 (P = .007 and P = .002, respectively) (Figure 3C). We observed no changes in total CD32 staining.
Validation of quantitative RT-PCR for FcγRII isoforms on the LightCycler. The products obtained in PCR were separated on 1% agarose gel. GUS, FcγRIIb2, and then FcγRIIa were loaded. The first 3 slots are the serial dilutions of the standard curve, followed by the nontemplate control, followed by duplicates of 2 samples.
Validation of quantitative RT-PCR for FcγRII isoforms on the LightCycler. The products obtained in PCR were separated on 1% agarose gel. GUS, FcγRIIb2, and then FcγRIIa were loaded. The first 3 slots are the serial dilutions of the standard curve, followed by the nontemplate control, followed by duplicates of 2 samples.
Variation in FcγRIIa/FcγRIIb2 mRNA ratios within healthy volunteers. (A) Neutrophils from 60 healthy volunteers were isolated, and FcγRIIa/FcγRIIb2 mRNA ratios were determined. Four nonoverlapping groups were observed. There was an equal distribution of males and females over the groups without bias of age toward the groups. There was a high prevalence of FcγRIIa/FcγRIIb2 mRNA ratios 1:1 (n = 23; 38%) and 2:1 (n = 17; 28%), whereas 3:1 (n = 6; 10%) and 4:1 (n = 14; 24%) were less frequently observed. (B) FcγRIIa/FcγRIIb2 mRNA ratios plotted against age. No correlation between age and FcγRIIa/FcγRIIb2 mRNA ratios was found (r2 = 0.006 987 and P = .564).
Variation in FcγRIIa/FcγRIIb2 mRNA ratios within healthy volunteers. (A) Neutrophils from 60 healthy volunteers were isolated, and FcγRIIa/FcγRIIb2 mRNA ratios were determined. Four nonoverlapping groups were observed. There was an equal distribution of males and females over the groups without bias of age toward the groups. There was a high prevalence of FcγRIIa/FcγRIIb2 mRNA ratios 1:1 (n = 23; 38%) and 2:1 (n = 17; 28%), whereas 3:1 (n = 6; 10%) and 4:1 (n = 14; 24%) were less frequently observed. (B) FcγRIIa/FcγRIIb2 mRNA ratios plotted against age. No correlation between age and FcγRIIa/FcγRIIb2 mRNA ratios was found (r2 = 0.006 987 and P = .564).
FcγRIIa/FcγRIIb2 mRNA ratio relates to elastase release by neutrophils in response to IgG complexes
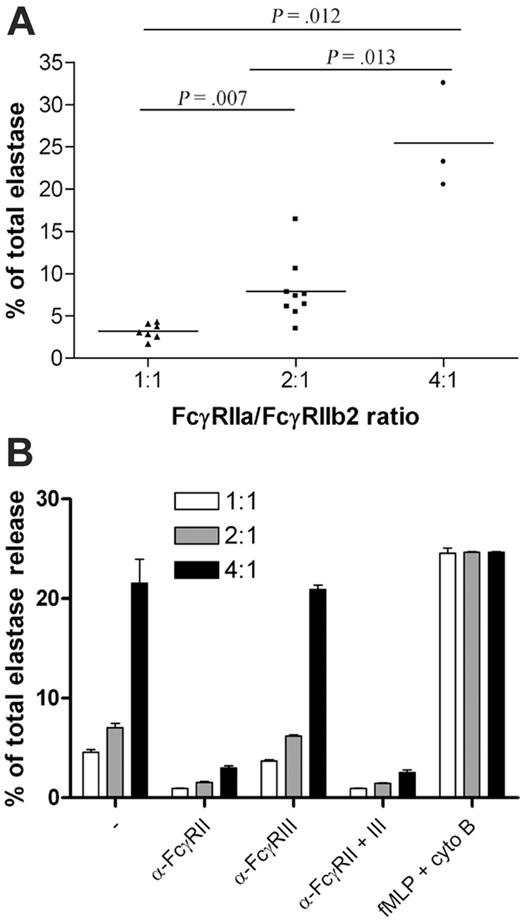
The release of elastase from the azurophil granules of neutrophils was also tested. We found a highly significant difference in elastase release in response to dimeric IgG (50 μg/mL) when neutrophils with a 1:1 ratio of FcγRIIa mRNA to FcγRIIb2 mRNA were compared with cells with a 2:1 ratio (Figure 4A; P = .007). In addition, the elastase release was even more pronounced in the group with a 4:1 ratio compared with the 2:1 (P = .013) or the 1:1 (P = .012) group. Again, blockade of FcγRIII had no effect, whereas anti-FcγRII fully attenuated the elastase release in response to 50 μg/mL dimeric IgG, irrespective of the ratio. Again, there was no significant difference in the responsiveness toward fMLP (in the presence of cytochalasin B) (Figure 4B).
Increase in CD11b exposure on neutrophils with different FcγRIIa/FcγRIIb2 mRNA ratio upon stimulation with polymeric IgG. (A) Histograms showing the up-regulation of CD11b on neutrophils stimulated with various concentrations of polymeric IgG. Representative examples of each group are shown. (B) Induction of CD11b accumulation in whole blood, obtained from several individuals selected from the white population, after stimulation with either 50 μg/mL dimeric IgG in the presence or absence of blocking Fab anti-FcγRII and/or blocking F(ab′)2 anti-FcγRIII or fMLP supplemented with cytochalasin B. Error bars represent SEM. (C) Whole blood, obtained from several individuals selected from the white population, was stimulated with 50 μg/mL dimeric IgG, and induction of CD11b accumulation was measured. The difference in exposure between the 3 groups of neutrophils tested was significant.
Increase in CD11b exposure on neutrophils with different FcγRIIa/FcγRIIb2 mRNA ratio upon stimulation with polymeric IgG. (A) Histograms showing the up-regulation of CD11b on neutrophils stimulated with various concentrations of polymeric IgG. Representative examples of each group are shown. (B) Induction of CD11b accumulation in whole blood, obtained from several individuals selected from the white population, after stimulation with either 50 μg/mL dimeric IgG in the presence or absence of blocking Fab anti-FcγRII and/or blocking F(ab′)2 anti-FcγRIII or fMLP supplemented with cytochalasin B. Error bars represent SEM. (C) Whole blood, obtained from several individuals selected from the white population, was stimulated with 50 μg/mL dimeric IgG, and induction of CD11b accumulation was measured. The difference in exposure between the 3 groups of neutrophils tested was significant.
Fluctuation of FcγRIIa/FcγRIIb2 mRNA ratio over time
Subsequently, the FcγRIIa/FcγRIIb2 mRNA ratio in neutrophils was measured 3 times at various time intervals (range, 2-12 months) in 10 individuals, 5 having neutrophils with a 1:1 ratio and the others with a 2:1 ratio. Whereas the individual levels of FcγRIIa and FcγRIIb2 mRNA fluctuated over time, we found no fluctuations in the ratio at any time point (mean and SEM of 0.9 ± 0.2 for the 1:1 ratio and 2.0 ± 0.5 for the 2:1 ratio). Upon stimulation with dimeric IgG, neither the CD11b up-regulation nor the elastase release responses of the neutrophils varied significantly over time (mean and SEM for CD11b, 3.0-fold ± 0.8-fold for the 1:1 ratio and 5.7-fold ± 1.5-fold for the 2:1 ratio; for elastase, 3.3% ± 0.5% for the 1:1 ratio and 11.4% ± 2.6% for the 2:1 ratio), which correlates with the fixed ratios of FcγRIIa/FcγRIIb2 mRNA.
Activation of neutrophils and PBMCs
The stable ratio in neutrophils was unexpected because of the earlier observations in mononuclear phagocytes that Th1 and Th2 cytokines affect the level of transcription and concomitantly the corresponding FcγRIIa/FcγRIIb2 mRNA ratio.7 To gain insight into the nature of the stability of the FcγRIIa/FcγRIIb2 mRNA ratio, isolated neutrophils were cultured for 4 hours in the presence or absence of known neutrophil activators (ie, GM-CSF,17 LPS, and LBP18 ) or cytokines (ie, IFNγ, IL-4, and TNFα) reported to influence the FcγRIIa/FcγRIIb2 mRNA ratio in monocytes.7,19
Elastase release from neutrophils with different FcγRIIa/FcγRIIb2 mRNA ratios upon stimulation with polymeric IgG. (A) Elastase release from neutrophils with different FcγRIIa/FcγRIIb2 ratios, obtained from several individuals selected from the white population, was measured after stimulation with 50 μg/mL dimeric IgG. The difference between the 3 groups tested was significant. (B) Whole blood, obtained from several individuals selected from the white population, was stimulated with either 50 μg/mL dimeric IgG in the presence or absence of blocking Fab anti-FcγRII and/or blocking F(ab′)2 anti-FcγRIII or fMLP supplemented with cytochalasin B, and elastase release was measured. Error bars represent SEM.
Elastase release from neutrophils with different FcγRIIa/FcγRIIb2 mRNA ratios upon stimulation with polymeric IgG. (A) Elastase release from neutrophils with different FcγRIIa/FcγRIIb2 ratios, obtained from several individuals selected from the white population, was measured after stimulation with 50 μg/mL dimeric IgG. The difference between the 3 groups tested was significant. (B) Whole blood, obtained from several individuals selected from the white population, was stimulated with either 50 μg/mL dimeric IgG in the presence or absence of blocking Fab anti-FcγRII and/or blocking F(ab′)2 anti-FcγRIII or fMLP supplemented with cytochalasin B, and elastase release was measured. Error bars represent SEM.
As indicated in Table 1, the FcγRIIa/FcγRIIb2 mRNA ratio in neutrophils did not change (P = .30). Again, the individual levels of FcγRIIa and FcγRIIb2 mRNA did change in comparison with the unstimulated control (ie, stimulation with GM-CSF resulted in a 10-fold increase in both FcγRIIa and FcγRIIb2 mRNA; IFNγ, a 5-fold increase; and IL-4, a 2-fold increase, thus leaving the ratios unaltered). Stimulation with TNFα as well as LPS with LBP did not alter the levels of FcγRIIa and FcγRIIb2 mRNA either. Furthermore, the cells were activated under all conditions applied, as indicated by the increased surface expression of CD11b, CD66b, and FcγRI (CD64) and the loss of FcγRIII (CD16). Here too, we did not observe changes in total levels of CD32 (data not shown).
Influence of the cytokine environment on the FcγRIIa/FcγRIIb2 mRNA ratio in neutrophils and PBMCs
. | FcγRIIa/FcγRIIb2 mRNA ratio . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | No stimulus . | GM-CSF, 50 U/mL . | LPS, 20 ng/mL . | IFNγ, 200 U/mL . | IL-4, 200 ng/mL . | ||||
| Donor 1 | |||||||||
| Neutrophils | 0.9 ± 0.04 | 0.9 ± 0.04 | 0.8 ± 0.08 | 0.9 ± 0.00 | 0.9 ± 0.00 | ||||
| PBMCs | 0.7 ± 0.12 | 0.2 ± 0.00 | 0.9 ± 0.03 | 0.7 ± 0.04 | 0.1 ± 0.00 | ||||
| Donor 2 | |||||||||
| Neutrophils | 0.9 ± 0.01 | 0.8 ± 0.00 | 0.9 ± 0.02 | 0.9 ± 0.05 | 0.9 ± 0.02 | ||||
| PBMCs | 0.7 ± 0.04 | 0.2 ± 0.02 | 0.5 ± 0.03 | 0.6 ± 0.02 | 0.1 ± 0.00 | ||||
| Donor 3 | |||||||||
| Neutrophils | 1.9 ± 0.02 | 2.0 ± 0.06 | 2.2 ± 0.07 | 1.9 ± 0.06 | 1.9 ± 0.07 | ||||
| PBMCs | 0.6 ± 0.02 | 0.1 ± 0.00 | 0.5 ± 0.01 | 0.4 ± 0.01 | 0.1 ± 0.00 | ||||
| Donor 4 | |||||||||
| Neutrophils | 1.9 ± 0.03 | 1.7 ± 0.02 | 1.9 ± 0.01 | 1.7 ± 0.01 | 1.9 ± 0.00 | ||||
| PBMCs | 1.6 ± 0.07 | 0.4 ± 0.01 | 1.1 ± 0.13 | 1.5 ± 0.07 | 0.2 ± 0.00 | ||||
. | FcγRIIa/FcγRIIb2 mRNA ratio . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
. | No stimulus . | GM-CSF, 50 U/mL . | LPS, 20 ng/mL . | IFNγ, 200 U/mL . | IL-4, 200 ng/mL . | ||||
| Donor 1 | |||||||||
| Neutrophils | 0.9 ± 0.04 | 0.9 ± 0.04 | 0.8 ± 0.08 | 0.9 ± 0.00 | 0.9 ± 0.00 | ||||
| PBMCs | 0.7 ± 0.12 | 0.2 ± 0.00 | 0.9 ± 0.03 | 0.7 ± 0.04 | 0.1 ± 0.00 | ||||
| Donor 2 | |||||||||
| Neutrophils | 0.9 ± 0.01 | 0.8 ± 0.00 | 0.9 ± 0.02 | 0.9 ± 0.05 | 0.9 ± 0.02 | ||||
| PBMCs | 0.7 ± 0.04 | 0.2 ± 0.02 | 0.5 ± 0.03 | 0.6 ± 0.02 | 0.1 ± 0.00 | ||||
| Donor 3 | |||||||||
| Neutrophils | 1.9 ± 0.02 | 2.0 ± 0.06 | 2.2 ± 0.07 | 1.9 ± 0.06 | 1.9 ± 0.07 | ||||
| PBMCs | 0.6 ± 0.02 | 0.1 ± 0.00 | 0.5 ± 0.01 | 0.4 ± 0.01 | 0.1 ± 0.00 | ||||
| Donor 4 | |||||||||
| Neutrophils | 1.9 ± 0.03 | 1.7 ± 0.02 | 1.9 ± 0.01 | 1.7 ± 0.01 | 1.9 ± 0.00 | ||||
| PBMCs | 1.6 ± 0.07 | 0.4 ± 0.01 | 1.1 ± 0.13 | 1.5 ± 0.07 | 0.2 ± 0.00 | ||||
Purified neutrophils and PBMC fractions were each stimulated in triplicate for 4 hours with the stimulants indicated. Data are presented as means ± SEM.
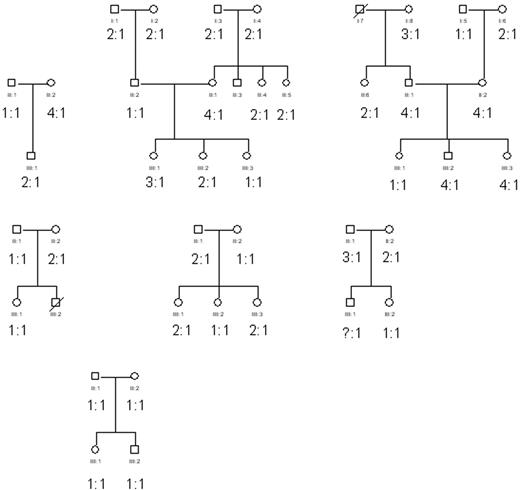
Inheritance of the FcγRIIa/FcγRIIb2 mRNA ratios. Neutrophils from several white families were obtained and subsequently the FcγRIIa/FcγRIIb2 mRNA ratios were determined as described in “Materials and methods.” The age within this group varied between 10 to 80 years. No obvious inheritance pattern was observed.
Inheritance of the FcγRIIa/FcγRIIb2 mRNA ratios. Neutrophils from several white families were obtained and subsequently the FcγRIIa/FcγRIIb2 mRNA ratios were determined as described in “Materials and methods.” The age within this group varied between 10 to 80 years. No obvious inheritance pattern was observed.
At the same time, we confirmed that the monocytes of the same individuals showed a significant up-regulation of the mRNA encoding FcγRIIb2 in response to IL-4 as well as GM-CSF compared with control cells (P = .001), thereby strongly decreasing the FcγRIIa/FcγRIIb2 mRNA ratio (Table 1). Of interest, the discrete FcγRIIa/FcγRIIb2 mRNA ratios in the donors' neutrophils were also observed within the PBMCs. However, the observed ratios in neutrophils did not seem to be linked to the FcγRIIa/FcγRIIb2 mRNA ratios observed in the PBMC fraction (Table 1, donor 3).
Inheritance patterns of the FcγRIIa/FcγRIIb2 mRNA ratio
To study the possibility of genetic factors underlying the FcγRIIa/FcγRIIb2 mRNA ratio in neutrophils, we investigated 42 individuals from 7 unrelated families with an age range of 10 to 80 years (Figure 5). No clear-cut inheritance pattern was observed.
FCGR2B promoter polymorphisms in relation to FcγRIIa/FcγRIIb2 mRNA ratios
Recently, 2 functional polymorphisms in the promoter region of the FCGR2B gene were shown to be linked to transcriptional activity.20 The promoter haplotypes affecting transcription are located at nucleotide (nts) –386 and –120, and comprise a G>C and a T>A transition, respectively. The most common “wild-type” –386G/–120T haplotype was termed 2B.1, and the second most frequent –386C/–120A haplotype was named 2B.4.
We investigated the potential influence of these haplotypes on the various FcγRIIa/FcγRIIb2 mRNA ratios observed in the neutrophils of our cohort of white volunteers. As indicated in Table 2, we found a strong association between the 1:1 FcγRIIa/FcγRIIb2 mRNA ratio and the 2B.4 haplotype, whereas the 3:1 and 4:1 FcγRIIa/FcγRIIb2 mRNA ratios seemed to have an absolute association with the 2B.1 haplotype with low transcriptional activity. Only one individual in the 2:1 FcγRIIa/FcγRIIb2 mRNA ratio group had a 2B.4 haplotype and another had a 2B.2 haplotype (–386C/–120T).
Association of FCGR2B haplotypes with FcγRIIa/FcγRIIb2 mRNA ratios in neutrophils
. | FcγRIIa/FcγRIIb2 mRNA ratio . | . | |
|---|---|---|---|
. | 1:1 . | 2:1 or higher . | |
| Genotype, no. subjects (% of group) | |||
| 2B.1/2B.1 | 6 (35) | 25 (96) | |
| 2B.1/2B.4 | 10 (59) | 1 (4) | |
| 2B.4/2B.4 | 0 (0) | 0 (0) | |
| Haplotype frequency, % | |||
| 2B.1 (-386G/-120T) | 68.8 | 98.0 | |
| 2B.4 (-386C/-120A) | 31.2 | 2.0 | |
. | FcγRIIa/FcγRIIb2 mRNA ratio . | . | |
|---|---|---|---|
. | 1:1 . | 2:1 or higher . | |
| Genotype, no. subjects (% of group) | |||
| 2B.1/2B.1 | 6 (35) | 25 (96) | |
| 2B.1/2B.4 | 10 (59) | 1 (4) | |
| 2B.4/2B.4 | 0 (0) | 0 (0) | |
| Haplotype frequency, % | |||
| 2B.1 (-386G/-120T) | 68.8 | 98.0 | |
| 2B.4 (-386C/-120A) | 31.2 | 2.0 | |
n = 44; Fisher exact test, OR 0.05; P < .001.
Discussion
To date, there are no monoclonal antibodies available that distinguish between the different FcγRII isoforms. We have therefore developed a highly sensitive RT-PCR for the quantitation of mRNA of FcγRII isoforms. It has been hypothesized that the ratio of activating versus inhibitory FcγRs determines the responsiveness of phagocytic cells to IgG agonists.7,8 In the present paper, we provide evidence for this hypothesis by showing that neutrophils of healthy individuals display an interindividual variation in the FcγRIIa/FcγRIIb2 mRNA ratio and corresponding differences in their responsiveness toward polymeric IgG. We observed 4 groups of ratios. Two independent activation markers were affected by the variation in the FcγRIIa/FcγRIIb2 mRNA ratio.
Under normal conditions, circulating neutrophils express both FcγRIIa and FcγRIIb.7 These cells are also known to express FcγRIIIb, a GPI-anchored molecule.6 The role of FcγRIIIb in activation of these cells has been debated. Some investigators have shown that this receptor is indeed capable of inducing signal transduction,11 possibly with the help of FcγRIIa,21 whereas others have suggested that FcγRIIIb does not contribute to effector functions.22 Our group has demonstrated previously that elastase release from neutrophils induced by dimeric IgG is fully dependent on FcγRII, and not on FcγRIIIb.16 In addition, FcγRIIIb–/– individuals have been reported to be clinically healthy.23 Using blocking anti-FcγRIIIb antibodies, we confirmed that elastase release and CD11b up-regulation on the cell surface of neutrophils upon stimulation with aIgG are independent of FcγRIIIb. Thus, with respect to the agonists applied in our study, FcγRIIIb seems redundant on neutrophils. We used only neutrophils that did not express FcγRI, because this marker may be induced by prior activation in vitro or in vivo.6 The observed variability in responsiveness to polymeric IgG was determined by the ratio of FcγRIIa and FcγRIIb (independent of FcγRIIIb) and stable over time, as was assessed for up to 1 year.
Recently, 2 independent studies have shown that the nonsynonymous single nucleotide polymorphism (SNP) in FCGR2B, which results in a single amino acid substitution, isoleucine to threonine at residue 232, in the transmembrane domain24,25 leads to exclusion of FcγRIIb from lipid rafts. Subsequently, exclusion of FcγRIIb from lipid rafts results in enhanced signaling of either the B-cell receptor or FcγRIIa.24,25 This could provide an alternative explanation for our findings. However, we made sure that our forward primer for FcγRIIb2 ligates behind the SNP at position 695, rendering our quantification of FcγRIIb2 mRNA independent of this polymorphism. Furthermore, when we analyzed individuals with different FcγRIIa/FcγRIIb2 mRNA ratios, we found no association of either 1:1, 2:1, 3:1, or 4:1 with FCGR2B-232T or FCGR2B-232I. Similarly, activation of neutrophils by IgG to up-regulate CD11b or release elastase was not associated with the FCGR2A SNPs for the well-characterized functional FcγRIIa H131R variants (data not shown). Thus, we showed that differences in sensitivity to immune complexes correlate with differences in the FcγRIIa/FcγRIIb2 mRNA ratios and not with genomic FCGR2A or FCGR2B polymorphisms.
To investigate the inheritance of the various categories of fixed FcγRIIa/FcγRIIb2 mRNA ratios in neutrophils, 7 pedigrees were tested. Of course, many genetic traits are subject to variable (co)dominant penetrance, rendering it difficult to elucidate the exact mode of transmission in the small sample size of families tested in the present study. Nonetheless, these findings are important because indications for either an autosomal dominant or recessive pattern of inheritance for the FcγRIIa/FcγRIIb2 mRNA ratios can be excluded by the findings within the pedigrees thus far tested.
To date, no known FCGR2A promoter haplotypes have been identified that associate with quantitative changes in FcγRIIa expression.26 A role for other factors determining the FcγRIIa/FcγRIIb2 mRNA ratios in neutrophils was considered. A recent study has shown that a polymorphism in the promoter region linked to transcriptional activity of the FCGR2B gene exists at nts –386 and –120.20 Four different haplotypes were identified: 2B.1 or “wild type” (–386G/–120T); 2B.2 (–386C/–120T), which may be a more frequent polymorphism in the promoter for the FCGR2C (pseudo)gene; 2B.3 (–386G/–120A); and 2B.4 (–386C/–120A). The 2B.4 haplotype was reported to have enhanced transcriptional activity compared with the 2B.1 haplotype. These haplotypes may determine the affinity of binding sites in the promoter region, such as for the transcription factors Yin-Yang (YY1) and GATA-4. The polymorphism at –386 was also reported to determine the binding affinity for the ubiquitous transcription factor AP-1.15 The differences in the FcγRIIa/FcγRIIb2 mRNA ratios in neutrophils may indeed be related to these polymorphisms. A strong association between the 2B.4 haplotype and the 1:1 FcγRIIa/FcγRIIb2 mRNA ratio was found, whereas the 3:1 and 4:1 FcγRIIa/FcγRIIb2 mRNA ratios were found to be strictly associated with the 2B.1 haplotype. The 2:1 FcγRIIa/FcγRIIb2 mRNA ratio group contained one individual with the 2B.4 haplotype and one with the 2B.2 haplotype. In line with Su et al, our findings indicate that the increased transcription of FCGR2B may be due to the 2B.4 haplotype, resulting in lower FcγRIIa/FcγRIIb2 mRNA ratios.27 However, the association of the promoter haplotypes with the corresponding FcγRIIa/FcγRIIb2 mRNA ratios was not absolute. In addition, we observed no clear-cut inheritance pattern of the FcγRIIa/FcγRIIb2 mRNA ratios, whereas we did find a Mendelian inheritance of the promoter haplotypes (data not shown). Probably, the FcγRIIa/FcγRIIb2 mRNA ratios depend not only on the promoter haplotypes of FCGR2B, but also on other, as-yet-unidentified genetic loci.
FcγRIIb has been implicated as an important regulator in antibody-mediated diseases.8,28,29 Furthermore, FcγRIIb has been demonstrated to clear immune complexes in the absence of inflammation.30,31 Therefore, a high FcγRIIa/FcγRIIb mRNA ratio might predispose to a more rapid progression to inflammation during immune-complex diseases. Whether these mRNA ratios in neutrophils have similar impact on the FcγRII-dependent phagocytosis of IgG-opsonized microorganisms was tested by using IgG-opsonized FITC-labeled S aureus. In contrast to the much smaller IgG dimers and aggregates, we found no significant differences between individuals with a 2:1 ratio and individuals with a 4:1 ratio, despite the fact that the phagocytosis of the IgG-opsonized bacteria was completely FcγRII dependent (data not shown). There are some possible explanations: (1) Opsonized bacteria are larger “complexes” than aggregated IgG and hence the threshold for cell activation is readily exceeded due to the density and activation signals generated by the IgG-opsonized bacteria. (2) FcγRIIb can also act as a phagocytic receptor, as reported in several papers.30,31 These effects might explain why we did not observe any differences between the 2 ratio groups investigated. (3) And lastly, another as-yet-unidentified receptor may be involved. Coligation of this receptor with FcγRII might enhance and optimize cell activation to maximal reactivity, independent of the ratio group. We are currently exploring these possibilities.
Of interest, we observed that the FcγRIIa/FcγRIIb2 mRNA ratio in neutrophils was stable over time. This is remarkable because cytokines have been shown to influence this balance in monocytes,7 as we confirmed in the present study. We found that none of the cytokines known to activate neutrophils influenced the FcγRIIa/FcγRIIb2 mRNA ratio in these cells. This lack of any alteration in the ratio upon cytokine treatment of neutrophils in vitro further supports the finding of a stable individual FcγRIIa/FcγRIIb2 mRNA ratio in neutrophils over time in vivo. There may be a good reason for the diversity in ratio among these phagocytes. Neutrophils are cells that are short-lived and need to respond rapidly in response to pathogens. In contrast, monocytes are long-lived cells that have the capacity to differentiate into more mature cells (ie, macrophages and dendritic cells). During their life span, monocytes may need to be regulated more stringently than neutrophils, and therefore to respond to exogenous stimuli such as IL-4 and IFNγ.
In conclusion, we have shown that the ratio between activating FcγRIIa and inhibitory FcγRIIb2 mRNA in neutrophils varies among individuals, is associated with FCGR2B promoter haplotypes, and is accompanied by differences in responsiveness of these cells to IgG complexes. In each individual, this mRNA ratio in neutrophils was constant over time, which suggests a fixed level of transcription for these receptors. Because the same 4 ratio groups were present in children, elderly, and black individuals, we suggest that age and racial background seem to be of little or no importance for the individual ratio. When these ratios are indeed independent of in vivo conditions of cytokine environment, inflammation, or exacerbations of clinical symptoms, these findings call for further studies on the balance of activating and inhibitory FcγRII receptors in various diseases in which neutrophils are supposed to play an important role.
Prepublished online as Blood First Edition Paper, March 21, 2006; DOI 10.1182/blood-2005-12-4997.
E.v.M. designed and performed research, analyzed data, and wrote the paper; W.B.B. performed research and analyzed data; J.G. performed research;
C.E.H. designed research; M.d.B. provided vital analytic tools; D.R. designed research and wrote the paper; and T.W.K. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal