Caspase-2 was reported to be involved in a number of apoptotic pathways triggered by various stimuli. However, the molecular mechanism of procaspase-2 activation in the course of apoptosis remains poorly defined. In this report, we demonstrate that procaspase-2 is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex (DISC) in human T- and B-cell lines. We show that procaspase-2 is activated at the DISC on CD95 stimulation. Despite its presence at the DISC, caspase-2 does not initiate apoptosis on CD95 stimulation in caspase-8–deficient cell lines. Taken together, our data reveal that caspase-2 is activated at the DISC but does not play an initiating role in the CD95-induced apoptosis.
Introduction
CD95 (APO-1/Fas) is a member of the death receptor family, a subfamily of the TNF-R superfamily.1-5 Crosslinking of CD95 with its natural ligand CD95L (FasL/CD178)6 or with agonistic antibodies such as anti–APO-17 induces apoptosis in sensitive cells. The death-inducing signaling complex (DISC) is formed within seconds after CD95 stimulation.8 The DISC consists of oligomerized, probably trimerized, CD95 receptors; the adaptor molecule FADD; 2 isoforms of procaspase-8 (procaspase-8/a and procaspase-8/b); procaspase-10; and c-FLIPL/S/R.5,9 The interactions between molecules at the DISC are based on homotypic contacts. The death domain (DD) of the receptor interacts with the DD of FADD, whereas the death effector domain (DED) of FADD interacts with the N-terminal tandem DEDs of procaspase-8, -10, and c-FLIPL/S/R. The binding of procaspase-8 to the DISC results in processing of the zymogen. As a result the active caspase-8 heterotetramer p102-p182 is released into the cytosol to propagate the apoptotic signal.10
Two CD95 signaling pathways were established.11 Type I cells are characterized by high levels of CD95 DISC formation and increased amounts of active caspase-8. Activated caspase-8 directly leads to the activation of downstream effector caspase-3 and -7. Type II cells are characterized by lower levels of CD95 DISC formation and, thus, lower levels of active caspase-8.11 In this case, signaling requires an additional amplification loop that involves the cleavage by caspase-8 of the Bcl-2-family protein Bid to generate truncated (t) Bid and subsequent tBid-mediated release of Cytochrome C from mitochondria. The release of Cytochrome C from mitochondria results in apoptosome formation, followed by activation of procaspase-9, which in turn cleaves downstream, effector caspases.12
Caspase-2 is referred to as an initiator caspase.13-15 It is characterized by the presence of a caspase activation recruitment domain (CARD) and is structurally related to the initiator caspase-9.16,17 The mechanism of procaspase-2 activation in apoptosis in contrast to other initiator caspases remains poorly defined. It was reported that caspase-2 is implicated in Cytochrome C release and is essential for drug-induced apoptosis in several human cell lines.18-20 On the basis of these data caspase-2 was proposed to be the initiator caspase in cytotoxic stress-induced apoptosis acting upstream of mitochondria. At the same time, others suggested that activation of caspase-2 occurs downstream of mitochondria.21 In addition, caspase-2 was reported to be a proximal mediator in the heat-shock–induced apoptosis.22
The role of caspase-2 in death receptor signaling is also contradictory. Caspase-2 was shown to be processed in the course of death receptor–mediated apoptosis.23,24 It was reported that this cleavage is mediated by caspase-3 and, accordingly, processing of caspase-2 takes place after activation of caspase-3. In contradiction to these data, caspase-2 was shown to be necessary for optimal TRAIL-mediated cleavage of Bid.25 Down-regulation of caspase-2 using RNA interference sufficiently inhibited TRAIL-induced apoptosis in type II cells. Moreover, it was demonstrated that in caspase-2–knockdown cell lines the sensitivity toward CD95-mediated apoptosis is decreased.26 In the latter work, it was suggested that caspase-2 influences activation of procaspase-8 at the DISC. However, the investigators failed to detect an association of caspase-2 with the DISC or with FADD and procaspase-8.
To clarify the contradictions in the literature, we analyzed the role of procaspase-2 in CD95-mediated apoptosis. We show that procaspase-2 is found at the CD95 DISC and is activated on CD95 stimulation in a number of human T- and B-cell lines. At the same time we demonstrate that procaspase-2 in the absence of procaspase-8 could not initiate cell death. Thus, we have shown that caspase-2 is activated at the DISC but does not prime CD95-induced apoptosis.
Materials and methods
Cell lines
The T-cell lines HUT78, CEM, Jurkat (caspase-8 deficient, clone I9.2), Jurkat clone A3, and the B-lymphoblastoid cell lines SKW6.4, Raji, BJAB were maintained in RPMI 1640 (Life Technologies, Germany), 10 mM HEPES (Life Technologies, Germany), 50 μg/mL gentamicin (Life Technologies, Karlsruhe, Germany), 10% fetal calf serum (Life Technologies) in 5% CO2. The caspase-8–deficient Jurkat cell line (clone I9.2) and Jurkat clone A3 were obtained from J. Blenis (Harvard Medical School).
Antibodies and reagents
Anti–caspase-2 monoclonal antibody (clone 35; mouse IgG1), anti-FADD monoclonal antibody (mouse IgG1) were purchased from Transduction Laboratories (Lexington, KY). Anti-CD95, anti-RAIDD polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Bid polyclonal antibody was from Biosource International (Nivelles, Belgium). The anti–caspase-8 mAb C15 (mouse IgG2b) recognizes the p18 subunit of caspase-8. Anti–APO-1 is an agonistic monoclonal antibody (IgG3, κ) recognizing an epitope on the extracellular part of CD95 (APO-1/Fas).7 The horseradish peroxidase–conjugated goat anti–mouse IgG1, IgG2a, and IgG2b were from Southern Biotechnology Associates (Manchester, United Kingdom). Leucine zipper (LZ)–CD95L was produced as described.27 ZVAD-fmk was purchased from Merck (Darmstadt, Germany). ZIETD-afc and zVDVAD-afc were from Molecular Probes (Leiden, The Netherlands). Caspase-2 siRNA and caspase-8 siRNA (SI00038542 and SI00299593, respectively) were purchased from Qiagen (Hilden, Germany). HiPerFect Transfection Reagent was purchased from Qiagen. All other chemicals used were of analytical grade and purchased from Merck or Sigma (Taufkirchen, Germany).
Flow cytometry analysis
The percentage of viable cells was determined by forward light scatter/sideward light scatter (FSC/SSC) using a fluorescence-activated cell scan (FACScan) Cytometer (BD, Heidelberg, Germany). A minimum of 10 000 cells per sample was analyzed. Specific cell death was calculated as follows: (percentage of experimental cell death – percentage of spontaneous cell death) / (100 – percentage of spontaneous cell death) × 100.
Immunoblotting
For immunoblotting analysis the cells were lysed in buffer A (20 mM Tris/HCl, pH 7.4, 1% Triton X-100, 10% glycerol, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [Sigma], protease inhibitor cocktail [Roche, Basel, Switzerland] for 15 minutes on ice and centrifuged [15 minutes at 14 000g]). Postnuclear supernatant equivalents of 0.5 × 106 cells or 25 μg protein as determined by the bicinchoninic acid (BCA) method (Pierce, Rockford, IL) were separated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto a nitrocellulose membrane (Amersham, Uppsala, Sweden).
Caspase activity assays
Cytosolic lysates were incubated with 50 μM site-specific tetrapeptide substrates (zIETD-afc for caspase-8, zVDVAD-afc for caspase-2) in a caspase assay buffer B (50 mM HEPES, 100 mM NaCl, 10 mM dithiothreitol, 0.1% [wt/vol] CHAPS, 10% [wt/vol] sucrose, pH 7.4) in a final volume of 200 μL. The release of the fluorogenic group AFC was determined after 1 hour of incubation at 37°C by a microplate fluorescence reader Wallach 1420 (Perkin Elmer, Langen, Germany) at the excitation wavelength of 405 nm and emission wavelength of 535 nm.
DISC analysis by immunoprecipitation and immunoblotting
The composition of CD95 DISC was determined as follows: 1 × 108 cells were either treated with 10 μg LZ-CD95L–containing supernatant or 1 μg/mL anti–APO-1 (IgG3) for 5 minutes (unless otherwise indicated) at 37°C, washed twice in 1 × PBS, and subsequently lysed in buffer A (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail [Roche], 1% Triton X-100 [Serva, Heidelberg, Germany], and 10% glycerol) (stimulated condition) or lysed without treatment (unstimulated condition). CD95 DISC was immunoprecipitated overnight with 2 μg anti–APO-1 and 30 μL Protein-A Sepharose. Beads were washed 5 times with 20 volumes of lysis buffer. The immunoprecipitates were analyzed on 12% PAAG. Subsequently, the gels were transferred to Hybond nitrocellulose membrane (Amersham Pharmacia Biotech, Freiburg, Germany), blocked with 5% nonfat dry milk in PBS/Tween (PBS plus 0.05% Tween 20) for 1 hour, washed with PBS/Tween, and incubated with the primary antibody in PBS/Tween at 4°C overnight. Blots were developed with a chemoluminescence method following the manufacturer's protocol (PerkinElmer Life Sciences, Rodgan, Germany).
Caspase-2 immunoprecipitation
The Caspase-2 immunoprecipitation was performed as described for DISC immunoprecipitation. Briefly, 1 × 108 cells were treated with 10 μg LZ-CD95L–containing supernatant for 10 minutes at 37°C, washed twice in 1 × PBS, and subsequently lysed in buffer A (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail [Roche], 1% Triton X-100 [Serva], and 10% glycerol). Caspase-2 was immunoprecipitated overnight with 5 μg anti–caspase-2 antibodies and 30 μL Protein-A Sepharose.
Caspase inhibition experiments
ZVAD-fmk (pan-specific caspase inhibitor) was added to the cells 30 minutes before the addition of anti–APO-1 or LZ-CD95L.
siRNA experiments
Transfections of siRNA were performed according to the manufacturer's protocol using HiPerFect Transfection Reagent (Qiagen).
Results
Procaspase-2 is processed during CD95-mediated apoptosis
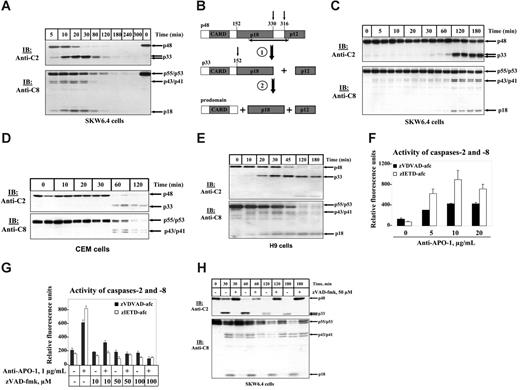
Procaspase-2 was reported to be processed in the course of CD95-mediated apoptosis.23,24 To further investigate these findings, B-lymphoblastoid SKW6.4 cells were stimulated with 5 μg/mL agonistic anti-CD95 (anti–APO-1) antibody for various time points. CD95-mediated processing of procaspase-2 was analyzed by Western blotting using anti–caspase-2 mAbs (Figure 1A-B). Cleavage of procaspase-2 to the p33 subunit was already observed 5 minutes after stimulation similar to caspase-8 processing (Figure 1A). Furthermore, maximal cleavage of both caspases appeared at 20 minutes after triggering of CD95. To analyze caspase processing at a slower kinetics we stimulated SKW6.4 cells with 200 ng/mL anti–APO-1 (Figure 1C). In this case, a significant increase in caspase processing could only be detected after 2 hours. In summary, we observed that the cleavage kinetics of caspase-2 and -8 were identical on strong and weak CD95 stimulation.
To confirm this observation in another cell line we analyzed the CD95-mediated processing of caspase-2 and -8 in CEM cells. CEM cells are referred to as type II cells that are characterized by lower levels of DISC formation and, subsequently, by different kinetics of cell death. Indeed, we observed a slower activation of caspases on stimulation with 5 μg/mL anti–APO-1 (Figure 1D). The first cleavage products of both caspases appeared only after 1 hour. Again the processing of both caspases took place simultaneously. We also observed parallel activation of caspase-8 and -2 in other cell lines: in H9 (type I cells) (Figure 1E), HUT78 (type I cells) (data not shown), and in Jurkat A3 (type II cells) (data not shown).
Processing of procaspases-2 and -8 in total cellular lysates on CD95 stimulation. (A) B-lymphoblastoid SKW6.4 cells were treated with 5 μg/mL agonistic anti–APO-1 antibody for indicated periods of time. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). (B) Two cleavage steps of procaspase-2 during apoptosis. At the first cleavage step p33 and small catalytical subunit (p12) are formed. As a result of the second cleavage step the CARD-containing prodomain is generated along with the large catalytical subunit. The epitope of anti–caspase-2 mAbs is shown by black arrow. (C) B-lymphoblastoid SKW6.4 cells were treated with 200 ng/mL agonistic anti–APO-1 antibody for indicated periods of time. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). (D) CEM cells were treated with 5 μg/mL agonistic anti-CD95 (anti–APO-1) antibody for indicated time points. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). (E) H9 cells were treated with 1 μg/mL agonistic anti-CD95 (anti–APO-1) antibody for indicated time points. (F) SKW6.4 cells were treated with 5, 10, and 20 μg/mL agonistic anti–APO-1 antibody for 1 hour. The activities of caspase-2 and -8 in cellular lysates were measured using zVDVAD-afc and zIETD-afc, correspondingly. (G) SKW6.4 cells were pretreated with indicated concentrations of zVAD-fmk for 30 minutes. Subsequently, SKW6.4 cells were stimulated with 1 μg/mL agonistic anti–APO-1 antibody for 1 hour. The activity of caspase-2 and -8 in cellular lysates was measured using zVDVAD-afc and zIETD-afc, respectively. (F-G) Error bars indicate SD. (H) SKW6.4 cells were treated with 1 μg/mL LZ-CD95L for indicated time points. ZVAD-fmk was used, when indicated, in a concentration of 50 μM. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8).
Processing of procaspases-2 and -8 in total cellular lysates on CD95 stimulation. (A) B-lymphoblastoid SKW6.4 cells were treated with 5 μg/mL agonistic anti–APO-1 antibody for indicated periods of time. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). (B) Two cleavage steps of procaspase-2 during apoptosis. At the first cleavage step p33 and small catalytical subunit (p12) are formed. As a result of the second cleavage step the CARD-containing prodomain is generated along with the large catalytical subunit. The epitope of anti–caspase-2 mAbs is shown by black arrow. (C) B-lymphoblastoid SKW6.4 cells were treated with 200 ng/mL agonistic anti–APO-1 antibody for indicated periods of time. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). (D) CEM cells were treated with 5 μg/mL agonistic anti-CD95 (anti–APO-1) antibody for indicated time points. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). (E) H9 cells were treated with 1 μg/mL agonistic anti-CD95 (anti–APO-1) antibody for indicated time points. (F) SKW6.4 cells were treated with 5, 10, and 20 μg/mL agonistic anti–APO-1 antibody for 1 hour. The activities of caspase-2 and -8 in cellular lysates were measured using zVDVAD-afc and zIETD-afc, correspondingly. (G) SKW6.4 cells were pretreated with indicated concentrations of zVAD-fmk for 30 minutes. Subsequently, SKW6.4 cells were stimulated with 1 μg/mL agonistic anti–APO-1 antibody for 1 hour. The activity of caspase-2 and -8 in cellular lysates was measured using zVDVAD-afc and zIETD-afc, respectively. (F-G) Error bars indicate SD. (H) SKW6.4 cells were treated with 1 μg/mL LZ-CD95L for indicated time points. ZVAD-fmk was used, when indicated, in a concentration of 50 μM. The cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8).
To verify that procaspase-2 is not only cleaved in the course of CD95-mediated apoptosis but also undergoes proteolytic activation we implemented fluorometric caspase activity assays with a fluorescent substrate of caspase-2, zVDVAD-afc. After treatment of SKW6.4 cells with different concentrations of anti–APO-1 antibody we observed an increase in fluorescence on activation that clearly indicated activation of caspase-2 (Figure 1F). Activation of caspase-2 was paralleled by activation of caspase-8 measured using zIETD-afc as a substrate (Figure 1F). Catalytic activation of both caspases, caspase-2 and -8, was inhibited by addition of the pancaspase inhibitor zVAD-fmk (Figure 1G).
To ensure that simultaneous activation of both caspases takes place also with a different trigger of CD95 we stimulated SKW6.4 cells with LZ-CD95L (Figure 1H). Again, we observed simultaneous appearance of caspase-2 and -8 cleavage products, and their processing was inhibited by zVAD-fmk.
Taken together, we demonstrate that kinetics of caspase-2 and caspase-8 activation is identical and that caspase-2 activation occurs at the early stages of CD95-mediated apoptosis.
Caspase-2 is recruited to the DISC
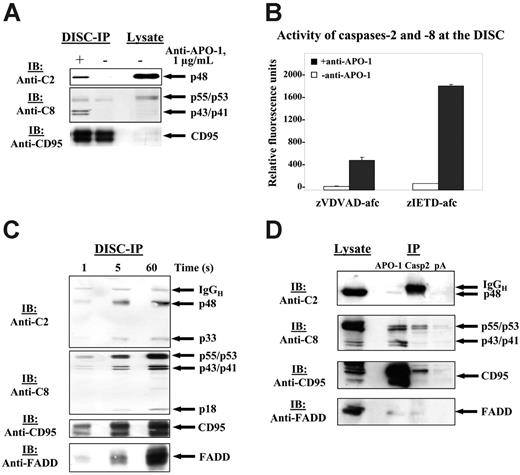
To determine at which step in CD95 signaling activation of procaspase-2 takes place we analyzed CD95 signaling starting from CD95 DISC formation. HUT78 cells were stimulated with agonistic anti–APO-1 antibody. The CD95 DISC was immunoprecipitated with anti–APO-1 antibody and analyzed by immunoblotting. In contrast to previous reports, procaspase-2 was detected at the CD95 DISC (Figure 2A).26,28 We probed the immunoprecipitated complex for the presence of other CD95 DISC components. Immunoblotting analysis revealed the presence of CD95 and procaspase-8/a and -8/b along with their cleavage products p43/p41 (Figure 2A).
To analyze whether procaspase-2 is catalytically activated at the DISC we tested an in vitro–isolated DISC for specific caspase-2 catalytic activity. CD95 receptor was immunoprecipitated with anti–APO-1 antibody and Protein-A beads both from CD95-stimulated as well as from unstimulated SKW6.4 cells. Subsequently, the beads, containing the CD95 DISC, were incubated with zVDVAD-afc, a fluorescent substrate of caspase-2 (Figure 2B). We detected an increase in fluorescence on stimulation, which indicates catalytic activity of caspase-2 at the CD95 DISC. As a positive control for this assay we measured caspase-8 activity of the same CD95 DISC using zIETD-afc, a fluorescent substrate of caspase-8. Activity of caspase-8 was observed only in the case of stimulated CD95, which demonstrates the specificity of immunoprecipitation. Thus, we have shown that caspase-2 is not only recruited to the CD95 DISC but also catalytically activated at this complex.
Procaspase-2 is recruited to the CD95 DISC and is catalytically activated at the DISC. (A) HUT78 cells were either stimulated with 1 μg/mL agonistic anti–APO-1 antibody for 5 minutes (+) or left untreated (–). CD95 was immunoprecipitated from 3 × 107 HUT78 cells using anti–APO-1 antibody and Protein-A Sepharose. Immunoprecipitates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, anti-CD95 polyclonal antibodies (C20). Total cellular lysates from 106 cells were loaded on the same gel. (B) SKW6.4 cells were stimulated with 1 μg/mL anti–APO-1 antibody for 5 minutes (+) or left untreated (–). CD95 was immunoprecipitated from 5 × 107 SKW6.4 cells using anti–APO-1 antibody and Protein-A Sepharose. Subsequently, washed Protein-A beads were split into 2 halves and incubated for 60 minutes together with zVDVAD-afc or zIETD-afc. Error bars indicate SD. (C) HUT78 cells were stimulated with 1 μg/mL anti–APO-1 antibody for 1, 5, and 60 seconds. Subsequently, the cells were lysed in Triton X-100 buffer, and DISCs were immunoprecipitated with Protein-A Sepharose (30 μL for each sample) without the addition of anti–APO-1. Immunoprecipitates were analyzed using 15% PAAG and subsequent immunoblotting with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, anti-FADD mAbs, and anti-CD95 polyclonal antibodies C20. (D) SKW6.4 cells were stimulated with LZ-CD95L for 15 minutes, then the cells were lysed. The lysates were divided into 3 equal parts, and immunoprecipitations were performed using anti–APO-1, anti–caspase-2 antibodies, and Protein-A beads as negative control. Lysates from the same experiments were used as a positive control. Immunoprecipitates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, and anti-CD95 polyclonal antibodies (C20).
Procaspase-2 is recruited to the CD95 DISC and is catalytically activated at the DISC. (A) HUT78 cells were either stimulated with 1 μg/mL agonistic anti–APO-1 antibody for 5 minutes (+) or left untreated (–). CD95 was immunoprecipitated from 3 × 107 HUT78 cells using anti–APO-1 antibody and Protein-A Sepharose. Immunoprecipitates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, anti-CD95 polyclonal antibodies (C20). Total cellular lysates from 106 cells were loaded on the same gel. (B) SKW6.4 cells were stimulated with 1 μg/mL anti–APO-1 antibody for 5 minutes (+) or left untreated (–). CD95 was immunoprecipitated from 5 × 107 SKW6.4 cells using anti–APO-1 antibody and Protein-A Sepharose. Subsequently, washed Protein-A beads were split into 2 halves and incubated for 60 minutes together with zVDVAD-afc or zIETD-afc. Error bars indicate SD. (C) HUT78 cells were stimulated with 1 μg/mL anti–APO-1 antibody for 1, 5, and 60 seconds. Subsequently, the cells were lysed in Triton X-100 buffer, and DISCs were immunoprecipitated with Protein-A Sepharose (30 μL for each sample) without the addition of anti–APO-1. Immunoprecipitates were analyzed using 15% PAAG and subsequent immunoblotting with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, anti-FADD mAbs, and anti-CD95 polyclonal antibodies C20. (D) SKW6.4 cells were stimulated with LZ-CD95L for 15 minutes, then the cells were lysed. The lysates were divided into 3 equal parts, and immunoprecipitations were performed using anti–APO-1, anti–caspase-2 antibodies, and Protein-A beads as negative control. Lysates from the same experiments were used as a positive control. Immunoprecipitates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, and anti-CD95 polyclonal antibodies (C20).
To examine the kinetics of procaspase-2 recruitment to the DISC we stimulated HUT78 cells with anti–APO-1 antibodies for 1, 5, and 60 seconds. Subsequently, Protein-A Sepharose beads were added to total cellular lysates, and the formed DISCs were immunoprecipitated. Immunoblotting analysis indicates that procaspase-2 is recruited to the DISC within seconds after stimulation and undergoes processing immediately (Figure 2C). Furthermore, we observed that DISC molecules, FADD, procaspase-8, and procaspase-2, are recruited to CD95 with similar kinetics. As soon as they are recruited to the DISC procaspase-2 and -8 undergo autocatalytic processing. Interestingly, we observe simultaneous cleavage of both procaspases, procaspase-2 and -8, at the DISC. This observation is consistent with our previous findings of similarity in kinetics of procaspase-2 and -8 in total cellular lysates.
To confirm that caspase-2 is a component of the CD95 DISC we stimulated SKW6.4 cells with LZ-CD95L, which was followed by immunoprecipitation using anti–caspase-2 antibody (Figure 2D). Indeed, we could coimmunoprecipitate the main components of CD95 DISC. However, it should be noted that the amounts of CD95, procaspase-8, and FADD were lower in the caspase-2 immunoprecipitation than in the anti–APO-1 immunoprecipitation, apparently because of lower immunoprecipitating efficiency of anti–caspase-2 antibody used in this experiment.
Taken together, these data indicate that caspase-2 is recruited to the DISC, which is followed by caspase-2 activation at the DISC.
Procaspase-2 is present at the DISC of several cell lines
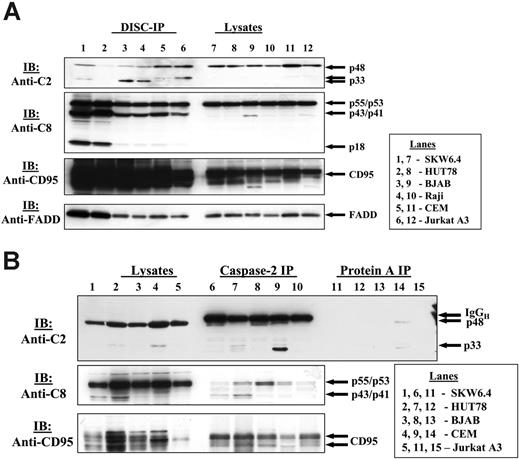
Following this finding, we analyzed CD95 DISCs, immunoprecipitated from different B- and T-cell lines. We could detect caspase-2 in CD95 DISCs of various cell lines (Figure 3A). Caspase-2 was detected in the CD95 DISC of type I cells (SKW6.4, HUT78, Raji, BJAB) as well as in the DISC of type II cells (CEM, Jurkat A3). Not only the full-length form of procaspase-2 was detected at the DISC, but also the p33 subunit that results from the first cleavage step of procaspase-2 (Figure 1B).23 Interestingly, the amount of caspase-2, detected at the DISC in HUT78 cells, was lower compared with the one in SKW6.4 cells despite similar recruitment of procaspase-8. However, we observed a slightly enhanced recruitment of procaspase-2 to the DISCs from Jurkat A3 and CEM cells referred to as type II cells.
The specificity of CD95 DISC formation was controlled, probing for other components of the DISC with specific antibodies against CD95, FADD, and procaspase-8. Immunoblotting revealed the presence of CD95 as well as FADD, and products of procaspase-8/a and -8/b processing (p55/p53, p43/p41, and p18). Notably, the amount of procaspase-2 that was recruited to the DISC from the lysates was always lower than the corresponding amounts of FADD and procaspase-8 (Figure 3A; data not shown). Interestingly, in unstimulated cells we could not detect an association of procaspase-2 with individual DISC components, c-FLIP, and procaspase-8, when performing immunoprecipitations using anti-FLIP mAb NF6 and anti–caspase-8 mAb N1 (data not shown).
Procaspase-2 is present at the CD95 DISC of different human cell lines. (A) SKW6.4, HUT78, BJAB, Raji, CEM, Jurkat A3 cells were stimulated with LZ-CD95L. CD95 was immunoprecipitated from 3 × 107 cells using anti–APO-1 antibody and Protein-A Sepharose. Immunoprecipitates along with total cellular lysates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, anti-FADD mAbs, and anti-CD95 polyclonal antibodies C20. (B) SKW6.4, HUT78, BJAB, CEM, and Jurkat A3 cells were stimulated with LZ-CD95L, which was followed by caspase-2 immunoprecipitation.
Procaspase-2 is present at the CD95 DISC of different human cell lines. (A) SKW6.4, HUT78, BJAB, Raji, CEM, Jurkat A3 cells were stimulated with LZ-CD95L. CD95 was immunoprecipitated from 3 × 107 cells using anti–APO-1 antibody and Protein-A Sepharose. Immunoprecipitates along with total cellular lysates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs, anti–caspase-8 mAbs C15, anti-FADD mAbs, and anti-CD95 polyclonal antibodies C20. (B) SKW6.4, HUT78, BJAB, CEM, and Jurkat A3 cells were stimulated with LZ-CD95L, which was followed by caspase-2 immunoprecipitation.
To further verify that caspase-2 is a component of the CD95 DISC complex in several human T- and B-cell lines we stimulated SKW6.4, HUT78, BJAB, CEM, and Jurkat A3 cells with LZ-CD95L, which was followed by immunoprecipitation using an anti–caspase-2 antibody. Indeed, we could coimmunoprecipitate components of the CD95 DISC: CD95 and procaspase-8 (Figure 3B).
RAIDD has been reported to interact with caspase-2 by homotypic contacts of the respective CARD domains.29,30 Because RAIDD also possesses a DD it seems likely to be the adaptor molecule for procaspase-2 at the DISC. To check this hypothesis we analyzed CD95 DISCs that were immunoprecipitated from SKW6.4, HUT78, CEM cells for the presence of RAIDD. However, RAIDD was not observed at the DISC despite its presence in total cell lysates (data not shown). Thus, we demonstrate that procaspase-2 is found at the CD95 DISC of several human T- and B-cell lines, and we did not find RAIDD as an adaptor of caspase-2 at the DISC.
Procaspase-2 does not initiate apoptosis in the absence of caspase-8
Initiator caspases undergo autocatalytic activation when they are brought into close proximity. Following activation, initiator caspases start the apoptotic caspase cascade. To address the question whether procaspase-2 can initiate CD95-mediated apoptosis in the absence of caspase-8, we examined the caspase-8–deficient cell line Jurkat A3 (Figure 4A). Consistent with published data the treatment of the caspase-8–deficient cells with LZ-CD95L did not result in apoptosis up to 24 hours (Figure 4A).24 As control the cells from the parental Jurkat cell line A3 were treated with the same amount of LZ-CD95L, which efficiently caused apoptosis (Figure 4A). Similarly, no apoptosis was observed in caspase-8–deficient cells on stimulation with anti–APO-1 (Figure 4B). SKW6.4 cells that were used as a positive control exhibit high sensitivity on stimulation with the same amount of anti–APO-1 antibody.
Procaspase-2 does not initiate apoptosis in the absence of procaspase-8. (A) Caspase-8–deficient Jurkat A3 cells and parental Jurkat A3 cells were stimulated with LZ-CD95L for indicated periods of time. Cell death was measured by FACS using FSC/SCC. (B) Caspase-8–deficient Jurkat A3 cells and SKW6.4 cells were stimulated with indicated concentrations of anti–APO-1 for 24 hours. Cell death was measured by FSC/SCC. (A-B) Error bars indicate SD. (C) Caspase-8–deficient Jurkat A3 cells and parental Jurkat A3 cells were stimulated with LZ-CD95L for indicated periods of time. The total cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). Unspecific bands, recognized by the anti–caspase-8 antibody in caspase-8–deficient Jurkat cells, are marked by stars. (D) Caspase-8–deficient and parental Jurkat A3 cells were stimulated with LZ-CD95L for indicated periods of time. CD95 was immunoprecipitated from 5 × 107 cells using anti–APO-1 antibody with Protein-A Sepharose. Immunoprecipitates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs and anti–caspase-8 mAbs C15. (E) Caspase-8–deficient Jurkat A3 cells were stimulated with LZ-CD95L for 1, 3, 4, and 6 hours. CD95 DISCs were immunoprecipitated from 3-, 4-, and 6-hour time points. Immunoprecipitates along with total cellular lysates were analyzed using immunoblotting with anti–caspase-2 mAbs and anti-FADD mAbs. (F) SKW6.4 and CEM cells were silenced using caspase-2 siRNA. Processing of procaspase-8 in these cells was analyzed after stimulation with 1 μg/mL anti–APO-1 for 1 hour. (G) SKW6.4 and CEM cells were silenced using caspase-8 siRNA. Processing of procaspase-2 as well as PARP cleavage were analyzed after stimulation with 1 μg/mL anti–APO-1 for 1 hour.
Procaspase-2 does not initiate apoptosis in the absence of procaspase-8. (A) Caspase-8–deficient Jurkat A3 cells and parental Jurkat A3 cells were stimulated with LZ-CD95L for indicated periods of time. Cell death was measured by FACS using FSC/SCC. (B) Caspase-8–deficient Jurkat A3 cells and SKW6.4 cells were stimulated with indicated concentrations of anti–APO-1 for 24 hours. Cell death was measured by FSC/SCC. (A-B) Error bars indicate SD. (C) Caspase-8–deficient Jurkat A3 cells and parental Jurkat A3 cells were stimulated with LZ-CD95L for indicated periods of time. The total cellular lysates were analyzed by immunoblotting using anti–caspase-2 mAbs (anti-C2) and anti–caspase-8 mAbs (anti-C8). Unspecific bands, recognized by the anti–caspase-8 antibody in caspase-8–deficient Jurkat cells, are marked by stars. (D) Caspase-8–deficient and parental Jurkat A3 cells were stimulated with LZ-CD95L for indicated periods of time. CD95 was immunoprecipitated from 5 × 107 cells using anti–APO-1 antibody with Protein-A Sepharose. Immunoprecipitates were subjected to 12% PAAG gels and immunoblotted with anti–caspase-2 mAbs and anti–caspase-8 mAbs C15. (E) Caspase-8–deficient Jurkat A3 cells were stimulated with LZ-CD95L for 1, 3, 4, and 6 hours. CD95 DISCs were immunoprecipitated from 3-, 4-, and 6-hour time points. Immunoprecipitates along with total cellular lysates were analyzed using immunoblotting with anti–caspase-2 mAbs and anti-FADD mAbs. (F) SKW6.4 and CEM cells were silenced using caspase-2 siRNA. Processing of procaspase-8 in these cells was analyzed after stimulation with 1 μg/mL anti–APO-1 for 1 hour. (G) SKW6.4 and CEM cells were silenced using caspase-8 siRNA. Processing of procaspase-2 as well as PARP cleavage were analyzed after stimulation with 1 μg/mL anti–APO-1 for 1 hour.
Following this assay, we characterized the CD95-mediated status of procaspase-2 in caspase-8–deficient cells. Procaspase-2 was present in caspase-8–deficient cells according to the immunoblotting analysis (Figure 4C). However, we did not monitor procaspase-2 processing on stimulation with LZ-CD95L up to 2 hours (Figure 4C). In the parental Jurkat A3 cells, both procaspase-8 and -2 were cleaved on addition of LZ-CD95L (Figure 4C).
Furthermore, immunoprecipitation experiments that were done in parallel from the same total cellular lysates stimulated with LZ-CD95L have shown that procaspase-2 is recruited to the CD95 DISC of caspase-8–deficient cells (Figure 4D). Procaspase-8 was indeed absent in this cell line, whereas it was recruited and processed at the DISC in the parental cell line (Figure 4D). Importantly, we did not observe processing of procaspase-2 at the DISC, neither on short (Figure 4D) nor on longer stimulation times up to 6 hours (Figure 4E). Thus, we have found procaspase-2 at the CD95 DISC of caspase-8–deficient cells. Caspase-8–deficient Jurkat A3 cells were resistant toward CD95-mediated apoptosis, and the resistance was accompanied by the absence of procaspase-2 processing. This finding indicates that procaspase-2 is not an initiator caspase in CD95-induced apoptosis.
To rule out that this effect is cell-type specific we used siRNA for caspase-8 and caspase-2 in several type I and type II cell lines. Caspase-2 silencing did not influence caspase-8 activation in either type I or type II cell lines (Figure 4F). Interestingly, caspase-2 was not activated on CD95-stimulation in caspase-8–/– cell lines (Figure 4G). Not only was caspase-2 not activated in caspase-8–/– cell lines, but PARP cleavage also did not take place (Figure 4G) nor did caspase-3 and Bid cleavage (data not shown), indicating defects in CD95-induced apoptosis. Taken together, these data show that caspase-8 is the initiator caspase in the CD95-induced apoptosis and not caspase-2.
Discussion
The role of procaspase-2 in apoptosis is contradictory. Structurally it belongs to the family of initiator caspases that undergo activation when being brought into close proximity and, subsequently, start the apoptotic process.13 However, the absence of a severe phenotype in caspase-2–deficient mice has complicated the assignment of its role in apoptosis.31 Nevertheless, in several studies procaspase-2 has been reported to function as the initiator caspase in apoptosis induced by genotoxic stress.18-20 Recently, there have been reports on caspase-2 in death receptor-mediated apoptosis, which indicate that its function is more intriguing and beyond the function of one of the downstream caspases. It has been demonstrated that procaspase-2 must be activated shortly after receptor stimulation upstream of Bid cleavage and that down-regulation of caspase-2 results in a decrease of sensitivity toward death receptor-mediated apoptosis.25,26 In this study, we address the question of the mechanism of procaspase-2 activation in CD95-mediated apoptosis. We have found the activating complex for procaspase-2, and we show that this complex is indeed the CD95 DISC. Moreover, we have demonstrated that procaspase-2 contributes to the amplification of the death signal through Bid and the mitochondrial pathway.
Notably, other groups did not detect procaspase-2 in CD95 DISC immunoprecipitations under native conditions, using different antibodies and different time points for the analysis of the formation of the CD95 DISC.26,28 As procaspase-2 is recruited to the CD95 DISC in lower amounts than procaspase-8 and FADD (Figure 3A), it seems likely that more sensitive immunoprecipitation methods are required using anti–APO-1 antibodies.
Interactions between the molecules at the DISC are based on the contacts between homotypic domains. FADD is recruited to the DISC by DD interactions, and procaspase-8 and -10 are recruited to the DISC by DED interactions.5 Procaspase-2 possesses a CARD domain that is well described as an important apoptotic domain that comprises 6 antiparallel α-helices in different orientation as compared with DD and DED.32-34 The question arises what the putative adaptor molecule could be that can dock procaspase-2 to CD95 DISC. A likely candidate for the adaptor molecule is RAIDD, one of the few molecules that contains both a DD and a CARD.30 Furthermore, RAIDD has been reported to interact with procaspase-2 in the recently reported PIDDosome.29 However, in our study we failed to detect the RAIDD at the DISC. Therefore, it is likely that there is another yet unidentified molecule, which contains both CARD and DD domains, that docks caspase-2 to the DISC.
Another possibility is that procaspase-2 is recruited to the DISC by heterotypic interactions between CARD and DD. Heterotypic contacts have been recently reported for the interactions of CARD of the newly identified molecule ARC with the DD of CD95.35 Only a few reports are available on heterotypic interactions so far; however, these interactions are consistent with the recent structural analysis showing similarities between binding surfaces of a DD and a DED from 2 functionally nonrelated proteins such as the Drosophilia Tube and the hamster PED.36 The presence of heterotypic interactions at the DISC involving CARD of caspase-2 and DD of CD95 would be consistent with our findings.
Procaspase-2 molecules have been reported to be activated by being brought into close proximity of each other through dimer formation.16 Thus, an intriguing question arises whether procaspase-2 alone can initiate CD95-mediated apoptosis. To address this question, we have examined caspase-8–deficient Jurkat A3 cells as well as caspase-8– and caspase-2–deficient cell lines obtained by using siRNA. We have found that in these cells procaspase-2 does not initiate cell death, even though it is present at the CD95 DISC. Furthermore, caspase-8 activation occurs in caspase-2–independent way. These findings contradict to recent reports showing that caspase-2 activation precedes caspase-8 activation in TNF-induced apoptosis37 and that caspase-2 is essential for caspase-8 activation in TRAIL-induced apoptosis.38 These differences might be explained by differences between CD95 and other death receptor signaling as well by other cell types applied in the above-mentioned studies.
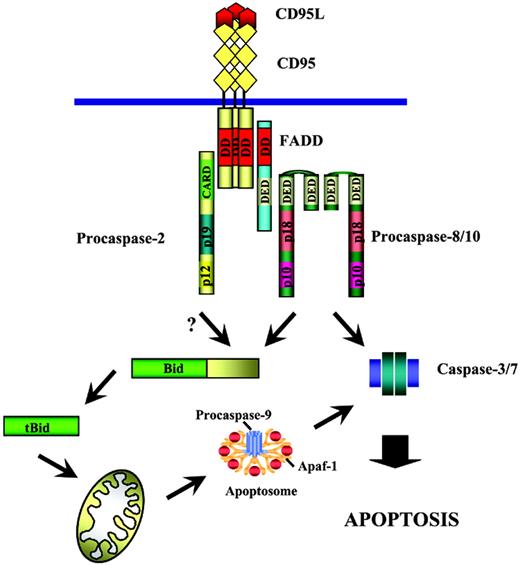
After activation at the DISC, caspase-2 likely contributes to further apoptotic cascade by processing its substrates. The Bid molecule was found to be the substrate for caspase-2 in vitro.18,39 It was also reported that in caspase-2–deficient cell lines TRAIL-mediated cleavage of Bid is impaired.25 Thus, it seems likely that after activation at the DISC caspase-2 along with caspase-8 contributes to the Bid cleavage (Figure 5), which leads to the amplification of the downstream apoptotic cascade. This “amplification” function of caspase-2 might be crucial in some types of cells in which the concentration of active caspase-8 is not high enough to trigger apoptosis. Then participation of caspase-2 would be indispensable.
Procaspase-2 in the CD95 signaling. Procaspase-2 is recruited to the DISC, which is followed by its activation at the DISC. Following activation at the DISC caspase-2 likely cleaves Bid, followed by translocation of tBid to mitochondria, with Cytochrome C release from mitochondria triggering the following apoptotic cascade.
Procaspase-2 in the CD95 signaling. Procaspase-2 is recruited to the DISC, which is followed by its activation at the DISC. Following activation at the DISC caspase-2 likely cleaves Bid, followed by translocation of tBid to mitochondria, with Cytochrome C release from mitochondria triggering the following apoptotic cascade.
Other large protein complexes, which involve procaspase-2, have been reported. Procaspase-2 on overexpression has been shown to associate with RAIDD in the TNFR1 complex.30,40 However, in a recent analysis of the endogenous TNFR1 signaling complex neither procaspase-2 nor RAIDD have been found.28 Recently, an interesting cytosolic complex of caspase-2 has been described that comprises the adaptor molecule RAIDD and the p53-induced death domain-containing protein (PIDD).29 This complex has been suggested to function under genotoxic stress. In addition, procaspase-2 was shown to be recruited to the TRAF1, TRAF2, and RIP-containing protein complex that activates NF-κB through the CARD domain of caspase-2.41 Moreover, there is increasing evidence that triggering of CD95 does not only result in death signals but also in NF-κB activation.42 It is possible that procaspase-2 is a missing link between the CD95 DISC and CD95-mediated NF-κB activation. This question should be further addressed in future studies.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-07-007096.
Supported by grants from the Deutsche Krebshilfe, the European Community, and the Wilhelm Sander Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank B. Zhivotovsky, E. Suri-Payer, R. Arnold, K. Gülow, and D. Brenner for discussion and critically reading the manuscript and Heidi Sauter for expert secretarial assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal