Toll-like receptors (TLRs) are able to interact with pathogen-derived products and their signals induce the coordinated activation of innate and adaptive immune mechanisms. Dendritic cells (DCs) play a central role in these events. As the different TLRs are able to trigger MyD88/TRIF-dependent and -independent signaling pathways, we wondered if the simultaneous activation of these signaling cascades would synergize with respect to DC activation and induce superior cytotoxic T-lymphocyte (CTL) activity in vivo. We observed that indeed the combined activation of MyD88-dependent and -independent signaling induced by TLR7 and TLR3 ligands provoked a more rapid and more sustained bone marrow–derived DC (BMDC) activation with regard to the secretion of proinflammatory cytokines, like IL-6 and IL-12p70, and the expression of costimulatory molecules like CD40, CD70, and CD86. Furthermore, in the presence of combined TLR ligand–stimulated DCs, CD4+ and CD8+ T cells were insensitive toward the inhibitory effects of regulatory T cells. Most importantly, peptide-loaded BMDCs stimulated by TLR ligand combinations resulted in a marked increase of CTL effector functions in wild-type mice in vivo. Thus, our results provide evidence that unlocking the full potential of DCs by advanced activation protocols will boost their immunogenic potential and improve DC-based vaccination strategies.
Introduction
Cytotoxic T lymphocytes (CTLs) play a major role in rejecting malignant or virus-infected cells1,2 by recognizing processed antigen epitopes presented by major histocompatibility complex (MHC) class I molecules via their T-cell receptor (TCR).3,4 In addition to the antigen-specific interaction, further costimulatory signals are crucial in order to achieve adequate CTL activation (reviewed in Banchereau and Steinman5 ). This costimulation is primarily provided by professional antigen-presenting cells (ie, dendritic cells [DCs] that express costimulatory molecules like CD40, CD80, CD86 as well as receptors of the tumor necrosis factor [TNF] superfamily)5-7 and produce inflammatory cytokines like IL-6, IL-12, and type 1 interferons.8,9 These costimulatory properties of DCs can be initiated by the triggering of Toll-like receptors (TLRs), which have an essential function in sensing microbial or viral infections.10 There are at least 11 different TLRs allowing the specific recognition of distinct conserved microbial or viral structures called pathogen-associated molecular patterns (PAMPs).11 Therefore, TLR-mediated signals have an essential impact on the induction and regulation of adaptive immune responses.12,13
The intracellular signaling pathway for most of the TLRs is quite well characterized and in common with the signaling for the IL-1 and IL-18 receptors that are crucially dependent on the adaptor molecule MyD88, ultimately leading to the activation of the transcription factor NF-κB.14-17 In addition to MyD88, TIRAP and TRIF have signaling function in TLR4- and TLR2-mediated activation, explaining the residual MyD88-independent activity after ligation of these TLRs.18-22 In contrast, TRIF is the only adaptor molecule responsible for TLR3-mediated activation and therefore TLR3 is the only TLR so far described acting completely independently of MyD88.23
Recently, Gautier et al9 demonstrated that stimulation of DCs with a combination of ligands for TLR3 and TLR7 leads to the induction of high amounts of IL-12p70 and production of type I interferons. In addition, Napolitani et al24 were able to show that combined TLR ligation cooperatively activates DCs in a way that not only IL-12 but also IL-23 secretion and the expression of Notch ligands Delta-4 and Jagged-1 are increased in DCs, leading to a T-helper 1 (Th1)–type polarization.
However, T-cell priming not only depends on the activation status of DCs but also is controlled by the activity of regulatory T cells. This cell type is able to suppress the function of CD4+ and CD8+ T cells in a contact-dependent manner.25,26 On the other hand, the inhibitory function of regulatory T cells (Treg's) themselves is influenced by the maturation status of DCs.27,28 In this regard, the synergistic effects of combined TLR stimulations of DCs on CD4+, CD8+ T cell, and Treg function in vitro and the translation of these observations to CTL activation in vivo have not been studied so far. Here, we demonstrate that there is a qualitative and quantitative synergy in activation of DCs by combined TLR ligation using distinct combinations of TLR ligands. Beyond this we are able to show for the first time that this synergistic activation of DCs converts to superior CD4 and CD8 T-cell responses while abrogating Treg-mediated suppression. These results provide a new insight into the complex cross talk of innate and adaptive immunity and might contribute to new DC-based vaccination strategies.
Materials and methods
Mice
C57BL/6 mice at the age of 6 to 8 weeks were used for DC immunization experiments as well as for the generation of bone marrow–derived DCs (BMDCs). TCR transgenic OT-II mice recognize ISQAVHAAHAEINEAGR from chicken ovalbumin (OVA323-339) and were kindly provided by Dr Christian Kurts (University of Bonn, Germany). St35 mice are transgenic for a TCR recognizing the H-2Db–restricted peptide SGPSNTPPEI from the Adenovirus Ad5 E1A protein (E1a234-243) and were generated as described previously.29 All animal procedures were conducted in accordance with the institutional guidelines.
Generation and activation of mouse bone marrow–derived dendritic cells
Mouse immature DCs were generated from bone marrow according to standard protocols.30,31 Minor modifications included a full replacement of culture medium containing GM-CSF (200 units/mL) on day 2 (removal of nonadherent cells), whereas on day 4 about 75% of medium/GM-CSF (200 units/mL) was replaced. Culture medium IMDM was supplemented with 5% FCS. On day 6 the immature DCs were activated with TLR ligands in titrated concentrations and combinations or left untreated. The following TLR stimuli were used: TLR2 agonist Palmitoyl-3-Cys-Ser-(Lys)4 (Pam3Cys; EMC Microcollections, Tübingen, Germany) and TLR3 agonist poly-(I:C) (Amersham, Freiburg, Germany). TLR4 was stimulated with LPS (Salmonella typhimurium; Sigma, Deisenhofen, Germany), TLR7/8 with R-848 (Invivogen, Toulouse, France), and TLR9 with the phosporothioate-stabilized oligonucleotide CpG 1668 (TIB; Molbiol, Hamburg, Germany). Where indicated, stimuli were removed by extensive washing (hereafter referred to as “stimulus removal”) and replaced by fresh medium containing no stimulus.
Detection of BMDC activation by cytokine release and surface markers
After 20 hours (40 hours in stimulus-removal experiments) of stimulation, culture supernatants were collected and frozen at –80°C for subsequent analysis. Cytokines or chemokines were analyzed by standard enzyme-linked immunosorbent assay (ELISA) protocols or by the Luminex detection system (Bio-Rad Laboratories, Munich, Germany) as indicated. All ELISA antibodies and recombinant standards were from BD Pharmingen (Heidelberg, Germany) and used according to the manufacturer's instructions. For detection, 3,3′, 5,5′ TMB liquid substrate (Sigma) was used. The optical density at 450 nm after adding 2M H2SO4 was analyzed using a SpectraFluorPlus reader from Tecan (Crailsheim, Germany). For cytokine measurement by the Luminex detection system, the Multicytokine Beadlite Kits (Biomol, Hamburg, Germany) were used according to the manufacturer's protocol.
After 2 days of incubation, BMDCs were analyzed by flow cytometry of viable (propidium iodide [PI]–negative) CD11c+ cells. The following monoclonal antibodies (mAbs) were used: allophycocyanin (APC)–conjugated anti-CD11c, FITC-conjugated anti-CD137 ligand/80/40, PE-conjugated anti-CD80, and Ox40 ligand/CD70 (all from BD Pharmingen).
BMDC immunizations
BMDCs (day 6 of differentiation) were stimulated with the TLR3 ligand poly-(I:C), the TLR7/8 ligand R-848, a combination of both, or left unstimulated for 6 or 20 hours as indicated. SIINFEKL peptide (100 nM) was loaded onto the stimulated DCs for 1 hour. The synthetic peptide SIINFEKL derived from chicken ovalbumin was kindly provided by Dr Stefan Stevanovic (Institute for Cell Biology, Department of Immunology, University of Tübingen, Germany). After removal of unbound peptide by washing twice with PBS, 3 × 105 DCs were injected intraperitoneally into C57BL/6 mice.
Flow cytometric analysis and in vivo cytotoxicity assay
Functional analyses of CTL responses induced by immunizations were performed by an in vivo cytotoxicity assay (12 hours) with differentially 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled (Molecular Probes, Eugene, OR; 4 μM and 0.4 μM CFSE, respectively) syngenic target cells 6 days after DC immunization. The CFSElo population was pulsed with 1 μM SIINFEKL, whereas the CFSEhi population was left without peptide. Target cells (2 × 107) were adoptively transferred into immunized mice at a 1:1 ratio (CFSElo/CFSEhi). Specific lysis was assessed as follows: specific lysis [%] = 100 × counts [CFSElo]/(counts [CFSElo] + counts [CFSEhi]). Twelve hours after intravenous injection of the target cells, mice were killed and spleens were processed to single-cell suspensions and stained with the indicated monoclonal antibodies for fluorescence-activated cell sorter (FACS) analysis. The following mAbs were used for analysis by flow cytometry: APC-Cy7–conjugated anti-CD8, PE-Cy7–conjugated anti-CD25, APC-conjugated anti-CD44 (all from BD Pharmingen), FITC-conjugated anti-CD44 (Immunotools, Friesoythe, Germany), PE-conjugated anti-CD62L (Immunotools), or APC-conjugated anti-CD62L (BD Pharmingen). PI was used to exclude nonviable cells from analysis. For detection of peptide-specific CD8+ T cells, PE-labeled SIINFEKL–H2-Kb tetramer was used (kindly provided by D. Busch, Munich, Germany). Where indicated, blood samples were collected after tail vein incision and incubated on ice with specific mAbs as indicated after a hypotonic lysis step. All analyses were performed with a FACSCanto flow cytometer and FACSDiva software (BD Pharmingen).
To assess intracellular IFN-γ production, splenocytes were restimulated ex vivo for 6 hours with or without SIINFEKL peptide (100 nM) in the presence of Brefeldin A (1 μg/mL; Sigma). Cells were then washed with FACS buffer and the surface stained with the indicated antibodies. Cells were treated with Cytofix/Cytoperm (BD Pharmingen) according to the manufacturer's protocol and finally an intracellular anti–IFN-γ antibody was added (BD Pharmingen). All washes after the permeabilization step and the intracellular staining itself were performed in PBS containing 0.1% BSA, 0.05% Saponin (Sigma).
MACS sorting
Regulatory T cells (CD4+CD25+) and conventional CD4+CD25– T cells were isolated from syngenic C57BL/6 mice and transgenic CD8+ T cells were isolated from OT-1 mice (specific for the Kb-restricted SIINFEKL) or St35 mice (specific for the Db-restricted SGPSNTPPEI) by magnetic-activated cell separation (MACS). Briefly, splenocytes were stained for the desired population with monoclonal antibodies coupled to magnetic beads and purification was performed according to the manufacturer's protocol. Purified cells were greater than 90% pure as judged by FACS analysis.
3H-thymidine proliferation assays
MACS-sorted CD4+CD25– T cells (2 × 104/well) were activated via soluble anti-CD3 mAb and by differentially TLR ligand–matured DCs (2 × 103/well) in the presence or absence of MACS-sorted and preactivated (αCD3/αCD28 for 2 days) CD4+CD25+ Treg's at a 1:1 ratio. After 3 days of coculture in 96-well round-bottom plates, the cells were pulsed with 3H-thymidine (0.5 μCi/well [0.0185 MBq/well]) and incorporation was measured after 18 hours by β-scintillation counting.
MACS-sorted OT-I CD8+ T cells (2 × 104/well) were cocultured in the presence or absence of indicated amounts of MACS-sorted and preactivated (αCD3/αCD28 for 2 days) CD4+CD25+ Treg's with differentially TLR-activated DCs (2 × 103/well), which had been pulsed with 5 nM SIINFEKL peptide. In addition, soluble anti-CD3 mAb was present in the culture. After 3 days of coculture in 96-well round-bottom plates, cells were 3H-thymidine pulsed, harvested after an additional 18 hours, and analyzed for proliferation by 3H-thymidine incorporation.
Statistical analyses
Statistical analyses of the data were performed by 2-way analysis of variance (ANOVA) for multivariate analyses. Only P values less than .001 were considered statistically significant.
Results
Effects of combined TLR agonists on BMDC activation
DCs are equipped with a wide variety of TLR specifically recognizing distinct microbial or viral structures. TLR ligation leads to DC activation characterized by the production of inflammatory cytokines like IL-6, IL-12, and RANTES as well as the upregulation of costimulatory molecules. Signaling after TLR-mediated activation involves pathways that are either dependent or independent of the adaptor protein MyD88. Therefore, we sought to explore the effects of combined TLR ligation on BMDCs.
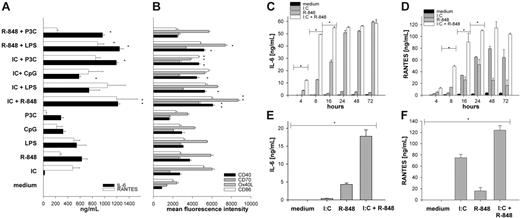
First, we assessed the responsiveness of mouse BMDCs to TLR ligation and incubated BMDCs with the TLR ligands Pam3Cys (TLR2 ligand), poly-(I:C) (TLR3 ligand), LPS (TLR4 ligand), R-848 (TLR7 ligand), and unmethylated CpG DNA (TLR9 ligand). All TLR agonists were used at their maximum effective dose for the induction of IL-6 production after 20 hours as a single stimulus determined by titration experiments (data not shown). As described previously,24,32 we found that TLR ligation led to the production of high amounts of IL-6 and RANTES (Figure 1A). MyD88-independent TLR3 ligation was more efficient in upregulating costimulatory molecules and inducing the secretion of RANTES33 but less effective in cytokine production of IL-6 and IL-12.9,24 Combined-TLR ligation of a MyD88-dependent stimulus with a MyD88-independent stimulus, however, led to a significantly enhanced production of IL-6 and RANTES (Figure 1A) as well as IL-12p70 and MCP-1 (not shown). A very similar effect was observed for the upregulation of the costimulatory molecules CD40 and CD86 as well as Ox40 ligand, CD70 (Figure 1B), and 4-1BBL (data not shown), again demonstrating the synergistic effect of combined TLR ligation. We found the most prominent synergy for the combination of TLR3 and TLR7 [poly-(I:C) plus R-848] and therefore used this TLR “superactivating” combination for the following investigations.
Combined stimulation of TLRs enhances production of IL-6 and RANTES and upregulation of DC activation markers. BMDCs from C57BL/6 mice were generated with GM-CSF for 6 days. On day 6, the immature DCs were activated with single-TLR ligands or various combinations thereof: Poly (I:C) (IC) 50 μg/mL, R-848 1 μg/mL, LPS 100 ng/mL, CpG ODN1668 (CpG) 1 μM, Pam3CysSKKK (P3C) 1 μg/mL. After 18 hours of stimulation, culture supernatants were collected for ELISA or Luminex analysis. (A) IL-6 (▪) and RANTES (□). (B) Two days after TLR ligand induced maturation expression of activation markers, CD40 (▪), CD70 (##), Ox40 ligand (##), and CD86 (□) were analyzed by FACS. The depicted results show mean and standard deviation from 4 independent experiments where mean fluorescence intensity as measure of surface expression of the respective activation marker was analyzed on viable CD11c+ cells. (C-D) Supernatants from BMDCs stimulated for the indicated period of time (4 to 72 hours) with medium (▪), I:C (##), R-848 (##), or I:C + R-848 (□) in combination were analyzed for IL-6 (C) and RANTES (D). (E-F) BMDCs were stimulated as described in “Generation and activation of mouse bone marrow–derived dendritic cells.” After 20 hours, supernatants were collected and the TLR ligand–containing medium was exchanged for medium without stimulus. Supernatants were collected again 20 hours later (after stimulus removal) and analyzed for the production of IL-6 (E) and RANTES (F) by ELISA or Luminex, respectively. All data are presented as mean and standard deviation of triplicate wells from 1 representative of at least 3 independent experiments for IL-6 and RANTES (A,C-F). *P < .001 by ANOVA among the respective single- and combined TLR ligand–stimulated cells.
Combined stimulation of TLRs enhances production of IL-6 and RANTES and upregulation of DC activation markers. BMDCs from C57BL/6 mice were generated with GM-CSF for 6 days. On day 6, the immature DCs were activated with single-TLR ligands or various combinations thereof: Poly (I:C) (IC) 50 μg/mL, R-848 1 μg/mL, LPS 100 ng/mL, CpG ODN1668 (CpG) 1 μM, Pam3CysSKKK (P3C) 1 μg/mL. After 18 hours of stimulation, culture supernatants were collected for ELISA or Luminex analysis. (A) IL-6 (▪) and RANTES (□). (B) Two days after TLR ligand induced maturation expression of activation markers, CD40 (▪), CD70 (##), Ox40 ligand (##), and CD86 (□) were analyzed by FACS. The depicted results show mean and standard deviation from 4 independent experiments where mean fluorescence intensity as measure of surface expression of the respective activation marker was analyzed on viable CD11c+ cells. (C-D) Supernatants from BMDCs stimulated for the indicated period of time (4 to 72 hours) with medium (▪), I:C (##), R-848 (##), or I:C + R-848 (□) in combination were analyzed for IL-6 (C) and RANTES (D). (E-F) BMDCs were stimulated as described in “Generation and activation of mouse bone marrow–derived dendritic cells.” After 20 hours, supernatants were collected and the TLR ligand–containing medium was exchanged for medium without stimulus. Supernatants were collected again 20 hours later (after stimulus removal) and analyzed for the production of IL-6 (E) and RANTES (F) by ELISA or Luminex, respectively. All data are presented as mean and standard deviation of triplicate wells from 1 representative of at least 3 independent experiments for IL-6 and RANTES (A,C-F). *P < .001 by ANOVA among the respective single- and combined TLR ligand–stimulated cells.
To further characterize the synergistic effects of combined TLR ligation on DCs, we performed a time course of TLR ligation and analyzed BMDC activation by the production of IL-6 and RAN-TES 4 to 72 hours after incubation. As depicted in Figure 1C-D, single-TLR ligation led to the production of detectable amounts of IL-6 (Figure 1C) or RANTES (Figure 1D) after 16 to 20 hours of incubation. In contrast, combined ligation of TLR3 and TLR7 induced cytokine release as early as 4 hours after TLR ligation and reached a maximum of IL-6 release already after 8 hours of stimulation, demonstrating that the observed synergy is present not only in terms of quantity of cytokine production but also in a qualitative manner inducing accelerated DC maturation.
Yang et al34 recently showed that sustained TLR ligation is needed in order to induce persistent cytokine production in DCs. To investigate the requirements for the sustained presence of TLR agonists after combined TLR stimulation, we incubated BMDCs with single-TLR ligands or combinations thereof and removed the stimuli after 20 hours by extensive washing and renewing the medium without stimulus. The production of cytokines was then analyzed after another 20 hours. BMDCs were able to produce only low amounts of IL-6 and RANTES after withdrawal of the stimulus after single-TLR ligation (Figure 1E-F). In contrast, DCs stimulated by combined TLR ligation were able to release significantly higher amounts of IL-6 (Figure 1E), RANTES (Figure 1F), and IL-12p70 (not shown), indicating that combined TLR activation enables DCs to become independent of the sustained TLR ligation.
Enhanced activation of CD4+ T cells by combined TLR ligand activated DCs in vitro and in vivo
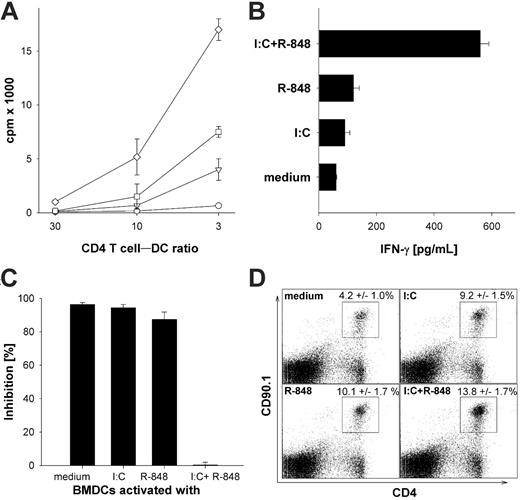
To analyze the effect of superactivated DCs on CD4 T cells, we first used TLR-activated BMDCs as antigen-presenting cells to induce activation of TCR transgenic CD4+ OT-II T cells in vitro. As described previously, nonactivated peptide-pulsed BMDCs induced little proliferation of TCR transgenic CD4+ OT-II T cells (Figure 2A) and only background levels of IFN-γ production (Figure 2B). Single ligation of TLR3 or TLR7 on BMDCs led to increased proliferation and production of considerable amounts of IFN-γ. However, we found that combined-TLR3/7–activated BMDCs induced by far the strongest T-cell proliferation and cytokine production in CD4+ T cells compared with single-TLR–activated BMDCs. This is in line with previous results showing that combined TLR ligation of human DCs leads to a Th1-type polarization of CD4+ T cells in vitro.24
Enhanced activation of TCR transgenic CD4+ T cells in vitro and in vivo by BMDCs stimulated with TLR ligand combinations. (A) MACS-sorted transgenic OT-II CD4+ T cells (7 × 103) were cocultured with titrated numbers of OVA323-339 peptide–pulsed (0.3 μM) DCs activated with the indicated TLR ligands as described in “Generation and activation of mouse bone marrow–derived dedritic cells.” Proliferation by 3H-thymidine incorporation was analyzed on day 4 of the culture. ○ indicates BMDCs stimulated for 24 hours with medium; □, poly-(I:C) 50 μg/mL; ▵, R-848 1 μg/mL; and ⋄, poly (I:C) + R-848. (B) MACS-sorted OT-II transgenic CD4+ T cells (5 × 104) were cocultured with 3 × 103 OVA323-339 peptide–pulsed DCs activated with the indicated TLR ligands as described in “Generation and activation of mouse bone marrow–derived detritic cells.” Culture supernatants were collected after 3 days of the coculture and IFN-γ secretion was measured by standard ELISA. (C) BMDCs were stimulated as described in “Generation and activation of mouse bone marrow–derived dedritic cells” for 24 hours and washed extensively before adding differentially stimulated BMDCs (5 × 103/well) to cocultures of MACS-sorted transgenic CD4+ T cells from C57BL/6 mice (5 × 104/well) and preactivated (αCD3/αCD28) CD4+CD25+ Treg's. CD4+ T cells were activated with an α-CD3 mAb as proliferative stimulus. The inhibition in percentage is referenced to the respective wells without regulatory CD4+CD25+ T cells. All results are presented as mean and standard deviation of triplicate wells of 1 of 3 independent experiments. (D) C57BL/6 mice were adoptively transferred with 106 splenocytes from TCR transgenic OT-II mice recognizing the epitope ISQAVHAAHAEINEAGR and expressing the congenic marker CD90.1. Mice were injected with 3 × 105 peptide-loaded DCs activated for 6 hours with the indicted TLR ligands as described in “Generation and activation of mouse bone marrow–derived dedritic cells.” After 4 days, the mice were killed and splenocytes were analyzed for expansion. Expression of CD90.1 and CD4 was analyzed on splenocytes of mice injected with BMDCs that were in vitro activated with the indicated TLR ligands. These results are representative of 2 independent experiments with 5 mice per group. The numbers are mean percentage with standard deviation after immunization with BMDCs that had been activated with the indicated stimuli from 2 experiments performed with 5 mice per group (n = 10). Analysis by ANOVA indicated a significant difference (P < .001).
Enhanced activation of TCR transgenic CD4+ T cells in vitro and in vivo by BMDCs stimulated with TLR ligand combinations. (A) MACS-sorted transgenic OT-II CD4+ T cells (7 × 103) were cocultured with titrated numbers of OVA323-339 peptide–pulsed (0.3 μM) DCs activated with the indicated TLR ligands as described in “Generation and activation of mouse bone marrow–derived dedritic cells.” Proliferation by 3H-thymidine incorporation was analyzed on day 4 of the culture. ○ indicates BMDCs stimulated for 24 hours with medium; □, poly-(I:C) 50 μg/mL; ▵, R-848 1 μg/mL; and ⋄, poly (I:C) + R-848. (B) MACS-sorted OT-II transgenic CD4+ T cells (5 × 104) were cocultured with 3 × 103 OVA323-339 peptide–pulsed DCs activated with the indicated TLR ligands as described in “Generation and activation of mouse bone marrow–derived detritic cells.” Culture supernatants were collected after 3 days of the coculture and IFN-γ secretion was measured by standard ELISA. (C) BMDCs were stimulated as described in “Generation and activation of mouse bone marrow–derived dedritic cells” for 24 hours and washed extensively before adding differentially stimulated BMDCs (5 × 103/well) to cocultures of MACS-sorted transgenic CD4+ T cells from C57BL/6 mice (5 × 104/well) and preactivated (αCD3/αCD28) CD4+CD25+ Treg's. CD4+ T cells were activated with an α-CD3 mAb as proliferative stimulus. The inhibition in percentage is referenced to the respective wells without regulatory CD4+CD25+ T cells. All results are presented as mean and standard deviation of triplicate wells of 1 of 3 independent experiments. (D) C57BL/6 mice were adoptively transferred with 106 splenocytes from TCR transgenic OT-II mice recognizing the epitope ISQAVHAAHAEINEAGR and expressing the congenic marker CD90.1. Mice were injected with 3 × 105 peptide-loaded DCs activated for 6 hours with the indicted TLR ligands as described in “Generation and activation of mouse bone marrow–derived dedritic cells.” After 4 days, the mice were killed and splenocytes were analyzed for expansion. Expression of CD90.1 and CD4 was analyzed on splenocytes of mice injected with BMDCs that were in vitro activated with the indicated TLR ligands. These results are representative of 2 independent experiments with 5 mice per group. The numbers are mean percentage with standard deviation after immunization with BMDCs that had been activated with the indicated stimuli from 2 experiments performed with 5 mice per group (n = 10). Analysis by ANOVA indicated a significant difference (P < .001).
Pasare and Medzhitov35 recently demonstrated that TLR-activated BMDCs are able to overcome Treg-mediated suppression of T-cell activation. To address whether combined TLR ligation of BMDCs has an impact on Treg-mediated suppression of T-cell activation, BMDCs were activated by poly-(I:C), R-848, or the combination thereof, then washed extensively to remove the remaining present TLR agonists. The different BMDCs were then added to cocultures of preactivated Treg's with OT-II CD4+ T cells, and the T-cell proliferation was analyzed. As depicted in Figure 2C, unstimulated or single-TLR–activated BMDCs were unable to break Treg-mediated suppression of CD4+ T-cell proliferation. However, CD4+ T cells activated by superactivated BMDCs were more or less completely refractory to Treg-mediated suppression in this setting. These results imply that Treg-mediated suppression of CD4+ T-cell proliferation can be overcome by “properly” activated DCs.
Next, we were interested in whether or not these results obtained from in vitro experiments were also relevant in vivo. Therefore, CD4+ TCR transgenic T cells from OT-II mice (expressing the congenic marker CD90.1) were adoptively transferred into C57BL/6 mice and subsequently immunized with the differentially TLR ligand–activated BMDCs pulsed with OVA323-339 peptide. We found an enhanced expansion of transgenic CD4+ T cells when the mice were treated with BMDCs after single-TLR ligation (Figure 2D). Conversely, as before in vitro, in the mice that had received BMDCs that had been activated by combined TLR ligation, the transgenic CD4+ T cells had expanded significantly more (P < .001 by ANOVA), suggesting that the observed effects might also be of relevance in vivo.
TLR superactivation of DCs induces superior CD8 T-cell responses in vitro and in vivo
To address whether combined TLR activation of DCs also has a direct impact on the activation of naive CD8+ T cells, we used TLR-activated BMDCs as APC to induce activation of TCR transgenic CD8+ St35 T cells in vitro. In parallel to the results obtained with CD4+ T cells, we found a very similar behavior of CD8+ T cells in terms of proliferation (Figure 3A) and the production of IL-2 (Figure 3B). CD8+ T cells stimulated with BMDCs after combined TLR ligation showed superior activation compared with single-TLR ligation. To assess whether TLR superactivated BMDCs can also break Treg-mediated suppression of CD8+ T-cell responses, BMDCs were again activated as before, washed extensively, and added to cocultures of preactivated Treg's with TCR transgenic CD8+ St35 T cells. As depicted in Figure 3C, unstimulated BMDCs were unable to break Treg-mediated suppression of CD8+ T-cell proliferation. Single-TLR–activated BMDCs were able to overcome Treg-mediated suppression only to some extent, whereas CD8+ T cells activated by superactivated BMDCs again were completely refractory to Treg-mediated suppression. To further explore the significance of combined TLR triggering in vivo, BMDCs activated by poly-(I:C) or R-848 alone or in combination for 6 hours in vitro were pulsed with the adenoviral E1a protein–derived peptide SGPSNTPPEI and used to immunize C57BL/6 mice after adoptive transfer of TCR transgenic St35 CD8+ T cells that are specific for this peptide and express the congenic marker CD45.1. T-cell expansion, expression of activation markers, and ex vivo production of IFN-γ were monitored 5 days later. We found that immunization with single-TLR–activated DCs resulted in considerable CD8+ T-cell activation in vivo. However, the CTL response upon immunization with TLR superactivated DCs was clearly superior as determined by the percentage of specific CTLs with an activated phenotype (CD44hi, Figure 3D; CD62Llo, not shown) and by the ex vivo production of IFN-γ as analyzed by intracellular FACS labeling (not shown; P < .001 by ANOVA). These results suggest that the combined-TLR activation of DCs affects not only CD4+ but also CD8+ T cells in vivo.
Enhanced activation of TCR transgenic CD8+ T cells in vitro and in vivo by BMDCs stimulated with TLR ligand combinations. (A) MACS-sorted transgenic St35 CD8+ T cells (5 × 104) were cocultured with 4 × 103, 1 × 103, or 2.5 × 102 SGP-peptide–pulsed medium-activated, poly-(I:C)–activated, R-848–activated, or superactivated DCs and proliferation (3H-thymidine incorporation) was measured on day 3 of the culture. ○ indicates BMDCs stimulated for 24 hours with medium; ▿, poly-(I:C) 50 μg/mL; □, R-848 1 μg/mL; or ⋄, poly (I:C) + R-848. (B) MACS-sorted transgenic St35 CD8+ T cells (5 × 104) were cocultured with DCs (1 × 103) activated with the indicated TLR ligands as described in “Generation and activation of mouse bone marrow–derived dendritic cells” and culture supernatant was collected after 2 days of coculture and analyzed by IL-2 ELISA. (C) BMDCs were stimulated as described in “Generation and activation of mouse bone marrow–derived dendritic cells” for 24 hours and washed extensively before adding differentially stimulated BMDCs (5 × 103/well) to cocultures of MACS-sorted transgenic CD8+ T cells from St35 mice (5 × 104/well) and preactivated (αCD3/αCD28) CD4+CD25+ regulatory T cells (Treg's). CD8+ T cells were stimulated with the adenoviral peptide SGP (0.5 nM). The inhibition in percentage is referenced to the respective wells without regulatory CD4+CD25+ T cells. All results are presented as mean and standard deviation of triplicate wells of 1 of 3 independent experiments. (D) C57BL/6 mice were adoptively transferred with splenocytes from 105 TCR transgenic St35 mice recognizing the H2-Db–restricted epitope SGPSNTPPEI and expressing the congenic marker CD45.1. Mice were injected with 3 × 104 peptide-loaded DCs activated for 6 hours with the indicted TLR ligands, as in Figure 1, in vitro intraperitoneally 1 day later. The frequency, phenotype, and ex vivo production of IFN-γ was analyzed on day 5 after injection in CD45.1+ cells in the spleens of host animals. Expression of the congenic marker CD45.1 (on transgenic St35 CD8+ T cells) and CD44 (gated on CD8+) splenocytes of mice injected with DCs that were in vitro activated with the indicated TLR ligands is shown. One representative dot plot for each immunization is depicted. The numbers are the mean percentage with standard deviation after immunization with BMDCs that had been activated with the indicated stimuli from 2 independent experiments performed with 3 mice per group (n = 6). Analysis by ANOVA indicated a significant difference (P < .001) among the different groups.
Enhanced activation of TCR transgenic CD8+ T cells in vitro and in vivo by BMDCs stimulated with TLR ligand combinations. (A) MACS-sorted transgenic St35 CD8+ T cells (5 × 104) were cocultured with 4 × 103, 1 × 103, or 2.5 × 102 SGP-peptide–pulsed medium-activated, poly-(I:C)–activated, R-848–activated, or superactivated DCs and proliferation (3H-thymidine incorporation) was measured on day 3 of the culture. ○ indicates BMDCs stimulated for 24 hours with medium; ▿, poly-(I:C) 50 μg/mL; □, R-848 1 μg/mL; or ⋄, poly (I:C) + R-848. (B) MACS-sorted transgenic St35 CD8+ T cells (5 × 104) were cocultured with DCs (1 × 103) activated with the indicated TLR ligands as described in “Generation and activation of mouse bone marrow–derived dendritic cells” and culture supernatant was collected after 2 days of coculture and analyzed by IL-2 ELISA. (C) BMDCs were stimulated as described in “Generation and activation of mouse bone marrow–derived dendritic cells” for 24 hours and washed extensively before adding differentially stimulated BMDCs (5 × 103/well) to cocultures of MACS-sorted transgenic CD8+ T cells from St35 mice (5 × 104/well) and preactivated (αCD3/αCD28) CD4+CD25+ regulatory T cells (Treg's). CD8+ T cells were stimulated with the adenoviral peptide SGP (0.5 nM). The inhibition in percentage is referenced to the respective wells without regulatory CD4+CD25+ T cells. All results are presented as mean and standard deviation of triplicate wells of 1 of 3 independent experiments. (D) C57BL/6 mice were adoptively transferred with splenocytes from 105 TCR transgenic St35 mice recognizing the H2-Db–restricted epitope SGPSNTPPEI and expressing the congenic marker CD45.1. Mice were injected with 3 × 104 peptide-loaded DCs activated for 6 hours with the indicted TLR ligands, as in Figure 1, in vitro intraperitoneally 1 day later. The frequency, phenotype, and ex vivo production of IFN-γ was analyzed on day 5 after injection in CD45.1+ cells in the spleens of host animals. Expression of the congenic marker CD45.1 (on transgenic St35 CD8+ T cells) and CD44 (gated on CD8+) splenocytes of mice injected with DCs that were in vitro activated with the indicated TLR ligands is shown. One representative dot plot for each immunization is depicted. The numbers are the mean percentage with standard deviation after immunization with BMDCs that had been activated with the indicated stimuli from 2 independent experiments performed with 3 mice per group (n = 6). Analysis by ANOVA indicated a significant difference (P < .001) among the different groups.
Combined-TLR activation of DCs induces a superior CTL activation in wild-type mice
We demonstrated in Figure 1 that combined TLR ligation resulted in a faster production of cytokines compared with single-TLR stimulation, suggesting that combined TLR ligation results in accelerated DC maturation. Therefore, we wondered whether DCs activated for a short duration by the combined TLR ligation would also show differences in the induction of T-cell responses in vivo compared with single-TLR–activated BMDCs. We reasoned that an adoptive transfer system of TCR transgenic T cells might not be suitable to estimate the biologic relevance or potency of the combined TLR ligation and therefore studied the primary wild-type CTL response upon DC vaccination. Thus we analyzed the effects of combined TLR ligation of DCs by immunizing wild-type C57Bl/6 mice without previous transfer of TCR transgenic cells. Specific CTL activation was analyzed by MHC class I tetramer staining for peptide-specific CD8+ T cells, production of IFN-γ ex vivo, and specific target cell lysis in vivo. As shown in Figure 4, we surprisingly found that immunization with peptide-loaded BMDCs activated for only 6 hours by combined TLR ligation was already sufficient to induce a strong peptide-specific (SIINFEKL) CD8 T-cell response as determined by specific MHC class I tetramer staining (Figure 4A) and CD44 upregulation and CD62L shedding (not shown). This was accompanied by a significant target cell lysis (Figure 4C) and the production of IFN-γ (Figure 4E). Six-hour single-TLR–activated BMDCs had only a marginal effect. This situation changed to some extent when BMDCs were stimulated for 20 hours with TLR ligands. Now injection of peptide-loaded BMDCs activated by single-TLR ligation were also able to activate CD8+ T cells in terms of the detection of antigen-specific CTLs (Figure 4B), in vivo target lysis (Figure 4D), and production of IFN-γ (Figure 4F) as well as CD62L shedding and CD44 upregulation (not shown); however, the superactivated DCs were still superior. These results demonstrate that the synergistic effect of combined TLR ligation on DCs translates into a significantly enhanced T-cell activation in wild-type mice.
Immunization with BMDCs stimulated with TLR ligand combinations results in superior CTL priming in vivo. DCs were stimulated with poly-(I:C), R-848, a combination of both, or left unstimulated for 6 hours or 20 hours. SIINFEKL peptide (100 nM) was loaded onto the stimulated DCs for 1 hour. After removal of unbound peptide, 3 × 105 BMDCs were injected intraperitoneally into C57BL/6 mice. Seven days after DC immunization, mice were killed and spleens were processed to single-cell suspensions and (A-B) stained for surface expression of CD8 and activation markers (CD44 and CD62L; not shown) as well as with H2-Kb SIINFEKL tetramer for specificity of the induced CD8+ T cells. The mean percentage with standard deviation of 3 independent experiments with 3 mice per group is shown. (C-D) To check for functionality in terms of peptide-specific lysis by CTLs an in vivo cytotoxicity assay was performed as described in “Flow cytometric analysis and in vivo cytotoxicity assay.” The mean percentage with standard deviation from 3 independent experiments is depicted. (E-F) To assess the IFN-γ production of the CD8+ T cells, splenocytes were restimulated in vitro for 6 hours with or without (data not shown; background levels were the same for all stimuli) SIINFEKL peptide in the presence of Brefeldin A (1 μg/mL) and analyzed by FACS. The mean percentage with standard deviation of IFN-γ–producing CD8+ cells is depicted. The results are summarized from 3 independent experiments. *P < .001 by ANOVA. **P < .05 by ANOVA.
Immunization with BMDCs stimulated with TLR ligand combinations results in superior CTL priming in vivo. DCs were stimulated with poly-(I:C), R-848, a combination of both, or left unstimulated for 6 hours or 20 hours. SIINFEKL peptide (100 nM) was loaded onto the stimulated DCs for 1 hour. After removal of unbound peptide, 3 × 105 BMDCs were injected intraperitoneally into C57BL/6 mice. Seven days after DC immunization, mice were killed and spleens were processed to single-cell suspensions and (A-B) stained for surface expression of CD8 and activation markers (CD44 and CD62L; not shown) as well as with H2-Kb SIINFEKL tetramer for specificity of the induced CD8+ T cells. The mean percentage with standard deviation of 3 independent experiments with 3 mice per group is shown. (C-D) To check for functionality in terms of peptide-specific lysis by CTLs an in vivo cytotoxicity assay was performed as described in “Flow cytometric analysis and in vivo cytotoxicity assay.” The mean percentage with standard deviation from 3 independent experiments is depicted. (E-F) To assess the IFN-γ production of the CD8+ T cells, splenocytes were restimulated in vitro for 6 hours with or without (data not shown; background levels were the same for all stimuli) SIINFEKL peptide in the presence of Brefeldin A (1 μg/mL) and analyzed by FACS. The mean percentage with standard deviation of IFN-γ–producing CD8+ cells is depicted. The results are summarized from 3 independent experiments. *P < .001 by ANOVA. **P < .05 by ANOVA.
Discussion
DCs are important cells in orchestrating innate and adaptive immune responses by their ability to sense danger signals (ie, via pattern recognition receptors such as TLRs) and to initiate potent CD4 and CD8 T-cell responses.27,36
Extending recent reports,9,24 we demonstrate here that combined-TLR ligation of DCs that triggers distinct signaling pathways results in an enhanced activation of these cells in a synergistic manner in terms of proinflammatory cytokine and chemokine production and also for expression of costimulatory molecules like CD40, Ox40 ligand, and CD70 (Figure 1). The latter has been reported to play an important role in mediating CD8 T-cell responses independent of a CD4 helper response.37 Beyond this, we are able to demonstrate here that combined-TLR ligation of DCs affects not only the quantity of cytokine or chemokine production but also the quality. This is reflected by a faster onset of IL-6 or RANTES secretion clearly detectable already 4 to 8 hours after adding the TLR ligand combination (Figure 1C-D). We also observe a more sustained production of IL-6 or RANTES after withdrawal of the stimuli (Figure 1E-F) as opposed to single-TLR ligation where persistent stimulation is needed for continuous cytokine production.34 We cannot differentiate whether this independence of the further presence of TLR ligands is mediated by a stronger initial signal after the combined TLR ligation or by the sustained activation of the signaling pathway after removal of TLR agonists. However, since a prolonged phosphorylation of Jun and an enhanced IκBζ mRNA synthesis upon combined-versus single-TLR ligation was demonstrated before,24 it appears more likely that sustained signaling is responsible for the enhanced production of cytokines.
Does the enhanced activation of DCs by combined TLR ligation directly influence priming of CD4+ and CD8+ T cells in vivo as observed by Napolitani et al24 for Th1-cell differentiation in vitro? To address this issue, we first used these TLR superactivated [poly-(I:C) and R-848] DCs to stimulate naive TCR transgenic CD4+ or CD8+ T cells in vitro and detected that the synergistic effect on DC activation provokes a superior T-cell activation with regard to proliferation (Figures 2, 3), production of IL-2 by CD8+ T cells or IFN-γ by CD4+ T cells, and lytic activity (data not shown). However, the superior stimulatory capacity of DCs activated by combined TLR stimuli is not limited to this experimental setting. Pasare and Medzhitov35 recently demonstrated that TLR-activated DCs are able to break Treg-mediated suppression of CD4+ T-cell responses. Therefore, we investigated whether or not DCs activated by combined TLR stimuli are also superior in preventing the Treg-mediated inhibition of CD4+ and CD8+ T-cell activation.
We found that single-TLR ligation on DCs was insufficient to break Treg-mediated suppression in our experimental setting using CD28/CD3 preactivated Treg's with a higher suppressive activity than naive Treg's used by Pasare and Medzhitov.35 Only DCs activated by combined TLR stimuli are able to prevent Treg-mediated suppression of CD4+ (Figure 2C) and CD8+ T-cell (Figure 3C) proliferation. The elevated levels of secreted IL-6 together with a so far unknown factor secreted by DCs38 can be expected to be involved in rendering CD4+ and CD8+ T cells refractory to the highly suppressive activity of preactivated Treg's.
To study whether our results obtained from in vitro experiments are also relevant in vivo, we used these TLR superactivated DCs after 6 hours of in vitro activation to immunize mice adoptively transferred with TCR transgenic CD4+ or CD8+ T cells and monitored T-cell behavior in vivo (Figures 2, 3). We demonstrate that immunizations with DCs stimulated with a combination of TLR3 and TLR7 ligands induce a much stronger CTL response as evidenced by CD8+ T-cell expansion and IFN-γ production. In a similar fashion, the expansion of CD4+ OT-II T cells is superior upon immunization with BMDCs that were activated by combined-TLR ligation in line with previous results obtained in vitro with human CD4+ T cells.24
Since we found that combined TLR ligation results in the release of a large amount of IL-6 as early as 8 hours after TLR stimulation, we asked whether this also leads to superior CTL activation in wild-type mice not supplied with TCR transgenic T cells. Therefore, we performed immunizations of wild-type mice with DCs after 6 or 20 hours of TLR ligation. We were surprised to find that already a 6-hour superactivation of DCs was sufficient to induce a significant CTL response in vivo. After 20 hours of stimulation, TLR superactivation is still superior but CTL activation is now also observed after single-TLR ligation of DCs (Figure 4). This result fits nicely to the observation shown in Figure 1C-D, where TLR superactivation induces an accelerated cytokine and chemokine secretion. T-cell priming will further benefit from the enhanced expression of costimulatory molecules, like CD40 and CD86. With regard to the superior CTL activity after immunization with superactivated DCs, the high levels of CD70 might also play an important role, as this molecule has been demonstrated to allow the initiation of CTL responses independently from CD4+ T cells.37,39 The mechanisms behind the synergistic effect described here are most likely based on the different intracellular pathways triggered by TLR3 and TLR7 ligands. Whereas the former triggers a MyD88-independent pathway involving the adaptor molecule TRIF and mediates induction of type I interferons via IRF3, the latter initiates the production of proinflammatory cytokines via MyD88, IRF5, IRF7, and NF-κB. Synergy between both pathways is enabled by the IRF3-initiated production of IFN-β, which in turn leads to higher IRF7 synthesis, allowing a more efficient TLR7 signaling.9 Our results clearly show that this synergistic activation of DCs has an important impact on the adaptive immune response initiated.
Taken together, we show that the synergistic effect of combined-TLR triggering on BMDCs includes a faster and more sustained secretion of proinflammatory cytokines, makes T cells less sensitive toward inhibition by regulatory T cells, and results in the induction of a superior CTL response in vivo. These results might be helpful in developing new DC-based vaccination strategies against malignant diseases and persistent viral infections.
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2005-10-4015.
Supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 432, B10 [H.S. and M.P.R.]; SFB 548 A6 [E.S.]).
T.W. and P.O. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal