Antiangiogenic agents and therapeutic strategies have entered the clinical oncology arena. The classical tumor size measurements defined to monitor efficacy of chemotherapy, however, might not be appropriate for these newer therapeutics. We previously found that circulating endothelial cells (CECs) were increased in number and more viable in cancer patients compared with control subjects. We investigated the correlation between CEC kinetics and clinical outcome in patients with advanced breast cancer receiving metronomic chemotherapy, a therapeutic strategy associated with antiangiogenic activity and anticancer efficacy. CEC number and viability were measured by flow cytometry in patients and in preclinical models. CECs were decreased in patients for whom no overall clinical benefit (defined as a clinical response or a stable disease) was observed compared with those who had a clinical benefit (P = .015). This difference was due to an increased fraction of apoptotic CECs in patients with a clinical benefit. Univariate and multivariate analyses indicated that CEC values greater than 11/μL were associated with a longer progression-free survival (P = .001) and an improved overall survival (P = .005). Preclinical models indicated that the source of apoptotic CECs was most likely the tumor vasculature. CEC kinetics and viability are very promising as predictors of clinical response in patients undergoing metronomic chemotherapy.
Introduction
Inhibitors of angiogenesis are a new clinical class of drugs with therapeutic potential in many diseases, including oncology.1,2 However, defining the most effective dose and schedule for such drugs or treatments remains a significant hurdle to their optimal use in the clinic. Thus, the classic maximum tolerable dose (MTD) approach, as defined in the past for cytotoxic drugs, might not be adequate or relevant for this class of drugs, which in general have considerably less toxicity than conventional cytotoxics used at MTD, and therefore may be used for prolonged periods to obtain inhibition of new vessel growth, and, in turn, tumor stabilization or shrinkage. At the present time, angiogenesis and antiangiogenic drug activity are empirically measured by microvascular density, measuring the circulating levels of angiogenic growth factors, or by functional approaches such as magnetic resonance imaging of blood flow and vascular permeability. However, these parameters are not always predictive, or are still in preclinical development.1-3
We have investigated, at the preclinical and clinical levels, the role of different surrogate markers of angiogenesis and vasculogenesis to understand whether they can be used to define the antivascular activity of some drugs or drug combinations and to predict response and/or survival in cancer patients receiving antiangiogenic treatment.4-6 Flow cytometry studies have indicated that circulating endothelial cells (CECs) are significantly increased in numbers and percentage viability in untreated cancer patients compared with healthy subjects.4 In the present study, 4-color flow cytometry has been used to enumerate CECs and circulating endothelial progenitors (CEPs) and to investigate their viability in metastatic breast cancer patients receiving metronomic chemotherapy treatment. Recent preclinical studies have demonstrated that this therapeutic strategy, involving administration of low doses of cytotoxic agents at close, regular intervals, with no extended breaks, has strong antiangiogenic activity, which contributes significantly to the antitumor activity of such regimens.7-10 This antiangiogenic effect, however should still be formally demonstrated at the clinical level.
Patients, materials, and methods
Patients
Breast cancer patients with metastatic disease were enrolled in a phase 3 study of metronomic chemotherapy administered according to Colleoni et al8 (cyclophosphamide [CTX] and methotrexate) with or without thalidomide. This was an institutional study of the European Institute of Oncology. Patients were required to have histologically confirmed metastatic breast carcinoma either pretreated or not after a previous line of chemotherapy for metastatic disease. Other inclusion criteria were measurable or evaluable disease, age of 80 years or less, Eastern Cooperative Oncology Group (ECOG) performance status less than 3, adequate bone marrow reserve defined as white blood cell count less than 4000 mm3 and platelet count more than 100 000 mm3, adequate renal function (serum creatinine < 120 μM) and hepatic function (serum bilirubin < 20 μM, aspartate aminotransferase [AST] [also known as serum glutamic oxaloacetic transaminase (SGOT)] < 60 IU/L). Any prior hormonal therapy or chemotherapy must have been discontinued at least 4 weeks before study entry. Each patient included in the study gave a written informed consent. The protocol was reviewed and approved by institutional review boards of the European Institute of Oncology.
Randomization was conducted after stratification according to pretreatment. Patients were randomized to methotrexate orally at a dose of 2.5 mg twice a day on days 1 and 4 every week (10 am, 5 pm) and cyclophosphamide orally at a dose of 50 mg a day (9 am) (Arm A) or the same treatment pus thalidomide administered orally at the dose of 200 mg/d (9 pm).
Assessment of response was performed according to World Health Organization (WHO) criteria after every 8 weeks of therapy. Complete remission (CR) was defined as the disappearance of all known lesions on 2 separate measurements at least 4 weeks apart. Partial remission (PR) was defined as a reduction of each lesion by at least 50%. Stable disease (SD) was defined as a decrease of less than 50% or an increase of less than 25% with no new lesions, and progressive disease as an increase of more than 25% or appearance of new lesions. Clinical benefit was defined as the proportion of patients who achieved CRs, PRs, or SDs for at least 24 weeks.
Evaluation of CECs by flow cytometry in patients
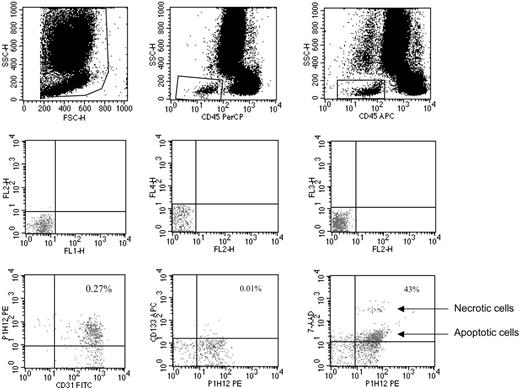
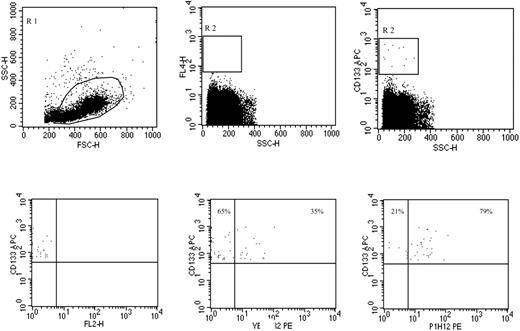
CECs and their progenitor subpopulation (CEPs) were evaluated as previously described.4,6 In brief, monoclonal antibodies including anti-CD45 used to exclude hematopoietic cells, anti-CD133 (a progenitor cell marker), P1H12 (CD146, an endothelial cell marker11 ), the apoptosis marker 7-aminoactinomycin D (7AAD),12 and appropriate analysis gates were used to enumerate viable and apoptotic CECs and CEPs (Figures 1, 2, 3). Cell suspensions were evaluated after red cell lysis by a FACSCalibur (BD, San Jose, CA). After acquisition of at least 100 000 cells per blood sample, analyses were considered as informative when adequate numbers of events (ie, > 100, typically 300-400 events) were collected in the CECs enumeration gates. CECs were defined as negative for hematopoietic marker CD45, positive for the endothelial marker P1H12 and negative for the progenitor marker CD133. CEPs (Figure 2) were depicted by the expression of CD133. Figure 3 shows the procedure followed to validate CEC/CEP enumeration and the evaluation of apoptosis by 7AAD.
Representative CEC enumeration by flow cytometry. Panels in the top row show the gate used to exclude platelets, dead cells, and debris, and the gate made to depict CD45-negative (ie, nonhematopoietic) cells. Panels in the middle row show the negative controls. Panels in the bottom row show the gate used to count CECs (left panel: CECs defined as CD45–, CD31+, P1H12+, and CD133–), the gate used to count CEPs (middle panel: CEPs defined as CD45– and CD133+), and the gate used to investigate CEC viability by 7AAD (right panel indicates how 7AAD used as in Philpott et al12 discriminates between apoptotic and necrotic cells). Antibodies used were FITC-labeled anti-CD31, PE-labeled P1H12 (CD146),4,12 and APC-labeled anti-CD133.
Representative CEC enumeration by flow cytometry. Panels in the top row show the gate used to exclude platelets, dead cells, and debris, and the gate made to depict CD45-negative (ie, nonhematopoietic) cells. Panels in the middle row show the negative controls. Panels in the bottom row show the gate used to count CECs (left panel: CECs defined as CD45–, CD31+, P1H12+, and CD133–), the gate used to count CEPs (middle panel: CEPs defined as CD45– and CD133+), and the gate used to investigate CEC viability by 7AAD (right panel indicates how 7AAD used as in Philpott et al12 discriminates between apoptotic and necrotic cells). Antibodies used were FITC-labeled anti-CD31, PE-labeled P1H12 (CD146),4,12 and APC-labeled anti-CD133.
Representative CEP phenotype evaluation by flow cytometry. Peripheral blood was processed by Ficoll to enrich for the mononuclear cell fraction including CEPs. Panels in the top row show the gate used to exclude platelets, dead cells, and debris (left panel), the negative control (middle panel), and the gate made on CD133+ cells, regardless of CD45 expression (right panel). Panels on the bottom show CD133 expression with an irrelevant PE-labeled antibody (left panel), and the frequencies of CD133+ VEGFR2+ (middle panel) and CD133+ P1H12+ (right panel) EPCs. Antibodies used were PE-labeled anti-VEGFR2, PE-labeled P1H12 (CD146),4,12 and APC-labeled anti-CD133.
Representative CEP phenotype evaluation by flow cytometry. Peripheral blood was processed by Ficoll to enrich for the mononuclear cell fraction including CEPs. Panels in the top row show the gate used to exclude platelets, dead cells, and debris (left panel), the negative control (middle panel), and the gate made on CD133+ cells, regardless of CD45 expression (right panel). Panels on the bottom show CD133 expression with an irrelevant PE-labeled antibody (left panel), and the frequencies of CD133+ VEGFR2+ (middle panel) and CD133+ P1H12+ (right panel) EPCs. Antibodies used were PE-labeled anti-VEGFR2, PE-labeled P1H12 (CD146),4,12 and APC-labeled anti-CD133.
Evaluation of CECs in preclinical models
Preclinical models of cancer-bearing animals undergoing treatment with metronomic chemotherapy were obtained by injecting intraperitoneally nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice of 6 to 8 weeks of age with 10 × 106 Namalwa or Granta 519 cells (American Type Culture Collection, Manassas, VA) as described previously.5,13,14 Namalwa cells were derived from an Epstein-Barr virus–positive (EBV+) Burkitt lymphoma, Granta 519 cells were derived from a mantle cell lymphoma in leukemic transformation. After injection, all of the mice that received transplants developed intraperitoneally measurable tumors in the injection site (the middle of the right posterior quadrant of the abdomen). Animals were evaluated for tumor growth every other day, and tumors were measured by calipers.5,13,14 In addition, 2 other tumor models were tested, a human melanoma (MeWo) implanted subdermally into nude mice, and a variant of the MDA-MB-231 human breast cancer cell line called LM2.4, which was selected for aggressive metastatic properties after orthotopic (intramammary fat pad) injection of the cells.15
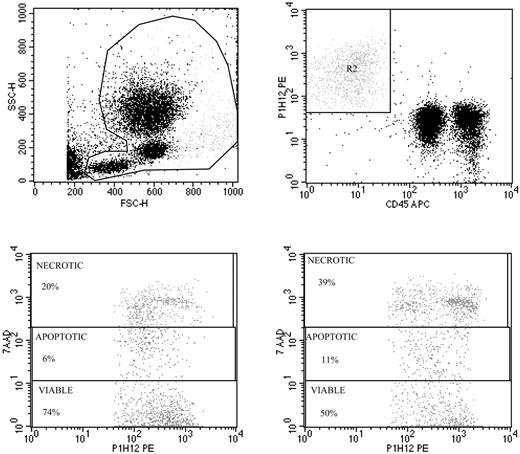
Validation of viable and apoptotic CEC enumeration. Representative spiking of peripheral blood with human umbilical vein endothelial cells (HUVECs) used to validate CEC enumeration by flow cytometry (top panels), and evaluation of apoptosis by 7AAD in HUVECs exposed to FAS ligand (bottom panels). Top panels show the gate used to exclude platelets, dead cells, and debris (left panel), and enumeration of HUVECs depicted as CD45–, P1H12+ cells (right panel). The detection limit of our procedure was 0.1 cells/μL, and specificity was more than 90%. Repeated CEC measurements in blood samples from more than 30 healthy subjects over time have shown a less than 14% variability. Panels of the bottom show representative 7AAD staining of HUVECs before (left) and after (right) exposure to FAS ligand.
Validation of viable and apoptotic CEC enumeration. Representative spiking of peripheral blood with human umbilical vein endothelial cells (HUVECs) used to validate CEC enumeration by flow cytometry (top panels), and evaluation of apoptosis by 7AAD in HUVECs exposed to FAS ligand (bottom panels). Top panels show the gate used to exclude platelets, dead cells, and debris (left panel), and enumeration of HUVECs depicted as CD45–, P1H12+ cells (right panel). The detection limit of our procedure was 0.1 cells/μL, and specificity was more than 90%. Repeated CEC measurements in blood samples from more than 30 healthy subjects over time have shown a less than 14% variability. Panels of the bottom show representative 7AAD staining of HUVECs before (left) and after (right) exposure to FAS ligand.
Tumor-bearing NOD/SCID mice (n = 12 per study group), were treated with CTX at the maximum tolerable dose (MTD) for NOD/SCID mice (75 mg/kg on days 3, 5, and 7; or 225 mg/kg per cycle of therapy repeated every 21 days13 ) or by using 2 metronomic low-dose schedules of CTX: the first according to Browder et al7 (170 mg/kg every 6 days) intraperitoneally in a site remote from the inoculated tumor; and the second according to Man et al9 using CTX in drinking water (approximately 20 mg/kg/d). All procedures involving animals were done in accordance with national and international laws and policies.
For CEC evaluation, mice were bled from the retro-orbital sinus. Following previously published flow cytometry protocol similar to the one used for the patients,4,6 monoclonal antibodies reacting with CD45 were used to exclude hematopoietic cells; CECs and CEPs were depicted as described previously5,13,14 using the endothelial murine markers vascular endothelial growth factor (VEGF) receptor 2 fetal liver kinase 1 (FLK-VEGFR2), CD13, and CD117 (BD). After red cell lysis, cell suspensions were evaluated by a FACSCalibur (BD) using analysis gates designed to exclude dead cells, platelets, and debris. After acquisition of at least 100 000 cells per sample, analyses were considered as informative when adequate numbers of events (ie, > 50, typically 100-200 events) were collected in the CEC and CEP enumeration gates. Percentages of stained cells were determined and compared with appropriate negative controls. Positive staining was defined as being greater than nonspecific background staining, and 7AAD used to enumerate viable, apoptotic, and dead cells.12
Statistical analysis
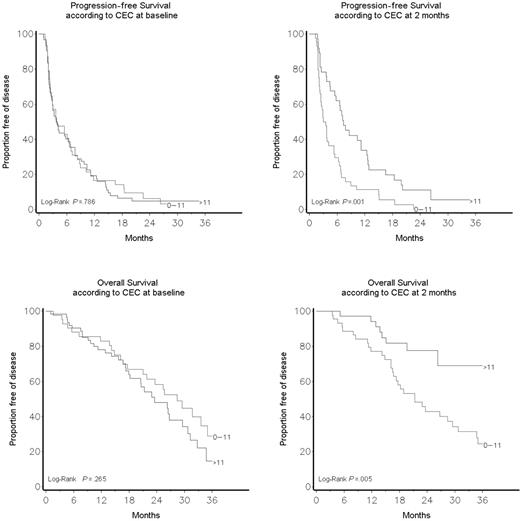
Statistical comparisons were performed using the t test, analysis of variance (ANOVA), and linear regression when data were normally distributed and the nonparametric analyses of Spearman and Mann-Whitney when data were not normally distributed. The Fisher exact test and the Mantel-Haenszel chi-square test for trend were used to assess the association between categorical and ordinal variables and treatment. Survival plots were drawn using the Kaplan-Meier method. The log-rank test was used to assess the survival difference between strata (Figure 4). The study was originally designed to detect differences in VEGF levels and not to detect differences in clinical benefit, an innovative concept originally used in clinical studies about endocrine therapy in advanced breast cancer. The response duration was measured from the date of achievement of response. Confidence intervals for the response rates were calculated using exact binomial methods. All tests were 2 sided. P values lower than .05 were considered as statistically significant.
Results
CEC kinetics correlate with progression-free and overall survival
There were 3 complete tumor remissions (CRs) in arm A (cyclophosphamide and methotrexate) and 3 in arm B (cyclophosphamide and methotrexate plus thalidomide), 15 partial tumor remissions (PRs) in arm A and 7 in arm B for an overall objective tumor responses of 20.9% (95% confidence intervals [CIs], 12.9%-31.0%) in arm A and 11.8% (95% CI, 5.8%-20.6%) in arm B. The clinical benefit (CR + PR + SD > 24 weeks) was 41.5% for both arms. Toxicity was generally mild. There was neither clinical benefit nor effect on CEC or CEP count and viability in patients receiving thalidomide in addition to the CM regimen (data not shown). These clinical data are described in detail elsewhere,16 and are beyond the scope of this work.
Progression-free and overall survival according to CEC count at baseline and after 2 months of therapy.
Progression-free and overall survival according to CEC count at baseline and after 2 months of therapy.
CECs and CEPs were evaluated at baseline in 104 of 171 enrolled patients (Figures 1, 2, 3). Of these 104 patients who had CECs and CEPs measured at baseline, 81 had also CECs and CEPs measured after 2 months of therapy. The reason for the reduction of the number of evaluated patients from baseline to 2 months was—in the large majority of patients—the lack of collection and/or shipment of blood samples to the laboratory. It was not related to the patients' characteristics, to the stage of the disease, to treatment modalities, or to baseline findings, and therefore did not prejudice the value of the results, but only reduced the statistical power of the study.
CEPs were always less than 5% of the CEC population, and no correlation was found between CEP number and the clinical outcome (data not shown). Data were evaluated also to see whether the total number of CD133+ progenitor cells (independently from CD45 and/or P1H12 expression) correlated with the clinical outcome, and, again, no correlation was found.
A CEC count greater than 11/μL (the 95% CI of CEC distribution in healthy controls4 ) after 2 months of therapy was associated with a significantly prolonged overall survival (P = .005). Table 1 shows patients' characteristics according to CEC count at baseline and after 2 months of therapy. A CEC count greater than 11/μL at baseline was associated with a higher number of metastatic sites (P = .04) and a higher frequency of lung involvement (P = .009). Significant correlations were found between lung metastases and more than 11 CECs/μL at baseline, and between the finding of 3 or more metastatic sites and less than 11 CECs/μL at baseline. No significant differences were found in age, performance status, number of previous treatment lines, hormone receptor, Ki67, Her-2/Neu, pT, pN, or epidermal growth factor receptor status.
Patient characteristics and stratification according to CEC count
. | CEC at baseline . | . | . | CEC at 2 mo . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 11/μL or less . | More than 11/μL . | P . | 11/μL or less . | More than 11/μL . | P . | ||||
| No. entered or eligible | 42 | 62 | 44 | 37 | ||||||
| Median age, y (range) | 54 (32-78) | 54 (34-78) | .71 | 53 (36-76) | 55 (34-73) | .87 | ||||
| Progressive disease at study entry, no. | ||||||||||
| No | 14 | 19 | 15 | 7 | ||||||
| Yes | 28 | 43 | .83 | 29 | 30 | .13 | ||||
| Baseline performance status, no. | ||||||||||
| 0 | 37 | 53 | 36 | 32 | ||||||
| 1 | 5 | 9 | .78 | 8 | 5 | .76 | ||||
| Pretreatment (chemotherapy), no. | ||||||||||
| No | 17 | 20 | 22 | 18 | ||||||
| Yes | 25 | 42 | .41 | 22 | 19 | > .999 | ||||
| Metastatic sites, no. | ||||||||||
| 1 | 17 | 34 | 22 | 22 | ||||||
| 2 | 13 | 21 | 14 | 15 | ||||||
| 3+ | 12 | 7 | .04* | 8 | 0 | .06 | ||||
| Tumor sites, no. | ||||||||||
| Lung | 4 | 20 | .009* | 12 | 7 | .44 | ||||
| Liver | 21 | 24 | .31 | 20 | 10 | .11 | ||||
| Bone | 26 | 26 | .07 | 19 | 14 | .66 | ||||
| Other sites | 24 | 23 | .048* | 19 | 19 | .51 | ||||
| Estrogen receptors, no. | ||||||||||
| Absent | 16 | 24 | 16 | 12 | ||||||
| Present | 23 | 32 | > .999 | 26 | 21 | > .999 | ||||
| Progesterone receptors, no. | ||||||||||
| Absent | 22 | 27 | 19 | 16 | ||||||
| Present | 17 | 28 | .53 | 21 | 15 | .81 | ||||
| Ki67, no. | ||||||||||
| Less than 20% | 14 | 18 | 9 | 14 | ||||||
| 20% or more | 8 | 9 | > .999 | 7 | 2 | .11 | ||||
| Her-2/Neu, no. | ||||||||||
| 0/+/++ | 24 | 27 | 17 | 18 | ||||||
| +++ | 2 | 6 | .45 | 4 | 2 | .66 | ||||
| pT, no. | ||||||||||
| pT1 | 6 | 17 | 11 | 8 | ||||||
| pT2 | 14 | 20 | 12 | 12 | ||||||
| pT3-4 | 4 | 4 | .17 | 4 | 1 | .70 | ||||
| pN, no. | ||||||||||
| pNo | 4 | 14 | 8 | 5 | ||||||
| pN1-pN2 | 17 | 27 | .25 | 21 | 14 | > .999 | ||||
| Epidermal growth factor receptor at baseline, no. | ||||||||||
| Less than 52 fmol/mg | 27 | 5 | 11 | 21 | ||||||
| Greater than or equal to 52 fmol/mg | 27 | 10 | .38 | 20 | 22 | .34 | ||||
| Median (range) | 56 (38-71) | 52 (39-64) | .07 | 52 (34-64) | 55 (39-71) | .12 | ||||
. | CEC at baseline . | . | . | CEC at 2 mo . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 11/μL or less . | More than 11/μL . | P . | 11/μL or less . | More than 11/μL . | P . | ||||
| No. entered or eligible | 42 | 62 | 44 | 37 | ||||||
| Median age, y (range) | 54 (32-78) | 54 (34-78) | .71 | 53 (36-76) | 55 (34-73) | .87 | ||||
| Progressive disease at study entry, no. | ||||||||||
| No | 14 | 19 | 15 | 7 | ||||||
| Yes | 28 | 43 | .83 | 29 | 30 | .13 | ||||
| Baseline performance status, no. | ||||||||||
| 0 | 37 | 53 | 36 | 32 | ||||||
| 1 | 5 | 9 | .78 | 8 | 5 | .76 | ||||
| Pretreatment (chemotherapy), no. | ||||||||||
| No | 17 | 20 | 22 | 18 | ||||||
| Yes | 25 | 42 | .41 | 22 | 19 | > .999 | ||||
| Metastatic sites, no. | ||||||||||
| 1 | 17 | 34 | 22 | 22 | ||||||
| 2 | 13 | 21 | 14 | 15 | ||||||
| 3+ | 12 | 7 | .04* | 8 | 0 | .06 | ||||
| Tumor sites, no. | ||||||||||
| Lung | 4 | 20 | .009* | 12 | 7 | .44 | ||||
| Liver | 21 | 24 | .31 | 20 | 10 | .11 | ||||
| Bone | 26 | 26 | .07 | 19 | 14 | .66 | ||||
| Other sites | 24 | 23 | .048* | 19 | 19 | .51 | ||||
| Estrogen receptors, no. | ||||||||||
| Absent | 16 | 24 | 16 | 12 | ||||||
| Present | 23 | 32 | > .999 | 26 | 21 | > .999 | ||||
| Progesterone receptors, no. | ||||||||||
| Absent | 22 | 27 | 19 | 16 | ||||||
| Present | 17 | 28 | .53 | 21 | 15 | .81 | ||||
| Ki67, no. | ||||||||||
| Less than 20% | 14 | 18 | 9 | 14 | ||||||
| 20% or more | 8 | 9 | > .999 | 7 | 2 | .11 | ||||
| Her-2/Neu, no. | ||||||||||
| 0/+/++ | 24 | 27 | 17 | 18 | ||||||
| +++ | 2 | 6 | .45 | 4 | 2 | .66 | ||||
| pT, no. | ||||||||||
| pT1 | 6 | 17 | 11 | 8 | ||||||
| pT2 | 14 | 20 | 12 | 12 | ||||||
| pT3-4 | 4 | 4 | .17 | 4 | 1 | .70 | ||||
| pN, no. | ||||||||||
| pNo | 4 | 14 | 8 | 5 | ||||||
| pN1-pN2 | 17 | 27 | .25 | 21 | 14 | > .999 | ||||
| Epidermal growth factor receptor at baseline, no. | ||||||||||
| Less than 52 fmol/mg | 27 | 5 | 11 | 21 | ||||||
| Greater than or equal to 52 fmol/mg | 27 | 10 | .38 | 20 | 22 | .34 | ||||
| Median (range) | 56 (38-71) | 52 (39-64) | .07 | 52 (34-64) | 55 (39-71) | .12 | ||||
pT and pN refer to the TNMUICC classification on primary tumory regional nodes and metastases. The definition of characteristics of the disease is focused on features related to the primary tumor (T), to the regional lymph nodes—including those of the ipsilateral axilla, supraclavicular, and internal mammary sites (N)—and of distant metastases.
As shown in Figure 4 and Table S1 (available on the Blood website; see the Supplemental Table link at the top of the online article), there was no correlation between baseline CEC count and response to therapy, and overall and progression-free survivals. On the other hand, there was a significantly better global response (P = .03) and a significantly improved clinical benefit (SD > 24 weeks + PR + CR) in patients who had a CEC count greater than 11/μL after 2 months of therapy (64.9% vs 29.6%, P = .002; Table 2). After a median follow-up of 17.4 months (range, 1.1-44.6 months), univariate and multivariate analyses (Table 3) indicated that patients who had a CEC count greater than 11/μL after 2 months of therapy had a significantly improved progression-free (P = .001) and overall survival (P = .005; Figure 4). Tertile CEC values after 4 weeks of therapy were still associated with significant differences in survival (data not shown).
Association between CECs at 2 months and response to therapy
. | . | CECs at 2 mo . | . | . | |
|---|---|---|---|---|---|
. | No. patients . | 11/μL or less, no. (%) . | More than 11/μL, no. (%) . | P . | |
| Global response | |||||
| Complete response | 5 | 1 (2.3) | 4 (10.8) | ||
| Partial response | 13 | 6 (13.6) | 7 (18.9) | ||
| Stable disease | 32 | 16 (36.4) | 16 (43.2) | ||
| Progressive disease | 31 | 21 (47.7) | 10 (27.0) | .03* | |
| CR + PR | 18 | 7 (15.9) | 11 (29.7) | ||
| SD + PD | 63 | 37 (84.1) | 26 (70.3) | .18 | |
| CR + PR + SD | 50 | 23 (52.3) | 27 (73.0) | ||
| PD | 31 | 21 (47.7) | 10 (27.0) | .07 | |
| Clinical benefit | |||||
| SD > 24 wk + PR + CR | 37 | 13 (29.6) | 24 (64.9) | ||
| SD < 24 wk + PD | 44 | 31 (70.4) | 13 (35.1) | .002* | |
| Response at 2 mo | |||||
| Response | 30 | 14 (34.2) | 16 (48.5) | ||
| Stable disease | 22 | 12 (29.3) | 10 (30.3) | ||
| Progression | 22 | 15 (36.6) | 7 (21.2) | .13 | |
. | . | CECs at 2 mo . | . | . | |
|---|---|---|---|---|---|
. | No. patients . | 11/μL or less, no. (%) . | More than 11/μL, no. (%) . | P . | |
| Global response | |||||
| Complete response | 5 | 1 (2.3) | 4 (10.8) | ||
| Partial response | 13 | 6 (13.6) | 7 (18.9) | ||
| Stable disease | 32 | 16 (36.4) | 16 (43.2) | ||
| Progressive disease | 31 | 21 (47.7) | 10 (27.0) | .03* | |
| CR + PR | 18 | 7 (15.9) | 11 (29.7) | ||
| SD + PD | 63 | 37 (84.1) | 26 (70.3) | .18 | |
| CR + PR + SD | 50 | 23 (52.3) | 27 (73.0) | ||
| PD | 31 | 21 (47.7) | 10 (27.0) | .07 | |
| Clinical benefit | |||||
| SD > 24 wk + PR + CR | 37 | 13 (29.6) | 24 (64.9) | ||
| SD < 24 wk + PD | 44 | 31 (70.4) | 13 (35.1) | .002* | |
| Response at 2 mo | |||||
| Response | 30 | 14 (34.2) | 16 (48.5) | ||
| Stable disease | 22 | 12 (29.3) | 10 (30.3) | ||
| Progression | 22 | 15 (36.6) | 7 (21.2) | .13 | |
Univariate and multivariate analysis: overall survival and disease-free survival
. | OS . | . | . | . | DFS . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Univariate . | . | Multivariate . | . | Univariate . | . | Multivariate . | . | ||||||
. | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | ||||||
| CECs at 2 mo | ||||||||||||||
| More than 11/μL | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| 0-11/μL | 2.76 (1.26-6.04) | .01* | 2.30 (1.02-5.19) | .04* | 1.93 (1.20-3.13) | .007* | 1.96 (1.19-3.24) | .009* | ||||||
| No. of metastatic sites | ||||||||||||||
| 1-2 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| 3+ | 2.65 (1.16-6.06) | .02* | 1.79 (0.76-4.23) | .18 | 1.33 (0.60-2.91) | .48 | 0.90 (0.39-2.06) | .80 | ||||||
| Liver metastasis | ||||||||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Yes | 1.89 (0.99-3.60) | .05 | 1.60 (0.83-3.07) | .16 | 1.12 (0.69-1.83) | .65 | 1.08 (0.66-1.77) | .77 | ||||||
. | OS . | . | . | . | DFS . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Univariate . | . | Multivariate . | . | Univariate . | . | Multivariate . | . | ||||||
. | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | ||||||
| CECs at 2 mo | ||||||||||||||
| More than 11/μL | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| 0-11/μL | 2.76 (1.26-6.04) | .01* | 2.30 (1.02-5.19) | .04* | 1.93 (1.20-3.13) | .007* | 1.96 (1.19-3.24) | .009* | ||||||
| No. of metastatic sites | ||||||||||||||
| 1-2 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| 3+ | 2.65 (1.16-6.06) | .02* | 1.79 (0.76-4.23) | .18 | 1.33 (0.60-2.91) | .48 | 0.90 (0.39-2.06) | .80 | ||||||
| Liver metastasis | ||||||||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Yes | 1.89 (0.99-3.60) | .05 | 1.60 (0.83-3.07) | .16 | 1.12 (0.69-1.83) | .65 | 1.08 (0.66-1.77) | .77 | ||||||
HR indicates hazard ratio.
All the results in the table (HR and P) are obtained from a Cox proportional hazard regression model. The first 2 colums are from a univariate model; the next 2 columns are from a multivariate model.
Apoptotic CECs, most likely released from tumor vessels, are the main contributors to the CEC count increases in responding patients
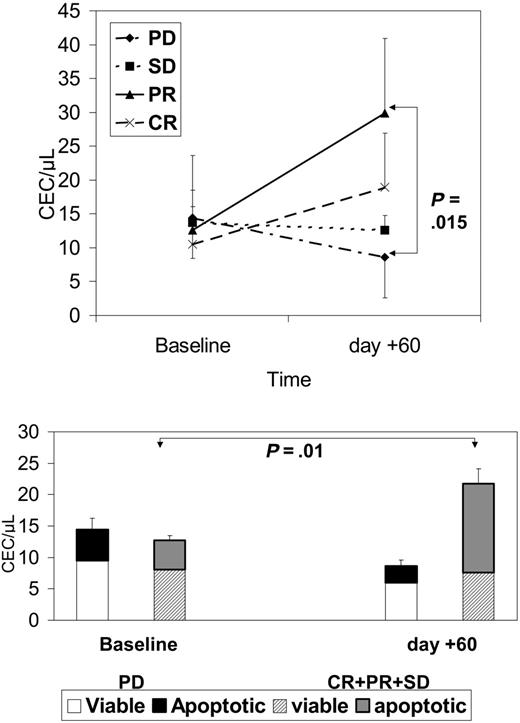
As shown in Figure 5, after 2 months of therapy there was a significant decrease in CEC count in patients without a clinical benefit. The rise in patients with a clinical benefit was generated by a significantly larger proportion of apoptotic CECs circulating in the blood of responders.
To explain the mechanism involved in detection of elevated apoptotic CECs in patients who respond to the treatment, and to determine whether this rise in apoptotic CECs was due to a release from the blood vessels in tumors or from some other source, we determined the levels of apoptotic CECs in normal or tumor-bearing mice treated with metronomic chemotherapy regimens. We enumerated CECs and measured their viability in 4 preclinical cancer models previously developed in our laboratory, and treated with MTD or metronomic chemotherapy regimens.
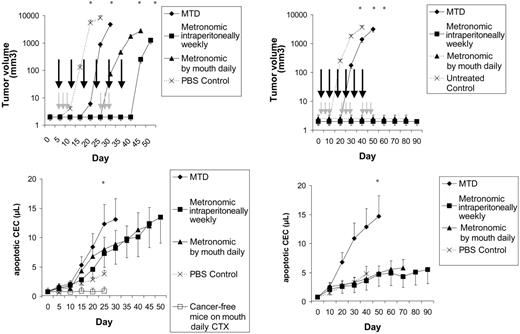
First, NOD/SCID mice were given injections of Namalwa or Granta 519 (Figure 6) cancer cells. We have studied these orthotopic models in the past for viable CEC/CEP kinetics and for sensitivity to metronomic chemotherapy.5,13,14 In these models, 2 metronomic regimens were associated with a release of apoptotic CECs in the peripheral blood (Figure 6). These CECs were most likely released from tumors, because in cancer-free mice receiving metronomic chemotherapy CEC count was stable (P < .01 vs cancer-bearing animals).
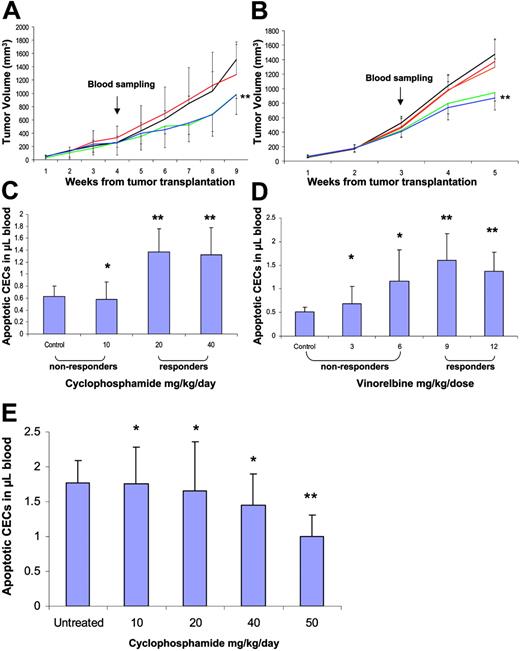
In separate studies, mice that received transplants of either MeWo human melanoma or a metastatic variant (LM2-4) of the MDAMB-231 human breast cancer were treated with escalating metronomic doses of CTX and Vinorelbine, respectively. We chose to escalate the metronomic doses in order to mimic a treatment process of responders and nonresponders. We reasoned that mice receiving lower than the optimal biological doses would not respond to the treatment, whereas those receiving the optimal dose or higher would show a tumor response, and respond to the treatment as previously reported for various metronomic chemotherapy regimens.17 The results in Figure 7 show that only when tumor-bearing mice were treated with the optimal or higher doses was a significant increase in the apoptotic CECs observed using 1-way ANOVA followed by the Student-Newman-Keuls test, similar to the results obtained with both Namalwa and Granta 519 human lymphoma models (Figure 6). However, when mice (in both tumor models) were treated using doses lower than the optimal dose where there was no effect on tumor growth control, significant increases in apoptotic CEC levels were not observed. As shown in Figure 7E, apoptotic CEC count was not increased in nontumor-bearing BALB/c mice treated with 10 to 40 mg/kg/d CTX for 1 week. The reduction in CEC count observed in mice treated with 50 mg/kg/d CTX is most likely due to the severe myelosuppression already described at this dose.17 These results reinforce our clinical data and provide a potential mechanism for the increase in apoptotic CECs.
CECs at baseline and after 2 months of therapy in patients with PD, SD, PR, or CR. Top panel shows absolute CEC counts; bottom panel shows how the fraction of apoptotic CECs contributed to the absolute CEC count in patients with or without clinical benefit. Results are expressed as mean ± SD.
CECs at baseline and after 2 months of therapy in patients with PD, SD, PR, or CR. Top panel shows absolute CEC counts; bottom panel shows how the fraction of apoptotic CECs contributed to the absolute CEC count in patients with or without clinical benefit. Results are expressed as mean ± SD.
CEC kinetics and viability in lymphoma-bearing mice. Left panels show tumor volume, day of MTD (gray arrows) and metronomic (black arrows) treatments, and CEC count in NOD/SCID mice that had been given injections of Namalwa and in cancer-free, control animals. Results are expressed as mean ± 1 SD (bars). n = 12 per study group. Right panels show tumor volume, day of MTD (gray arrows) and metronomic (black arrows) treatments, and CEC count in NOD/SCID mice that had been given injections of Granta 519. Results are expressed as mean ± 1 SD (bars). n = 12 per study group. *P < .01.
CEC kinetics and viability in lymphoma-bearing mice. Left panels show tumor volume, day of MTD (gray arrows) and metronomic (black arrows) treatments, and CEC count in NOD/SCID mice that had been given injections of Namalwa and in cancer-free, control animals. Results are expressed as mean ± 1 SD (bars). n = 12 per study group. Right panels show tumor volume, day of MTD (gray arrows) and metronomic (black arrows) treatments, and CEC count in NOD/SCID mice that had been given injections of Granta 519. Results are expressed as mean ± 1 SD (bars). n = 12 per study group. *P < .01.
CEC kinetics and viability in melanoma- and breast cancer–bearing mice. Six-week-old mice received transplants of either a human melanoma cell line (MeWo) (A) or a metastatic breast cancer cell line (LM2-4) (B). Tumors were monitored on a weekly basis. When tumors reached 200 mm3, mice were treated with 0, 10, 20, or 40 mg/kg/d of cyclophosphamide (black, red, green, and blue, respectively), or 0, 3, 6, 9, or 12 mg/kg 3 times a week of vinorelbine (black, red, orange, green, and blue, respectively). After 1 week of treatment, mice were bled from the retro-orbital sinus and blood was assessed for the levels of apoptotic CECs (panels C and D, respectively). Panel E shows apoptotic CEC counts in nontumor-bearing BALB/c mice treated with CTX for 1 week. The reduction in CEC count observed in mice treated with 50 mg/kg/d CTX is most likely due to the severe myelosuppression described in this same model by Shaked et al.17 *P > .05 indicates nonsignificant; **P < .05 indicates statistically significant from control using t test statistical analysis. Results are expressed as mean ± SD.
CEC kinetics and viability in melanoma- and breast cancer–bearing mice. Six-week-old mice received transplants of either a human melanoma cell line (MeWo) (A) or a metastatic breast cancer cell line (LM2-4) (B). Tumors were monitored on a weekly basis. When tumors reached 200 mm3, mice were treated with 0, 10, 20, or 40 mg/kg/d of cyclophosphamide (black, red, green, and blue, respectively), or 0, 3, 6, 9, or 12 mg/kg 3 times a week of vinorelbine (black, red, orange, green, and blue, respectively). After 1 week of treatment, mice were bled from the retro-orbital sinus and blood was assessed for the levels of apoptotic CECs (panels C and D, respectively). Panel E shows apoptotic CEC counts in nontumor-bearing BALB/c mice treated with CTX for 1 week. The reduction in CEC count observed in mice treated with 50 mg/kg/d CTX is most likely due to the severe myelosuppression described in this same model by Shaked et al.17 *P > .05 indicates nonsignificant; **P < .05 indicates statistically significant from control using t test statistical analysis. Results are expressed as mean ± SD.
Discussion
The concepts of induction of dose-limiting toxicity (DLT) and “objective tumor response” have driven early clinical anticancer chemotherapy drug development for decades. Alone, these tools might not be sufficient to evaluate the early clinical development of the new generation of antiangiogenic, antistroma, or other types of molecularly targeted drugs (especially in phase 1 monotherapy trials), since such drugs frequently have optimal therapeutic activity (in terms of tumor shrinkage or stable disease) below a maximum tolerated dose, when such a dose can be defined.1-3 New surrogate pharmacodynamic markers of drug activity are needed, and in this regard a simple blood test would be ideal.3,14,16-18
After the observations were noted that CECs are increased in the peripheral blood of cancer patients at diagnosis, and that CEC numbers were reduced to normal values in patients achieving a CR,4 we investigated in preclinical models whether CEC number and/or viability were modulated by drugs with antiangiogenic activity. Using either MTD CTX or endostatin as paradigms of drugs with tumor-cell cytotoxic versus endothelium-restricted activity, we observed that in mice given CTX at the MTD, most of the circulating apoptotic cells were hematopoietic and not endothelial in nature, and a relevant proportion of CECs were still viable. In contrast, in mice given endostatin, all of the increase in circulating apoptotic cells was in the endothelial cell compartment, and most CECs were apoptotic or dead.5 These observations suggested that CEC count and viability might have a predictive potential in clinical trials involving antiangiogenic treatment strategies.
Tumor vessel inhibition and/or regression can be achieved using drugs with a selective and restrictive action on tumor vessels, or by long-term administration of low doses of chemotherapy drugs such as CTX, methotrexate, or others at close regular intervals.7-10 In preclinical models, this latter therapeutic strategy, called “metronomic chemotherapy,” can sometimes actually induce regression of tumors resistant to the same drugs given at the MTD.7 When the same drug was given at a lower, more frequent dose, cancer regression was attributed to the inhibition of the drug-sensitive endothelial cell compartment of the tumors. We have shown recently that low-dose CTX and methotrexate can induce tumor regression in some patients with advanced breast cancer who were heavily pretreated with chemotherapy, and that this is associated with only minimal host toxicity.8
In the present study, univariate and multivariate analyses found that in patients with advanced breast cancer receiving metronomic chemotherapy, the CEC count after 2 months of therapy was a particularly good predictor of disease-free and overall survival after a prolonged follow-up for more than 2 years. Patients who had a CEC count greater than 11/μL after 2 months of metronomic chemotherapy had a significantly improved survival. Consistent with what we have previously seen in our preclinical studies,5 in patients with a clinical benefit the increase in CEC count was mostly due to an increase in their apoptotic fraction.
We previously described13 that in some preclinical models metronomic chemotherapy suppressed the levels of viable CECs and CEPs. In the present study, we have extended the parameters of CEC evaluation and found a substantial increase in apoptotic CECs, similar to the human clinical trial. Of note, in the same preclinical paper,13 we demonstrated that an MTD regimen of the same drug causes a short-time suppression of viable CECs and CEPs immediately after drug administration, which is followed by a robust increase in viable cells. Thus, an MTD regimen indeed has a possible antiangiogenic effect, at least in the beginning of each treatment cycle, which is similar to metronomic chemotherapy. But, it is very ephemeral. However, as opposed to MTD, metronomic chemotherapy regimen maintains the low levels of viable CECs for a long period of time.
We analyzed preclinical models of cancer-bearing and cancer-free animals to address the question of whether the rise in apoptotic CECs was due to endothelial cells from the tumor vasculature, from the normal vasculature, or from both. In mice receiving phosphate-buffered saline (PBS) and in mice receiving MTD CTX the rise in CEC count paralleled the rise in tumor growth (r = .711), whereas in mice receiving metronomic CTX, the rise in CECs was observed on day 15 onward despite an absence of tumor growth (P < .001 vs mice receiving MTD CTX). Thus, these results suggest different mechanisms for the rise of CEC count in mice in which enlarging tumors were escaping from MTD chemotherapy compared with mice in which metronomic therapy was able to control tumor growth. Also, one of the possible effects of the antiangiogenic activity of metronomic chemotherapy might be on tumor vessel remodeling, the so-called “vessel normalization” hypothesis.19 In this case, part of the remodeling process would likely cause a shedding of apoptotic endothelial cells from cancer vessels. The lack of an apoptotic CEC rise in cancer-free animals suggests that the cancer-associated vasculature is most likely the sole or predominant source of the rise in apoptotic CECs seen in our patients. The mechanisms of this release deserve further investigation, but the drop of circulating VEGF levels observed in patients with clinical benefit,16 might explain at least in part the increase in apoptotic CECs seen in these patients. Other mechanisms include direct killing of endothelial cells by prolonged exposure to metronomic chemotherapy or induction of thrombospondin-1.9,10,13,14,16-21
In the present clinical study, patients with clinical benefit had an increase in the apoptotic CEC count during therapy. A CEC increase has been already reported in cancer-bearing animals treated with some antiangiogenic drugs18 and in clinical data22 (John V. Heymach, personal communication, October 2005). Clinical studies in the field measured CECs after therapy and not during therapy as in the current study. Moreover, virtually all previous preclinical and clinical studies in the field investigated the number of viable CECs or the total number of CECs without discriminating between viable or apoptotic cells. In other preclinical and clinical studies, the use of antiangiogenic drugs or other therapeutic strategies was associated with either an increase18 or a decrease in CEC counts or in the CEC subpopulation expressing a progenitor phenotype.13,14,16,20-22 There are several different possible explanations for these findings. First, there is evidence that different compartments contribute to the total CEC count. A compartment is provided by the tumor vasculature, another by the normal vasculature, and a third by release from endothelial progenitor cell niches, most likely the bone marrow. The seminal recent study by Beaudry et al18 has already indicated that antiangiogenic drugs might have dual effects on these compartments, such as the enhancement in CEC counts paralleling the inhibition of marrow-derived endothelial progenitors observed in mice treated with the small-molecule VEGFR-2 antagonist, ZD6474.18 Second, some of these studies focused only on viable CECs while excluding apoptotic CECs from the count.23
In conclusion, our data indicate that CEC count and viability are very promising tools to select patients with cancer who might benefit from the new generation of antiangiogenic therapies that are entering the clinical arena. Considering the highly promiscuous phenotype of CECs and of their progenitor subpopulation,4,24 and that the currently available studies were carried out with 4-color flow cytometry, the recent availability of 6-color flow cytometry might represent a major step forward in this area. In fact, 6-color flow cytometry will allow the simultaneous investigation of a larger number of relevant parameters such as CEC expression of activation (eg, CD105) or lineage-specific (eg, VEGFR3) antigens that might offer significantly more insights into their biology, and potential as useful surrogate pharmacodynamic markers of drug activity and early tumor responsiveness/patient benefit.
Prepublished online as Blood First Edition Paper, March 16, 2006; DOI 10.1182/blood-2005-11-4570.
Supported in part by AIRC (Associazione Italiana per la Ricerca sul Cancro), ISS (Istituto Superiore di Sanità), and the sixth EU Framework Programme (Integrated Project “Angiotargeting” contract no. 504743) in the area of “Life sciences, genomics and biotechnology for health.” F.B. is a scholar of the US National Blood Foundation. R.S.K. is a recipient of a Tier I Canada Research Chair and is supported by a grant from the National Institutes of Health (CA-41223) and the Canadian Institute for Health Research (CIHR).
M.C., A.G., R.S.K., and F.B. designed the research; P.M., M.C., A.C., L.O., G.P., A.A., and Y.S. performed the research; P.M. analyzed data; and P.M., Y.S., R.S.K., and F.B. wrote the paper.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal