Abstract
Neutrophil transmigration into tissue is a multiple-step process that results from a coordinated rearrangement of the cytoskeleton and adhesion complexes. Assembly and disassembly of actin and adhesion structures dictate motility behavior, while polarity and gradient sensing provide directionality to the cell movement. Here, using mice deficient in the CDC42 regulator CDC42 GTPase-activating protein (CDC42GAP), we demonstrate that CDC42 activity separately regulates neutrophil motility and directionality. CDC42GAP–/– neutrophils showed increased motility, while directed migration was defective. Podosome-like structures present at the leading edge in wild-type neutrophils were significantly reduced in CDC42GAP–/– cells. CDC42GAP–/– neutrophils also showed increased lateral and tail filopodia-like formation, and excess membrane protrusions. We further suggest that CDC42GAP-mediated extracellular signal–regulated kinase (ERK) activity regulates motility associated with podosome-like structures at the cell leading edge, while CDC42GAP-induced p38MAPK phosphorylation regulates directed migration by antagonizing filopodia assembly. Overall, this study reveals that CDC42 activity regulates both motility and directionality in neutrophils, but via distinct mitogen-activated protein kinase (MAPK) pathways.
Introduction
Neutrophils are critical in the inflammatory process. The recruitment of neutrophils to sites of inflammation requires a series of highly regulated adhesive and chemotactic events. These cellular activities result from the activation of signals from various receptors, including adhesion molecules such as selectins and integrins, and chemokine receptors. Failure to regulate any of these events may lead to abnormal innate immune responses, including immunodeficiency or aberrant inflammatory reactions. Although the process of neutrophil extravasation has been well studied, the intracellular molecular events that ultimately regulate these processes still remain to be understood in detail, particularly in physiologic settings.
Cell migration is a multiple-step process that results from a coordinated rearrangement of the cytoskeleton and adhesion complexes.1 Upon chemoattractant stimulation, cells polarize and form lamellipodia containing F-actin at the leading edge oriented toward the source of stimulation and a contractile uropod. The lamellipodia are stabilized to the substratum by the assembly of adhesion complexes, which mature into focal adhesions. These complexes serve as traction forces necessary for the translocation of the cell body. Completion of cell migration requires actin disruption to allow both adhesion complex turnover at the leading edge and retraction of the tail. Thus, the cytoskeleton, through assembly and disassembly of F-actin and adhesion structures, is believed to dictate motility behavior, while polarity and gradient sensing give direction to the cell movement.
The key integrators of signals emanating from chemokine receptors and integrin molecules that coordinate these processes are members of the small Rho GTPase family, including Rac, CDC42, and Rho.2 Rho GTPases cycle between inactive GDP-bound and active GTP-bound forms. Guanine nucleotide exchange factors (GEFs) promote the activation of GTPases by stimulating the exchange of GDP to GTP, while GTPase-activating proteins (GAPs) accelerate hydrolysis of GTP, returning the GTPase to an inactive form.3 Activation of CDC42 controls the formation of filopodia and is critical for gradient sensing.4 Rac induces membrane protrusion via lamellipodia.4 In addition, both Rac and CDC42 regulate the formation of focal complexes that stabilize the leading edge of the cell. Activation of Rho generates contractility via stress fiber and focal adhesions.4,5 The details of the regulation of cell migration are highly cell-type specific. The use of gene-targeted mice provides an approach to study the role of Rho GTPases in primary cells in a lineage-specific fashion and has provided important information regarding the physiologic function of these proteins in multiple blood cell types.6-16
CDC42 is a well-known regulator of cell chemotaxis. In the neutrophil cell line HL60, or in macrophages, CDC42 inhibitors prevent cells from maintaining polarity and a persistent leading edge.17,18 In addition, CDC42 inhibitors induce formation of multiple short leading edges containing filamentous actin.18 These observations suggest that CDC42 activity is required to properly orient the cytoskeleton responses with respect to the direction of the chemoattractant. Using a mouse genetically deficient in the CDC42 GEF PIXα, which leads to loss of CDC42 activity, Li et al19 demonstrated that, upon activation of G-coupled receptors, free Gβ binds to p21-activated kinase (PAK), which then interacts with PIXα. This binding stimulates CDC42 activity and, in turn, leads to activation of PAK. The PIXα/PAK/CDC42 axis is essential for direction sensing and persistent polarized migration toward the source of stimulation by stimulating assembly of F-actin at the leading edge of the cell and by regulating the localization of an antagonist of F-actin assembly, PTEN, at the rear of the cell.19 Thus, CDC42 activity serves as a gradient sensor during neutrophil chemotaxis. The best-studied CDC42 effectors are Wiskott-Aldrich syndrome protein (WASp) and PAK, both of which are known to be involved in cytoskeleton reorganization.4,20 Although considerable progress has been made in understanding the role of CDC42 in physiologic settings, very little is known about the role of CDC42 upon integrin engagement during neutrophil migration and the associated adhesive structures or the consequences of a hyperactivity of CDC42 in these cells.
We have recently demonstrated that mice deficient in the CDC42 regulator CDC42GAP, which represents the gain of CDC42 activity, display defects in hematopoietic stem cell migration, adhesion, and F-actin reorganization. These changes were associated with abnormal erythropoiesis.21 In the present study, we examined the consequences of gain of CDC42 activity on neutrophil migration and provide evidence that CDC42 activity is important during inflammatory responses downstream of integrin molecules by regulating both random movement and directed migration via distinct mitogen-activated protein kinase (MAPK) signaling pathways.
Materials and methods
Generation of animal model
Cells from day-14.5 fetal liver CDC42GAP–/– mice21 and wild-type (WT) littermates were transplanted into lethally irradiated C57BL/6 recipients (1175 cGy in a split dose; Jackson Laboratories, Bar Harbor, ME). Animals were used for experiments 5 weeks after bone marrow reconstitution. All animals were bred in the Cincinnati Children's Research Foundation pathogen-free animal facility. All experimental procedures were approved by the institutional animal committee.
Neutrophil migration in vivo
Mice were challenged with 3% thioglycollate (Sigma, St Louis, MO) by intraperitoneal injection (1 mL). Peritoneal lavages were performed 4 and 18 hours after challenge.6 Cells were counted by hemocytometer. Neutrophil content was evaluated after cytospin preparation of the cells and Diff Quick staining (Dade Berhing, Deerfield, IL).
Neutrophil isolation
Neutrophils were isolated from bone marrow cells by percoll gradient as previously described.6 Neutrophils were also generated after culture of low-density bone marrow cells as previously described.12,15 Neutrophil purity was similar between genotypes and estimated between 65% and 75% depending on experiments. No differences in the phenotype of the cells were observed between the 2 methods of neutrophil isolation.
Neutrophil migration assays in vitro
Neutrophil migration was evaluated in triplicate using a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MD) as described previously.6,12,15 In the chemotaxis assay, fMLP (1 μM; Sigma) is added in the lower chamber. In the chemokinesis assay, fMLP is added in the upper chamber with the cells and in the lower chamber. Numbers of migrated cells were determined by counting 3 randomly chosen fields.
Migration was also evaluated using 3-μm transwell chambers (Corning Inc, Corning, NY) coated with fibrinogen (25 μg/mL; Sigma) in chemokinesis and chemotaxis assays. The cells (4 × 105) were diluted in 100 μL Hanks balanced salt solution (HBSS; Invitrogen, Carlsbad, CA), 1 mM Ca2+, and 1 mM Mg2+ with or without 10 μM fMLP, and migration toward fMLP was allowed for 3 hours. The migrated cells in the bottom well were counted with a hemocytometer.
To examine neutrophil transendothelial migration, human umbilical vein endothelial cells (HUVECs) (4 × 103) were seeded in the upper chamber of the transwell, and the cells were grown for 4 days at 37°C until confluence. Neutrophils (5 × 105) in 100 μL HBSS, 1 mM Ca2+, and 1mM Mg2+ with or without 10 μM fMLP were allowed to migrate toward fMLP for 3 hours. The migrated cells in the bottom well were counted with a hemocytometer.
Time-lapse video microscopy was performed in a Zigmond chamber (Neuro Probe, Gaithersburg, MD). After adhesion onto glass cover slips, the cells were mounted onto the Zigmond chamber. Migration was allowed in a gradient of 10 μM fMLP diluted in HBSS, 1% gelatin, and 1mM HEPES (pH 7.6) for 30 minutes. Microscope images were recorded at 55 interval with a Zeiss microscope (Oberkochen, Germany) at 10 ×/0.3 NA oil objective magnification equipped with an ORCA-ER camera (Hamamatsu, Tokyo, Japan) and driven by Openlab software (Improvision, Lexington, MA). A translocation rate of 23 to 25 freshly isolated neutrophils, shown in Figure 2, or 50 neutrophils generated by in vitro culture (not shown) from each genotype and from independent videos were analyzed using Openlab.
Neutrophil adhesion assay
Neutrophils (2 × 105) were allowed to adhere to fibrinogen (25 μg/mL) for 30 minutes in HBSS, 1 mM Ca2+, and 1 mM Mg2+ with or without 10 μM fMLP at 37°C, as previously described.12
Integrin receptor expression
To determine β2-integrin expression upon stimulation, neutrophils (5 × 105) were incubated with 10 μM fMLP for 30 minutes at 37°C. The cells were washed with HBSS at 4°C, and stained with phycoerythrin (PE)–labeled antimouse antibodies against Mac-1 (CD11b, M1/70) or CD18 (C71/16) (Pharmingen, San Diego, CA) at 4°C. Integrin expression was analyzed by flow cytometry using Facscanto (Becton Dickinson, Mountain View, CA). Fluorescence intensity is reported as median channel fluorescence (MCF) in arbitrary units.
Immunofluorescence
To characterize F-actin assembly and adhesion structures upon integrin ligation, neutrophils (5 × 104) were prestimulated with 10 μM fMLP in HBSS, 1 mM Ca2+, and 1 mM Mg2+ and seeded onto fibrinogen-coated slides for 30 minutes at 37°C. The cells were then fixed with 2% paraformaldehyde and stained with rhodamine-labeled phalloidin (Molecular Probes, Eugene, OR) or mouse antivinculin (V-11-5; Sigma) followed by anti–mouse Alexa488 (Molecular Probes). A Z series of fluorescence images was captured with a Leica DMIRB fluorescence microscope (Wetzlar, Germany) at 63×/0.70 NA magnification (air objective) and an ORCA-ER C4742-95 camera (Hamamatsu) equipped with a deconvolution system (Leica, CA) driven by Openlab software.12,15 The Z series was analyzed by deconvolution using Volocity (Improvision). Images were acquired with Openlab 4.0.3 (Improvision) and analyzed using Volocity (Improvision).
MAPK activities
Neutrophils (1-2 × 106) were first stimulated with 10 μM fMLP for 0, 1, and 5 minutes at 37°C. The cells were lysed in Triton-based buffer and analyzed for phospho-p42/p44MAPK and phospho-p38MAPK by immunoblot.12 The membranes were stripped and reprobed with p42/p44MAPK and p38MAPK for loading controls (all antibodies [Abs] were from Cell Signaling, Beverly, MA).
To assess MAPK activities upon integrin ligation, the cells (14 × 106) were resuspended in HBSS, 1 mM Ca2+, and 1 mM Mg2+ with 10 μM fMLP, and seeded onto fibrinogen-coated plates for 5, 15, and 30 minutes. The nonadherent cells were removed and the adherent cells were recovered from the plates using cold cell-dissociation buffer. The cells were lysed and analyzed for MAPK activities as described in the paragraph above.
To determine the role of extracellular signal–regulated kinaseMAPK (ERKMAPK) and p38MAPK in neutrophil migration, neutrophils were preincubated with 2 to 10 μM of the MAPK kinase (MEK) inhibitor U0126 (Calbiochem, San Diego, CA) or 10 μM of the p38MAPK inhibitor SB203580 (Calbiochem) or 10 μM DMSO for 30 minutes at 37°C. Migration assays and F-actin structure analysis were performed as described under “Neutrophil migration assays in vitro” and “Immunofluorescence” with similar inhibitor concentration.
Rho GTPase activity
Neutrophils were stimulated on fibrinogen-coated plates and 10 μM fMLP in HBSS, Ca2+, and Mg2+ for 10 minutes. The cells were lysed onto plates with Mg2+-based lysis buffer and assessed for Rac and CDC42 pull-down assay using PAK-PBD (Upstate, Charlottesville, VA) or for RhoA using Rhotekin-PBD (Upstate), as previously described.12 Total cell lysates were analyzed for CDC42, Rac, or RhoA expression with anti-CDC42 or anti-Rac (Transduction Laboratories, San Diego, CA), or anti-RhoA (Santa Cruz Biotechnology, Santa Cruz, CA) as a loading control.
Results
Gain of CDC42 activity: animal model
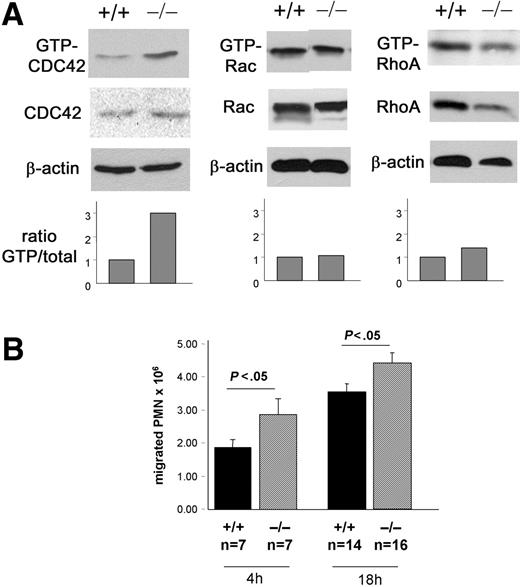
CDC42GAP-deficient mice have been previously described.21 Due to perinatal lethality in most CDC42GAP–/– mice, day-14.5 CDC42GAP–/– fetal livers were used to reconstitute hematopoiesis in irradiated congenic WT animals. Complete donor chimerism was demonstrated in these mice (not shown). CDC42GAP protein was barely detectable in neutrophils derived from the bone marrow of mice reconstituted with CDC42GAP–/– cells by immunoblot (data not shown), similar to that of CDC42GAP-deficient animals.21 In addition, CDC42GAP–/– neutrophils demonstrated a significant increase in GTP-bound CDC42 compared with that of WT without interfering with Rac and RhoA activities (Figure 1A), which is consistent with CDC42GAP-deficient animals.21 Mice reconstituted with CDC42GAP–/– cells did not show any abnormalities in peripheral blood or bone marrow neutrophil numbers or in bone marrow–derived neutrophil differentiation in vitro (data not shown). Therefore, mice reconstituted with CDC42GAP–/– hematopoietic cells represent an animal model of hematopoietic-specific gain of CDC42 activity.
The loss of CDC42GAP function is associated with increased neutrophil recruitment during inflammation
To address the role of CDC42 activity in neutrophils during inflammation, we elicited neutrophils into the peritoneal cavity 4 and 18 hours after thioglycollate instillation.6 The number of neutrophils recruited into the peritoneal cavities of CDC42GAP–/– reconstituted mice after challenge was slightly but significantly higher than WT controls at both time points (Figure 1B). Thus, deregulation of CDC42 activity leads to modest increased neutrophil response to inflammatory stimulus in vivo.
The loss of CDC42GAP function is associated with increased motility, while directed migration is defective
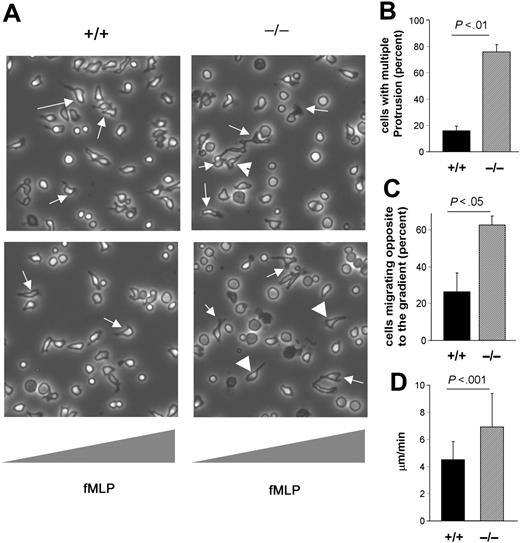
To dissect the role of CDC42GAP in neutrophil migration, we next examined the migration of CDC42GAP–/– neutrophils using time-lapse videomicroscopy. In response to the fMLP gradient, WT cells polarized with a single membrane extension that rapidly oriented toward the agonist gradient. Migration appeared coordinated with membrane extension at the leading edge and tail retraction oriented in a straight line, allowing the cells to efficiently translocate toward the source of stimulation (Figure 2A; see also Video S1, available on the Blood website by clicking on the Supplemental Videos link at the top of the online article). In contrast, CDC42GAP–/– neutrophils showed multiple surface extensions in various directions and abnormal filopodia in the tail in a subset of cells (Videos S2-S3; Figure 2A-B). In addition, the altered leading edge was accompanied by a change in cell direction and, as a result, a significant number of CDC42GAP–/– cells migrated in a direction away from the agonist gradient (Video S2 and Figure 2A,C). The speed of migration of CDC42GAP–/– cells that successfully oriented toward the fMLP gradient was significantly faster than that of WT cells (Figure 2D). Therefore, proper regulation of CDC42 activity appears to be important in vitro to maintain an appropriate direction toward the agonist gradient and to regulate the velocity of cell migration.
Model of gain of CDC42 activity. C57BL/6 cells mice reconstituted with WT or CDC42GAP–/– embryonic day (E)–14.5 fetal liver cells. The reconstituted animals were used 5 weeks after bone marrow reconstitution. (A) CDC42, Rac, and RhoA activities of bone marrow–derived neutrophils. Rho GTPase activity was assessed by the standard pull-down assay using the PAK-binding domain for CDC42 and Rac or the Rhotekin-binding domain for RhoA. The histogram represents the relative ratio of GTP-Rho GTPase versus total protein. β-actin was used as loading control. (B) Neutrophil recruitment into peritoneal cavities after challenge with 3% thioglycollate. Peritoneal lavages were performed 4 and 18 hours after challenge, and total cells were enumerated with a hemocytometer. Neutrophil content was evaluated after cytospin preparation of the cells and Diff Quick staining. Mean ± SEM from 3 independent experiments.
Model of gain of CDC42 activity. C57BL/6 cells mice reconstituted with WT or CDC42GAP–/– embryonic day (E)–14.5 fetal liver cells. The reconstituted animals were used 5 weeks after bone marrow reconstitution. (A) CDC42, Rac, and RhoA activities of bone marrow–derived neutrophils. Rho GTPase activity was assessed by the standard pull-down assay using the PAK-binding domain for CDC42 and Rac or the Rhotekin-binding domain for RhoA. The histogram represents the relative ratio of GTP-Rho GTPase versus total protein. β-actin was used as loading control. (B) Neutrophil recruitment into peritoneal cavities after challenge with 3% thioglycollate. Peritoneal lavages were performed 4 and 18 hours after challenge, and total cells were enumerated with a hemocytometer. Neutrophil content was evaluated after cytospin preparation of the cells and Diff Quick staining. Mean ± SEM from 3 independent experiments.
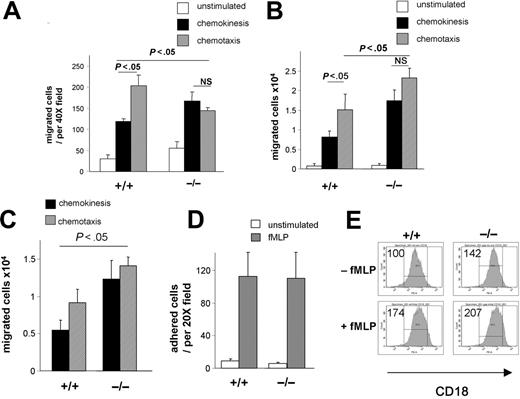
To further understand the role of CDC42 activity in neutrophil migration, we quantified migration in vitro using Boyden chamber assays. Chemokinesis was assessed in a uniform concentration of fMLP in both wells of the chamber, while chemotaxis was assessed in a gradient in which fMLP was placed only in the lower chamber. The number of WT cells that migrated in response to fMLP during chemotaxis was significantly higher than the number of neutrophils exhibiting fMLP-induced chemokinesis (Figure 3A). In contrast, the numbers of migrated CDC42GAP–/– neutrophils were similar in both assays (Figure 3A). This difference was due both to an increased CDC42GAP–/– neutrophil chemokinesis, and a significantly diminished CDC42GAP–/– neutrophil chemotaxis compared with that of WT controls.
Neutrophils transmigrate across the endothelial barrier. To better mimic a transmigration process, fMLP-stimulated neutrophil migration was examined in transwells precoated with fibrinogen, a major β2-integrin ligand. In this assay, CDC42GAP–/– neutrophils demonstrated increased number of migrated cells during both fMLP-induced chemokinesis and chemotaxis compared with that of WT cells (Figure 3B). This observation is consistent with an increased motility in CDC42GAP–/– neutrophils compared with that of WT. In addition, WT cells but not CDC42GAP–/– cells demonstrated significantly more migrated neutrophils in chemotaxis versus chemokinesis (Figure 3B). This observation is also consistent with a defective directed migration in CDC42GAP–/– cells compared with WT cells. Similar results were obtained using a transendothelial migration assay across HUVECs (Figure 3C), reinforcing the physiologic relevance of CDC42-mediated neutrophil migration. Of note, in transwell assays, despite an apparent defective directed migration, more CDC42GAP–/– cells migrated during chemotaxis than did the WT, which is likely due to the overall increased motility associated with CDC42GAP deficiency. This effect was not observed in the Boyden chamber assay. The apparent difference between the Boyden chamber and the transwell assays may reflect distinct integrin dependency of the assays to support cell migration. Indeed, migration is a complex process which requires coordination of signals from both chemokine and integrin receptors to control both chemokinesis and chemotaxis responses. Despite an apparent lack of specific substratum, migration measured in Boyden or Zigmond chambers also requires integrin engagement. However, these various assays may engage distinct integrins. Since migration can be differentially regulated depending on specific integrins,22 a change in integrin engagement could change the level of migration and therefore account for different level of cell migration measured by the Boyden chamber and transwell assays.
Loss of CDC42GAP expression is associated with increased motility while directed migration is defective. Neutrophil migration was examined by time-lapse video microscopy in a gradient of 10 μM fMLP in a Zigmond chamber. (A) Representative images of cells during the course of migration; fMLP concentration increases from left to right. Videos S1-S3 have more representative images. WT cells orient and migrate toward the source of fMLP (arrows). CDC42GAP–/– cells displayed abnormal membrane protrusion (arrows) and some cells migrated away from the gradient (arrowheads). (B) The percentage of cells displaying more than one membrane protrusion was enumerated. (C) The percentage of cells that migrated away from high fMLP concentration is indicated in the histogram. (D) The average translocation rate was measured using Openlab. The result from freshly isolated neutrophils is shown. Similar results were obtained from neutrophils generated in in vitro culture. All histograms represent the mean ± SD; n = 23 to 25 cells from 2 to 3 independent videos.
Loss of CDC42GAP expression is associated with increased motility while directed migration is defective. Neutrophil migration was examined by time-lapse video microscopy in a gradient of 10 μM fMLP in a Zigmond chamber. (A) Representative images of cells during the course of migration; fMLP concentration increases from left to right. Videos S1-S3 have more representative images. WT cells orient and migrate toward the source of fMLP (arrows). CDC42GAP–/– cells displayed abnormal membrane protrusion (arrows) and some cells migrated away from the gradient (arrowheads). (B) The percentage of cells displaying more than one membrane protrusion was enumerated. (C) The percentage of cells that migrated away from high fMLP concentration is indicated in the histogram. (D) The average translocation rate was measured using Openlab. The result from freshly isolated neutrophils is shown. Similar results were obtained from neutrophils generated in in vitro culture. All histograms represent the mean ± SD; n = 23 to 25 cells from 2 to 3 independent videos.
To determine whether the abnormal migration of CDC42GAP–/– neutrophils was related to abnormal adhesion, we analyzed adhesion to fibrinogen. Adhesion to fibrinogen was similar between each genotype (Figure 3D). In addition, CD18 integrin was expressed in CDC42GAP–/– cells at a level similar to that in WT cells, both before and after stimulation with fMLP (Figure 3E). These data indicate that the abnormal migration of CDC42GAP–/– cells was not due to altered adhesion or integrin receptor expression.
These results reflect an overall increased cell motility in CDC42GAP–/– neutrophils while directed migration is defective, suggesting a role for CDC42GAP in both random movement and directed migration in neutrophils in physiologically relevant in vitro models downstream of chemokine and/or integrin receptors.
The loss of CDC42GAP function is associated with abnormal assembly of filopodia and F-actin–containing podosome-like structures
Migration requires a coordinated rearrangement of the cytoskeleton and associated adhesion structures.1 To determine whether altered CDC42 activity effected cytoskeleton rearrangement, F-actin reorganization was examined in neutrophils prestimulated with fMLP on fibrinogen-coated slides. In WT cells, integrin ligation induced formation of a lamellipodium at the leading edge, accompanied in some cells by a punctuated pattern of F-actin located behind the cell's leading edge (Figure 4A). Some WT neutrophils also demonstrated the formation of filopodia concentrated at the leading edge (not shown). Consistent with the increased membrane protrusions observed by time-lapse microscopy, a significant number of CDC42GAP–/– cells displayed increased filopodia-like formations all around the cell (Figure 4A,C), including the tail (Figure S1). A subset of CDC42GAP–/– neutrophils demonstrated multiple “heads” or leading edges (Figure S1). Finally, the punctuate organization of F-actin behind the leading edge of WT cells was absent in a significant number of CDC42GAP–/– cells (Figure 4A).
CDC42GAP regulates both random movement and directed migration. (A) Neutrophil migration using the Boyden chamber. Migration was evaluated without stimulation, in uniform concentration, or in a gradient of 1 μM fMLP to measure chemokinesis or chemotaxis, respectively. The histogram represents the number of migrated neutrophils per field, mean ± SD; representative experiment in triplicate of 3 independent experiments is shown. (B) Neutrophil migration using transwells coated with fibrinogen. Migration was evaluated without fMLP or in uniform concentration or in a gradient of 10 μM fMLP. The histogram represents the total number of migrated neutrophils recovered from the bottom well, mean ± SD; representative experiment in triplicate from 3 independent experiments is shown (n = 9). (C) Neutrophil migration was evaluated using transendothelial migration across HUVECs in uniform concentration or in a gradient of 10 μM fMLP. The histogram represents the total number of migrated neutrophils recovered from the bottom well, mean ± SD; representative experiment in triplicate from 3 independent experiments is shown. (D) Adhesion to fibrinogen. Neutrophils were allowed to adhere to fibrinogen for 30 minutes in the absence or in the presence of 10 μM fMLP. Histogram represents the number of adherent cells counted per field, mean ± SD; representative experiment in triplicate of 3 independent experiments is shown. (E) β-2 integrin expression as assessed by flow cytometry before and after stimulation with 10 μM fMLP. The numbers indicate the relative median channel fluorescence.
CDC42GAP regulates both random movement and directed migration. (A) Neutrophil migration using the Boyden chamber. Migration was evaluated without stimulation, in uniform concentration, or in a gradient of 1 μM fMLP to measure chemokinesis or chemotaxis, respectively. The histogram represents the number of migrated neutrophils per field, mean ± SD; representative experiment in triplicate of 3 independent experiments is shown. (B) Neutrophil migration using transwells coated with fibrinogen. Migration was evaluated without fMLP or in uniform concentration or in a gradient of 10 μM fMLP. The histogram represents the total number of migrated neutrophils recovered from the bottom well, mean ± SD; representative experiment in triplicate from 3 independent experiments is shown (n = 9). (C) Neutrophil migration was evaluated using transendothelial migration across HUVECs in uniform concentration or in a gradient of 10 μM fMLP. The histogram represents the total number of migrated neutrophils recovered from the bottom well, mean ± SD; representative experiment in triplicate from 3 independent experiments is shown. (D) Adhesion to fibrinogen. Neutrophils were allowed to adhere to fibrinogen for 30 minutes in the absence or in the presence of 10 μM fMLP. Histogram represents the number of adherent cells counted per field, mean ± SD; representative experiment in triplicate of 3 independent experiments is shown. (E) β-2 integrin expression as assessed by flow cytometry before and after stimulation with 10 μM fMLP. The numbers indicate the relative median channel fluorescence.
Loss of CDC42GAP functions is associated with abnormal assembly of podosome-like structures and filopodia. F-actin assembly and organization of adhesion structures. (A) Neutrophils were prestimulated with 10 μM fMLP and seeded on fibrinogen-coated slides for 30 minutes. The cells were then fixed and stained with rhodamine-phalloidin (in red). Figure S1 shows more representative pictures. Two representative images of each genotype from 3 independent experiments are shown. (B) Neutrophils were prestimulated with fMLP and seeded on fibrinogen-coated slides for 30 minutes. The cells were then fixed and stained with rhodamine-phalloidin (in red) and antivinculin followed by anti–mouse Alexa488 (in green). Two representative images of each genotype from 3 independent experiments are shown. (C) The percentage of cells displaying more than 1 membrane protrusion and increased filopodia was quantified. (D) The percentage of cells displaying podosome-like structures was quantified. All histograms represent the mean ± SD from 3 independent experiments.
Loss of CDC42GAP functions is associated with abnormal assembly of podosome-like structures and filopodia. F-actin assembly and organization of adhesion structures. (A) Neutrophils were prestimulated with 10 μM fMLP and seeded on fibrinogen-coated slides for 30 minutes. The cells were then fixed and stained with rhodamine-phalloidin (in red). Figure S1 shows more representative pictures. Two representative images of each genotype from 3 independent experiments are shown. (B) Neutrophils were prestimulated with fMLP and seeded on fibrinogen-coated slides for 30 minutes. The cells were then fixed and stained with rhodamine-phalloidin (in red) and antivinculin followed by anti–mouse Alexa488 (in green). Two representative images of each genotype from 3 independent experiments are shown. (C) The percentage of cells displaying more than 1 membrane protrusion and increased filopodia was quantified. (D) The percentage of cells displaying podosome-like structures was quantified. All histograms represent the mean ± SD from 3 independent experiments.
Because the punctuate pattern of F-actin was reminiscent of podosomes seen in macrophages and osteoclasts, WT cells were stained for vinculin-associated adhesion structures. In WT cells, punctuate staining of F-actin was surrounded by a ring of vinculin (Figure 4B). These podosome-like structures were, in general, organized in clusters behind the cell's leading edge. In some WT cells, podosome-like structures assembled in a ring-like pattern (Figure 4B). However, in contrast with other cell types that display podosomes, under our experimental conditions the formation of a peripheral belt of podosomes was not observed in neutrophils. In CDC42GAP–/– cells, vinculin was located at the periphery of the cells (Figure 4B). Some CDC42GAP–/– cells displayed F-actin associated with vinculin in a dispersed manner in the cells (Figure 4B). The number of CDC42GAP–/– neutrophils displaying podosome-like structures at the leading edges of cells was significantly reduced compared with WT cells (Figure 4B,D). These results indicate that CDC42 activity regulates filopodia formation and a cell-adhesion structure in neutrophils that morphologically resembles podosomes.
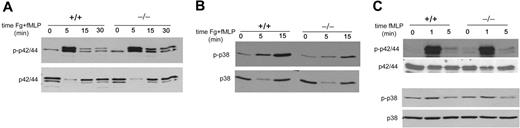
CDC42GAP regulates MAPK signaling in neutrophils. (A-B) Neutrophils were prestimulated with fMLP and seeded on fibrinogen for the indicated time. The cells were lysed and analyzed for phosphorylated p42/44 followed by total p42/44 (A) and for phosphorylated p38 followed by total p38 (B). (C) Neutrophils were stimulated with fMLP alone for the indicated time and examined for phosphorylated p42/44 and phosphorylated p38. Representative blot from 2 to 3 independent experiments is shown.
CDC42GAP regulates MAPK signaling in neutrophils. (A-B) Neutrophils were prestimulated with fMLP and seeded on fibrinogen for the indicated time. The cells were lysed and analyzed for phosphorylated p42/44 followed by total p42/44 (A) and for phosphorylated p38 followed by total p38 (B). (C) Neutrophils were stimulated with fMLP alone for the indicated time and examined for phosphorylated p42/44 and phosphorylated p38. Representative blot from 2 to 3 independent experiments is shown.
CDC42GAP regulates MAPK signaling in neutrophils
The MAPK family has been largely implicated in the regulation of cell migration.23 ERKMAPK regulates focal adhesion turnover and velocity of migration in fibroblasts,24-27 while p38MAPK inhibits filopodia formation and stimulates chemotaxis.28 To dissect the signaling pathways by which CDC42 activity regulates neutrophil migration and to determine whether CDC42GAP acts downstream of integrin versus chemokine receptors, we studied the activity of these MAPKs in CDC42GAP–/– neutrophils after integrin ligation and fMLP stimulation or after fMLP stimulation alone. Upon integrin ligation, ERK activity peaked at 5 minutes both in WT and CDC42GAP–/– neutrophils. In WT cells, ERK phosphorylation was transient, and the level of ERK activity returned to near baseline after 15 minutes. CDC42GAP–/– cells demonstrated significantly higher levels of phosphorylated ERK for up to 30 minutes compared with that of WT controls (Figure 5A). In contrast, the amount of phosphorylated p38MAPK was decreased in CDC42GAP–/– cells at both 5 and 15 minutes of stimulation compared with that in WT cells (Figure 5B). Abnormal MAPK activities associated with CDC42GAP deficiency were not observed when the cells were stimulated with fMLP alone (Figure 5C). Thus, these results suggest that CDC42GAP integrates these MAPK activities downstream of integrin receptors combined with chemokine receptors and that integrins can mediate signals via CDC42GAP for both random movement and directed migration.
CDC42GAP-mediated ERK activity regulates chemokinesis and podosome-like assembly, while CDC42GAP-mediated p38MAPK activity regulates chemotaxis and restrains filopodia-like formation
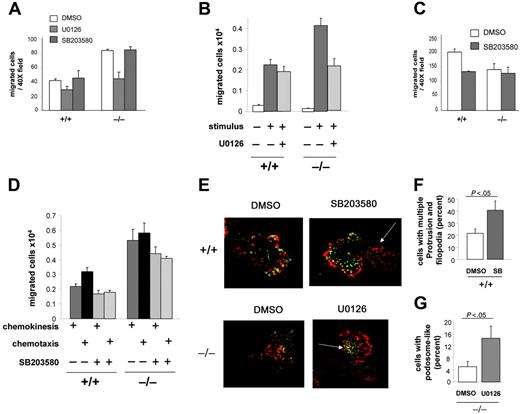
To further understand the roles of ERK and p38MAPK in CDC42GAP-mediated neutrophil migration, we treated WT and CDC42GAP–/– cells with pharmacologic inhibitors of MEK and p38MAPK. We analyzed fMLP-induced chemokinesis and chemotaxis in Boyden chambers and transwells coated with fibrinogen.
We first examined whether the increased chemokinesis in CDC42GAP-deficient cells was associated with high ERK or low p38MAPK activities in these cells. In the Boyden chamber assay, CDC42GAP–/– cells treated with U0126, a MEK inhibitor, demonstrated neutrophil chemokinesis similar to that of WT cells (Figure 6A). In contrast, treatment with the p38MAPK inhibitor SB203580 did not alter chemokinesis of WT cells or the abnormal chemokinesis responses of CDC42GAP–/– cells (Figure 6A). U0126 treatment in CDC42GAP–/– cells also rescued increased chemokinesis of CDC42GAP–/– cells to WT levels in fibrinogen-coated transwells (Figure 6B). In this assay, SB203580 treatment did not increase chemokinesis responses of WT cells (Figure 6D). These data indicate that high ERK activity but not low p38MAPK activity in CDC42GAP-deficient cells correlates with increased chemokinesis and suggest that the ERK but not the p38MAPK pathway may regulate CDC42GAP-mediated chemokinesis.
CDC42GAP-mediated ERK activity regulates chemokinesis and podosome-like assembly, while CDC42GAP-mediated p38MAPK activity regulates chemotaxis and restrains filopodia-like formation. Neutrophils were treated with either the MEK inhibitor U0126 or the p38MAPK inhibitor SB203580 and subjected to migration assays or F-actin and vinculin reorganization. (A) Chemokinesis assessed in the Boyden chamber as in Figure 3A. (B) Chemokinesis in the presence of U0126 assessed in transwells coated with fibrinogen as in Figure 3B. (C) Chemotaxis in the presence of SB203580 assessed in the Boyden chamber. (D) Chemokinesis and chemotaxis in the presence of SB203580 assessed in transwells coated with fibrinogen. All histograms represent the mean ± SD; a representative experiment in triplicate from 3 independent experiments is shown. (E) Neutrophils were prestimulated and seeded on fibrinogen for 30 minutes and examined for F-actin assembly and vinculin structures as in Figure 4B. Note the presence of filopodia in the tail in WT cells treated with SB203580 (arrow). Note the presence of podosome-like structures at the leading edge of the cells in CDC42GAP-deficient cells treated with U0126 (arrow). Representative images from 3 independent experiments are shown. (F) The percentage of WT cells treated with SB203580 displaying more than 1 membrane protrusion and increased filopodia was quantified. (G) The percentage of CDC42GAP-deficient cells treated with U0126 displaying podosome-like structures was quantified. Histograms represent the mean ± SD of 3 independent experiments.
CDC42GAP-mediated ERK activity regulates chemokinesis and podosome-like assembly, while CDC42GAP-mediated p38MAPK activity regulates chemotaxis and restrains filopodia-like formation. Neutrophils were treated with either the MEK inhibitor U0126 or the p38MAPK inhibitor SB203580 and subjected to migration assays or F-actin and vinculin reorganization. (A) Chemokinesis assessed in the Boyden chamber as in Figure 3A. (B) Chemokinesis in the presence of U0126 assessed in transwells coated with fibrinogen as in Figure 3B. (C) Chemotaxis in the presence of SB203580 assessed in the Boyden chamber. (D) Chemokinesis and chemotaxis in the presence of SB203580 assessed in transwells coated with fibrinogen. All histograms represent the mean ± SD; a representative experiment in triplicate from 3 independent experiments is shown. (E) Neutrophils were prestimulated and seeded on fibrinogen for 30 minutes and examined for F-actin assembly and vinculin structures as in Figure 4B. Note the presence of filopodia in the tail in WT cells treated with SB203580 (arrow). Note the presence of podosome-like structures at the leading edge of the cells in CDC42GAP-deficient cells treated with U0126 (arrow). Representative images from 3 independent experiments are shown. (F) The percentage of WT cells treated with SB203580 displaying more than 1 membrane protrusion and increased filopodia was quantified. (G) The percentage of CDC42GAP-deficient cells treated with U0126 displaying podosome-like structures was quantified. Histograms represent the mean ± SD of 3 independent experiments.
We next examined the potential role of p38MAPK in CDC42-mediated chemotaxis. In the Boyden chamber assay, the number of migrated cells in SB203580-treated WT cells was similar to that of CDC42GAP–/– cells (Figure 6C). In the transwell assay, SB203580-treated WT cells showed diminished chemotaxis (Figure 6D). SB203580 treatment of CDC42GAP–/– cells did not change the overall increased migration of these cells compared with that of WT neutrophils, although the level of cell migration of both WT and CDC42GAP–/– cells was diminished compared with that of the same cells treated with the vehicle control. Overall, these results indicate that low p38MAPK activity in CDC42GAP-deficient cells correlates with defective directed migration and suggest that p38MAPK may regulate chemotaxis mediated by CDC42GAP.
Finally, to correlate CDC42GAP-mediated migration and MAPK activities with cytoskeleton and adhesion structure reorganization, cells treated with the inhibitors were analyzed for F-actin and vinculin-associated adhesion structures. Clusters of podosome-like structures were rescued in CDC42GAP–/– cells treated with U0126 (Figure 6E,G), suggesting that high ERK activity in CDC42GAP-deficient cells correlates with the absence of these structures. In addition, WT cells treated with SB203580 developed filopodia-like structures in the tail (Figure 6E-F), and some cells displayed multiple leading edges (not shown). These changes were similar to the phenotype of CDC42GAP–/– cells. However, the formation of clusters of podosome-like structures was not abrogated in WT cells by SB203580 treatment. These results suggest that low p38MAPK activity in CDC42GAP-deficient cells correlates with increased filopodia-like formation but not the absence of podosome-like structures.
In conclusion, this study suggests that regulation of CDC42 activity via CDC42GAP regulates both random movement and directed migration, but via distinct MAPK signals. CDC42 activity appears to mediate neutrophil chemokinesis via ERK-dependent podosome-like structures, while CDC42-dependent directed migration may require p38MAPK signals to antagonize lateral membrane protrusion and filopodia formation.
Discussion
Migration is a multiple-step process regulated by Rho GT-Pases.1,2 Upon stimulation, cells polarize and form a lamellipodium of F-actin at the leading edge, which is stabilized to the substratum by adhesion complexes. These complexes mature into focal adhesions, which give the contractile forces necessary for cell translocation. Completion of cell movement requires adhesion turnover at the leading edge and retraction of the tail. Thus, assembly and disassembly of adhesion structures dictate motility behavior, while polarity and gradient sensing give the direction of the cell movement. Using mice deficient in the CDC42 regulator CDC42GAP, we present evidence that, in primary neutrophils, CDC42 activity plays a physiologic and important role in both motility and directionality via distinct MAPK signaling pathways.
We and others have suggested a role for CDC42 activity in cell motility. In various cell lineages,17,29,30 CDC42 activity has been associated with increased the velocity of nondirectional migration.17,29,30 Our data confirm and extend these previous reports. First, our study highlights the physiologic relevance of CDC42 activity in neutrophil motility, since the increased neutrophil motility associated with deregulation of CDC42 activity likely accounts for the modest increase of neutrophil recruitment into peritoneal cavities of thioglycollate-injected mice. In addition, our study suggests that CDC42 mediates neutrophil motility via a specialized adhesion structure related to podosomes, which is regulated by ERKMAPK downstream of integrins in combination with chemokine receptors. Indeed, integrin engagement by fibrinogen led to increased CDC42GAP-deficient neutrophil motility and alteration in podosome-like structures. Finally, ERK activity in CDC42GAP-deficient neutrophils was enhanced by integrin ligation and fMLP stimulation, but not by fMLP alone, which correlated with the increased motility and altered podosome-like structures in the same cells. Podosomes, which are characterized by a conical F-actin core surrounded by a ring of vinculin, are found in highly motile cells,31 in nontransformed cells such as monocytes, dendritic cells, macrophages, and osteoclasts, and in neutrophils32,33 and eosinophils.34 Podosomes are known for their role in matrix bone degradation. However, since they are linked to the extracellular matrix via integrins, podosomes are also thought to establish dynamic contacts necessary for effective cell migration.35 The key regulator of podosomes is the product of the gene mutated in WASp,36 a specific CDC42 effector. In macrophages and dendritic cells, loss of WASp results in defects in podosome assembly and motility.36-39 In our study, clusters and rings of podosome-like structures were present at the leading edge of WT cells. The number of cells displaying this structure was low, which may be due to their short life and dynamic phenotype.31 Podosome-like structures were defective in CDC42GAP-deficient cells. However, the apparent loss of podosomes may result from a higher podosome turnover. Indeed, CDC42GAP-deficient cells demonstrated increased motility, and motility is directly correlated with adhesion turnover. In addition, the increased motility and altered podosomes were likely due to sustained ERK activity. ERK localizes at focal adhesions and promotes motility by regulating focal adhesion turnover.24-27,40 We were unable to detect phosphorylated ERK in podosomes by immunofluorescence (not shown). However, like focal adhesions, ERK can localize to podosomes via integrin ligation and contributes to osteoclast motility.41 Finally, a complex including betaPIX, SPIN90, Nck, and WASp that regulates stable adhesion can be dynamically modulated by adhesion-dependent ERK activity.42 Thus, we hypothesize that CDC42 activity via CDC42GAP may regulate neutrophil motility via ERK-induced podosome turnover at the cell's leading edge.
In this study, we also suggest that CDC42 plays an important role in chemotaxis by restraining membrane extension. We report that alteration in CDC42 activity led to increased membrane protrusions and filopodia-like formations around the cell. Our study is consistent with the view that CDC42 is required to properly orient the cytoskeleton responses toward the chemoattractant.18,43 Using cells deficient in the CDC42 GEF Pixα, CDC42 has been shown to form a complex with PIXα and PAK and to regulate the intracellular localization of Akt (at the front) and of PTEN (at the back) necessary to polarize F-actin in the gradient of stimulation, and therefore sense the gradient.43 Thus, CDC42 is thought to be part of the “chemical compass” during chemotaxis to make the cell front distinct from the back.44 The “chemical compass” allows the formation of a leading edge of F-actin by the cooperation of Pi3K signals45,46 and the CDC42 pathway,43 while inhibitory signals, including PTEN43,47 and myosin II,48,49 negatively regulate actin polymerization and membrane protrusions in areas outside the leading edge.49 In our study, we suggest that CDC42 also regulates chemotaxis via p38MAPK, which serves as an inhibitory signal of membrane extensions during chemotaxis. Indeed, p38MAPK was reduced in CDC42GAP–/– cells and correlated with the defective chemotactic responses and increased filopodia. Thus, the CDC42-mediated p38MAPK pathway may contribute to inhibitory signals by restraining filopodia to the leading edge and providing positional information to direct migration.49,50 Interestingly, the CDC42-mediated p38MAPK pathway appears downstream of integrin molecules in combination with chemokine receptors, suggesting that CDC42 may integrate signals from both G-coupled chemokine and integrin receptors to regulate the “chemical compass.” α4 integrin–mediated signals can be polarized and can signal at the back of the cell to limit the activation of Rac and the formation of pseudopodia, thus restraining the formation of pseudopodia to the cell front.51 In our study, migration measured by Boyden chambers or fibrinogen-coated transwells may engage various integrins. Fibrinogen mainly but not exclusively binds to CD11b/CD18. The identification of the integrin from which CDC42GAP transduces inhibitory signals would be interesting to investigate further. A role for p38MAPK downstream of tumor necrosis factor α (TNFα) in neutrophil migration by antagonizing filopodia was previously described. This effect appeared to be independent from CDC42,28 suggesting that CDC42-mediated cell migration is agonist specific. Alternatively, since CDC42GAP possesses multiple signaling domains in addition to the GAP domain, we cannot fully exclude the possibility that CDC42GAP regulates p38MAPK independently on CDC42. The mechanism by which p38MAPK antagonizes filopodia remains to be established. Whether p38MAPK activity contributes to control PTEN52 or myosin II–related functions in neutrophils would be interesting to investigate further.
The function of CDC42 is likely to be complex. PIXα-deficient cells did not show any change in migration rate. The PIXα/PAK/CDC42 pathway was described downstream of the G-coupled receptor.19 CDC42GAP/CDC42 activity also appears to regulate migration downstream of integrin molecules. Thus, GEFs and GAPs may regulate GTPase activity downstream of specific receptors.53
A role for MAPK in cell migration has been largely documented. However, the mechanism by which CDC42 could regulate MAPK activities during neutrophil migration remains to be determined. In fibroblasts or hematopoietic stem cells, alteration of CDC42 activity by CDC42GAP abrogates Janus kinase (JNK) MAPK pathway without impairing either the ERK or the p38MAPK pathway in response to serum or growth factors.21,54 This observation reinforces the fact that CDC42 functions may be receptor specific. The CDC42 effectors, WASp and/or PAK, have been largely implicated in podosome assembly and/or adhesion complex turnover. WASp also plays a role in preventing lateral membrane protrusions.55 P38MAPK or ERK can be activated downstream of WASp/WAVE proteins56 or PAK.57,58 The role of WASp and/or PAK in CDC42-mediated MAPK activity downstream of integrin molecules in neutrophil migration is currently under investigation.
In conclusion, this study provides strong evidence that CDC42 activity is important for both random movement and directed migration. Our study highlights the physiologic relevance of CDC42 activity in neutrophil motility since alteration of CDC42 activity led to an overall increased, albeit modest, in vivo neutrophil recruitment into peritoneal cavities despite a defective directed migration. These data reinforce the importance of having a proper cycle between GTP-bound and GDP-bound CDC42 during neutrophil migration. Therefore, this study provides new insights in CDC42 functions in neutrophils, which could be useful for defining CDC42 as a molecular target to generate new therapeutic agents for a broad variety of diseases.
Authorship
Contributions: K.S., H.X., and S.A. performed research; Y.Z. contributed vital new reagents by providing the CDC42GAP knock-out mouse, analytical tools, and advice on the work; and M.-D.F. designed and performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 24, 2006; ZDOI 10.1182/blood-2006-03-013789.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Shelli Homan and Victoria Summey-Harner for animal husbandry. We also thank Amgen (Thousand Oaks, CA) for reagents. We are grateful to Dr Harmut Geiger (Cincinnati, OH) for providing us with HUVECs and for his help in the transendothelial assay.
This work was supported by the American Society of Hematology Scholar Award to M.-D.F.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal