Abstract
Dendritic cells (DCs) are important sentinels within innate immunity, monitoring the presence of infectious microorganisms. They operate in 2 different maturation stages, with transition from immature to mature DCs being induced by activation of toll-like receptors (TLRs). However, TLRs are also expressed on precursor cells of DCs. Here we analyzed the effects of TLR stimulation during the process of granulocyte-macrophage–colony-stimulating factor (GM-CSF)–mediated in vitro generation of immature DCs from precursor cells. We show that TLR triggering deviated phenotypic and functional differentiation from CD14+ monocytes to CD1a+ DCs. Similar results were obtained when differentiation of murine myeloid DCs from bone marrow cells was analyzed. The inhibitory effects were independent of soluble factors. TLR stimulation in DC precursor cells induced proteins of the suppressor of cytokine signaling family (SOCS), which correlated with loss of sensitivity to GM-CSF. Overexpression of SOCS-1 abolished GM-CSF signal transduction. Moreover, forced SOCS-1 expression in DC precursors mimicked the inhibitory effects on DC generation observed for TLR stimulation. The results indicate that TLR stimulation during the period of DC generation interferes with and deviates DC differentiation and that these effects are mediated particularly by SOCS-1.
Introduction
Innate immunity represents the first line of defense against invading pathogens. Tissue-resident immune cells, including macrophages and dendritic cells (DCs), are the first immune cells to encounter infectious agents. Recognition of infecting pathogens within these cells is performed by now well-known pattern recognition receptors responding to conserved microbial structures.1 Among these receptors, toll-like receptors (TLRs) have been identified as playing a pivotal role and indeed, both macrophages and dendritic cells express TLRs.2-4 In macrophages, TLR stimulation activates the antimicrobial weaponry, including increased phagocytosis5 ; in dendritic cells, migration into lymph nodes, antigen presentation, cytokine secretion, and subsequent activation of adaptive immunity6 are enhanced. Interestingly, both of these primary pathogen-encountering cells can develop from monocytes, which thus serve as common progenitors. Differentiation is regulated by cytokines from the environment and immune cells among which macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage–colony-stimulating factor (GM-CSF) are of ultimate importance.
Although it is well accepted that TLR stimulation activates macrophages and dendritic cells, there is only limited information as to a role of TLRs during differentiation processes. Thus, it is largely unknown whether TLR stimulation affects the replenishment of tissue-resident macrophages and dendritic cells from monocytes. This question is of significance as it might suggest a role for infectious stimuli to influence and guide the cellular composition of inflammatory responses.
It has been reported that migration of dendritic cells from local tissue to lymph nodes can be inhibited by intradermal administration of either whole Salmonella typhimurium or simply by LPS in a TLR-4–dependent manner.7 Another study showed that administration of LPS during the early stage of differentiation of human monocytes to DCs diminished the generation of CD1a+ DCs.8 This was confirmed by another group demonstrating that this blocking effect of LPS depends on activation of mitogen-activated protein kinase p38.9 However, the exact mechanisms of these inhibitory effects still remain to be identified. Secreted cytokines such as IL-109 have been suggested but seem to play only a minor role.7
Myeloid dendritic cell differentiation depends on the activity of GM-CSF, a cytokine that signals through Janus kinases (JAKs) and signal transducers and activators of transcription (STATs), especially JAK2 and STAT5.10 Suppressor of cytokine signaling proteins (SOCS) have been identified as intracellular feedback inhibitors of JAK/STAT signaling.11-13 Furthermore, SOCS-1 has been shown to negatively regulate JAK2 and GM-CSF signaling by inhibiting phosphorylation14 as well as proteasomal targeting15 of JAK2. Accordingly, SOCS-1 knockout mice, in spite of a lethal phenotype due to hypersensitivity16,17 toward IFN-γ, also exhibit hyperresponsiveness to GM-CSF.18,19
We and others have shown that TLR stimulation in innate immune cells results in the induction of various SOCS members, thereby regulating the responsiveness to cytokine stimulation as shown in detail for IFN-γ.20-22 We now tested the hypothesis that by means of induction of SOCS proteins, TLR-dependent signals influence differentiation of DCs from precursor cells.
We show that in human as well as murine precursor cells, TLR stimulation inhibits DC differentiation. We exclude paracrine factors to be responsible for this effect but show that SOCS proteins are induced. Moreover, induced or transfected SOCS-1 inhibits GM-CSF signaling and also mimics the inhibitory effects of LPS during in vitro DC generation.
Materials and methods
Reagents and cells
Highly purified lipopolysaccharide from Salmonella minnesota was kindly provided by U. Seydel (Borstel, Germany). Completely phosphorothioate-modified CpG-oligodeoxynucleotide (ODN) no. 1668 (TCC ATG ACG TTC CTG ATG CT) was purchased from TIB Molbiol (Berlin, Germany). Pam3Cys-SK4 and R-FSL (an analog of RR-MALP-2) were purchased from EMC Microcollections (Tübingen, Germany), and Poly [(I:C)] and Zymosan A (from Saccharomyces cervisiae) were from Sigma-Aldrich (Munich, Germany). Recombinant cytokines were purchased from Tebu (Frankfurt, Germany). Cell culture medium was Clicks/RPMI 1640 (Biochrom, Berlin, Germany) supplemented with 10% endotoxin-free fetal calf serum (FCS; BioWest, East Sussex, United Kingdom), antibiotics, and 50 μM β-mercaptoethanol (murine cells). RAW264.7 macrophages stably overexpressing SOCS proteins were established by cotransfection of SOCS expression plasmids and a neomycin resistance cassette as described by us previously.22
Mice
Balb/c mice were purchased from Harlan-Winkelmann, Borchen, Germany. TLR9–/– mice and wild-type littermates were received from H. Wagner (Munich, Germany).
Generation of human DCs
Human peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of healthy donors by standard Ficoll-Paque density gradient centrifugation and washed 3 times in phosphate-buffered saline (PBS) supplemented with 5 mM EDTA. CD14+ cells were positively selected by magnetic associated cell sorting (AutoMACS, program: possel; Miltenyi Biotec, Bergisch-Gladbach, Germany). CD14+ cells were seeded at a density of 2 × 106 cells/mL in 24-well, flat-bottom cell culture plates. Cultures were supplemented with 10 ng/mL recombinant human (rh) GM-CSF and 500 IU/mL rhIL-4. LPS (10 ng/mL) was added where indicated. Cells were cultured at 37°C in a humidified atmosphere in the presence of 5% CO2 for 6 days. Then, cells were harvested by incubation on ice for 30 minutes, followed by intensive washing to remove all cells. The procedure was controlled by light microscopy.
Murine bone marrow–derived dendritic cells
Dendritic cells were prepared from female, 4- to 10-week-old BALB/c mice as described,23 with minor modifications. Briefly, bone marrow was collected from femurs and tibiae of mice. Cells were counted and 4 × 106 cells were seeded into 80 cm2 tissue-culture flasks in culture medium supplemented with 200 U/mL GM-CSF. At day 6 fresh medium was added and at day 9 nonadherent, immature dendritic cells (CD11c+, GR-1–) were harvested. Culture supernatant of a GM-CSF transfected cell line24 was used equally as a source of GM-CSF. Where indicated, bone marrow cells were stimulated once at day 0 with either 100 ng/mL LPS or 100 nM CpG-ODN.
Transwell experiments
Bone marrow cells (7.5 × 105) from TLR9–/– knockout mice were seeded in 12-well cell cuture plates. Transwell inserts (0.4 μm; Costar Corning, Schiphol, The Netherlands) were placed into each well and 3 × 105 wild-type (wt) cells were added. Subsequently, cells were differentiated with GM-CSF in either the absence or presence of LPS or CpG-ODN for 9 days prior to flow cytometric analysis. Additionally, cells from the different mice were incubated separately. Cells from C3H/HeJ and C3H/HeN mice were used similarly.
Determination of cytokine secretion
Cell-free supernatants were harvested and analyzed for cytokines by commercially available enzyme-linked immunosorbent assay (ELISA) kits (OptEIA; Becton Dickinson, Heidelberg, Germany).
Cell viability
Cells were stained with propidium iodide (PI) and annexin V and analyzed by flow cytometry following the manufacturer's guidelines (Annexin V–FLUOS-Staining-Kit; Roche, Mannheim, Germany).
Mixed lymphocyte reaction
Murine T lymphocytes were prepared from spleens by CD90 MACS separation (Miltenyi). T cells (75 × 103) were incubated with graded numbers of day-10 murine DCs for 96 hours. Human mixed lymphocyte reactions (MLRs) were performed either in allogenic or autologous settings. Highly purified T lymphocytes (2 × 105; obtained by CD3-AutoMACS sorting) were incubated with graded numbers of Mitomycin C–pretreated antigen-presenting cells (APCs) from a different donor (allogenic MLR) for 96 hours. For autologous assays, the CD14-depleted cell fraction was frozen and thawed at day 6. T cells were isolated and experiments were assayed as with freshly isolated cells. For activation of autologous DC/T-cell cocultures, 0.1 ng/mL streptococcal pyrogenic exotoxin C (Toxin Technology, Sarasota, FL) was added. During the last 16 hours, cultures were labeled with 22.5 kBq (0.61 μCi) 3H-thymidine to measure cell proliferation. Thymidine incorporation was evaluated by β-scintillation counting.
Quantitative RT-PCR
Total RNA from 1 × 106 cells was isolated using a HighPure RNA-kit (Roche, Mannheim, Germany), which included DNaseI digestion. RNA (1 μg) was reverse-transcribed with a cDNA synthesis kit (MBI Fermentas, St Leon-Rot, Germany) and used as template in the quantitative polymerase chain reaction (PCR) mix according to the manufacturer's standard protocol (reagents from AbGene, Hamburg, Germany; on ABI Prism 7700, Applied Biosystems, Darmstadt, Germany). The primer sequences have been previously published22 or are available on request. Quantifications were made using fluorogenic probes (FAM/TAMRA; Eurogentec, Seraing, Belgium). Specificity of reverse transcription (RT)–PCR was controlled by no template and no RT controls. PCR efficiencies for all reactions were determined and were in a similar range. Quantitative PCR results are expressed relative to the housekeeping genes β-actin or GAPDH (1/2ΔCt).
Flow cytometry
Murine DCs were stained with antibodies against MHC-class II (2G9), CD11b (M1/70), CD11c (HL3) (BD Pharmingen, Heidelberg, Germany), or F4/80 (CI:A3-1; Serotec, Düsseldorf, Germany) according to standard protocols. Antibodies for staining of human cells were CD1a (H3149), CD14 (M5E2), CD40 (5C3), CD80 (L307.4), CD86 (IT2.2), and HLA-DR (G46-6) (BD Pharmingen). For the analysis of phosphorylated STAT-5, cells were fixed with 2% paraformaldehyde/PBS for 30 minutes at room temperature. Subsequently, cells were permeabilized in 90% methanol at –20°C overnight. Afterward, cells were incubated with 5 μL phosphotyrosine–specific STAT-5 antibody (Cell Signaling Technologies, Beverly, MA) in 100 μL of volume for 2 hours at room temperature followed by a secondary antibody. Cells were analyzed on a FACS Canto (BD Biosciences, Heidelberg, Germany).
Phagocytosis assays
Phagocytosis was measured using FITC-labeled latex beads of 1.1-μm size (Sigma, Taufkirchen, Germany), which were incubated for 90 minutes (murine) or 45 minutes (human) with cells at 4°C and 37°C. Phagocytotic activity is displayed as the difference of mean fluorescence intensities. Dead cells were excluded by propidium iodide staining.
Virus production and infection
Full-length SOCS constructs with BglII/Xho ends were amplified from cloning vectors by PCR using respective primers and were cloned into the bicistronic vector pMSCV (BD Clontech, Heidelberg, Germany) engineered to express enhanced green fluorescence protein (eGFP). Retroviral expression vectors were transiently transfected into AmphoPack293-cells (BD Clontech) using the calcium-phosphate method. Medium was exchanged 24 hours later to Dulbecco modified Eagle medium (DMEM)/10% Serum Supreme (Cambrex, Apen, Germany) and cells were incubated further at 32°C. Again, 24 hours and 48 hours later, the supernatants were collected and frozen in liquid nitrogen to use for the infection of murine bone marrow cells. Murine bone marrow cells (1.5 × 106) were plated in 6-well plates and spin-infected (2 hours, 1000 g; Hereaus Megafuge 1.0R, from Hereaus, Hanau, Germany) on days 1, 2, and 3 by the addition of virus supernatants in the presence of 8 μg/mL protamine-sulfate and GM-CSF. Medium was exchanged after 8 hours of incubation with virus supernatant.
Statistical analysis
Data are shown as the mean plus or minus the standard deviation (SD). Significant difference was evaluated by the unpaired Student t test with 2-tailed distributions. P values below .05 were considered to be significant and are indicated by one asterisk.
Results
LPS inhibits the development of immature DCs from human monocytes
In the current study, human DCs were generated using CD14+ monocytes from peripheral blood that were cultured for 6 days with GM-CSF and IL-4. Addition of LPS at day 0 to this cytokine cocktail led to the development of cells that mimicked monocytes morphologically (light microscopy, data not shown). These similarities in morphology were also apparent in flow cytometric analysis (forward and side scatter; Figure 1A): cells with additional LPS in the culture medium were smaller and showed a lower degree of granulation in comparison to cells not exposed to LPS. Block of DC generation was confirmed by analysis of surface molecules. LPS-treated cells failed to express the DC marker CD1a (Figure 1A); also, LPS led to a retention of CD14 expression whereas GM-CSF and IL-4 treatment resulted in partial loss of CD14. The frequency of CD1a+ cells under usual culturing conditions varied from about 30% to 80%, depending on the donor. LPS addition at the start of the culture period resulted in reduced numbers of CD1a+ cells in each individual donor (compare also Figure 1D). Moreover, LPS-treated cells showed weaker expression of costimulatory molecules CD40, CD80, and CD86, as well as of MHC class II (Figure 1B). GM-CSF receptor expression was not altered (data not shown). These LPS-induced blocking effects were dose-dependent and even observed at low doses of 100 pg/mL LPS (Figure 1C). The inhibition of DC generation was also observed when TLRs -1/2, -2/6, and -3 were stimulated by addition of lipopeptides (Pam3cysSK4, FSL), zymosan, or poly[(I:C)], yet the inhibitory effects were different in magnitude (Figure 1D).
TLR stimulation inhibits GM-CSF– and IL-4–induced development of DCs from human CD14+ monocytes. Human CD14+ monocytes were cultivated for 6 days with GM-CSF and IL-4 (indicated as GI) in either the presence or absence of 10 ng/mL LPS (indicated as GI+LPS). CD14+ cells left unstimulated for 6 days are indicated as “none.” (A) Cell size and granula, and expression of CD1a and CD14, were measured by flow cytometry (representatives of at least 20 experiments). (B) Histograms of indicated surface molecules from either control DCs (open) or LPS-treated cells (filled). Data are from one representative experiment out of 4 experiments. (C) Expression of CD1a and CD14 was measured in cultures that were treated with graded amounts of LPS as indicated (1 out of 4 experiments). (D) Monocytes from 3 different donors (marked by circles, rectangles, and triangles) were cultivated either with GM-CSF+IL-4 alone (GI) or in the presence of an additional 10 μg/mL Pam3 CysSK4, 1 μg/mL FSL, 50 μg/mL poly[(I:C)], 50 μg/mL zymosan, or 10 ng/mL LPS. Cells were analyzed for expression of CD1a at day 6. (E) Cell numbers from LPS-treated cultures were determined as mean values of triplicate counts (n = 8). (F) Cells were analyzed for phagocytosis of FITC-labeled latex beads. Shown is ΔMFI (37°C-4°C) of 1 of 4 experiments with similar results. (G) Cells were restimulated at day 6 with 50 ng/mL LPS (▦) or left unstimulated (□) and analyzed for secretion of IL-6, TNF, IL-12p40, and IL-10 after overnight incubation (displayed as mean and SD from 1 of 3 donors with similar results). (H) DCs either treated with LPS or not during the differentiation period and completely untreated CD14+ cells were assayed for their capacity to induce proliferation of allogenic CD3-sorted T lymphocytes (triplicates of 1 of 3 similar experiments). (I) Similarly, cells were assayed in an autologous MLR with CD3-sorted T lymphocytes (triplicates of 1 of 3 similar experiments). To induce proliferation, 0.1 ng/mL superantigen SPEC was added to autologous cocultures.
TLR stimulation inhibits GM-CSF– and IL-4–induced development of DCs from human CD14+ monocytes. Human CD14+ monocytes were cultivated for 6 days with GM-CSF and IL-4 (indicated as GI) in either the presence or absence of 10 ng/mL LPS (indicated as GI+LPS). CD14+ cells left unstimulated for 6 days are indicated as “none.” (A) Cell size and granula, and expression of CD1a and CD14, were measured by flow cytometry (representatives of at least 20 experiments). (B) Histograms of indicated surface molecules from either control DCs (open) or LPS-treated cells (filled). Data are from one representative experiment out of 4 experiments. (C) Expression of CD1a and CD14 was measured in cultures that were treated with graded amounts of LPS as indicated (1 out of 4 experiments). (D) Monocytes from 3 different donors (marked by circles, rectangles, and triangles) were cultivated either with GM-CSF+IL-4 alone (GI) or in the presence of an additional 10 μg/mL Pam3 CysSK4, 1 μg/mL FSL, 50 μg/mL poly[(I:C)], 50 μg/mL zymosan, or 10 ng/mL LPS. Cells were analyzed for expression of CD1a at day 6. (E) Cell numbers from LPS-treated cultures were determined as mean values of triplicate counts (n = 8). (F) Cells were analyzed for phagocytosis of FITC-labeled latex beads. Shown is ΔMFI (37°C-4°C) of 1 of 4 experiments with similar results. (G) Cells were restimulated at day 6 with 50 ng/mL LPS (▦) or left unstimulated (□) and analyzed for secretion of IL-6, TNF, IL-12p40, and IL-10 after overnight incubation (displayed as mean and SD from 1 of 3 donors with similar results). (H) DCs either treated with LPS or not during the differentiation period and completely untreated CD14+ cells were assayed for their capacity to induce proliferation of allogenic CD3-sorted T lymphocytes (triplicates of 1 of 3 similar experiments). (I) Similarly, cells were assayed in an autologous MLR with CD3-sorted T lymphocytes (triplicates of 1 of 3 similar experiments). To induce proliferation, 0.1 ng/mL superantigen SPEC was added to autologous cocultures.
GM-CSF is a known survival factor for human CD14+ monocytes. In our experiments, the cell number from LPS-stimulated cultures was only half as high as that from cultures stimulated with GM-CSF and IL-4, and was thus within the range of completely unstimulated cells (Figure 1E). LPS stimulation of freshly isolated CD14+ monocytes for 48 hours resulted in an increase in annexin V–staining cells (GM-CSF+IL-4, 20.2% ± 1.9%; GM-CSF+IL-4+LPS, 41.0% ± 4.5%), and thus LPS-induced early apoptosis might contribute to the reduced cell number of LPS-coincubated cells at day 6.
Cells generated in the presence of LPS were further characterized for their functional properties. Phagocytotic activity of differently generated cells was tested by measuring the uptake of FITC-labeled latex beads (Figure 1F). These experiments revealed that the phagocytotic activity of LPS-generated cells was much higher than that of GM-CSF/IL-4–generated DCs and comparable to completely unstimulated CD14+ cells. Furthermore, restimulation of cells on day 6 with LPS showed an impressively altered cytokine profile of LPS-treated cells. Although they were still able to secrete IL-6, they failed to produce IL-12p40 and TNF-α (Figure 1G). In contrast, IL-10 was inducible in comparable amounts from both cell types. The capacity to induce T-cell activation, a hallmark of DCs, was examined in allogenic and autologous mixed lymphocyte reactions. The autologous MLR was set up using the superantigen streptococcal pyrogenic exotoxin C (SPEC). In allogenic MLR, LPS-generated APCs turned out to be weak activators of T-cell proliferation, comparable to nondifferentiated CD14+ cells (Figure 1H). In contrast, DCs cultivated with GM-CSF/IL-4 were strong T-cell stimulators. However, in the autologous setting LPS-treated cells were able to induce T-cell proliferation albeit at slightly lower efficacy (Figure 1I).
TLR stimulation inhibits DC generation from murine bone marrow cells
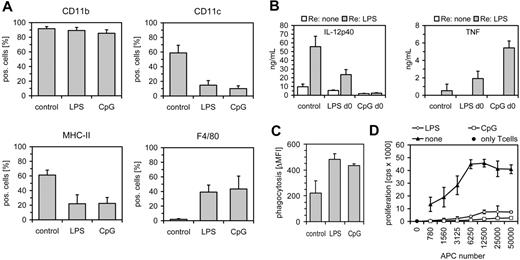
Next we wanted to analyze whether the results obtained in the human monocyte/DC system corroborate in a murine DC model. Therefore, we generated myeloid DCs from bone marrow cells (BMCs) by addition of GM-CSF. In parallel, BMCs were stimulated once at day 0 with either LPS or CpG-ODNs, which signal via TLR4 and TLR9, respectively. Cells were analyzed at day 9. In contrast to conventional DCs expressing CD11c to about 60% to 70%, LPS or CpG treatment reduced CD11c+ DCs to 15% and 10% (Figure 2A). These results were concordant with a reduced expression of MHC-class II. However, each cell type expressed the myeloid marker CD11b equally and LPS- or CpG-treated cells had an increased F4/80 expression. Compared with DC generation by GM-CSF alone, cell yields at day 9 were lower when cells were treated with LPS (49% ± 27% of control cells) or CpG-DNA (41% ± 32%) at the beginning of the culture period. Functionally, LPS- or CpG-treated cells showed reduced levels of IL-12 upon restimulation at day 9 with LPS, but were more effective in the production of TNF-α (Figure 2B). Furthermore, these cells showed an increased phagocytotic capacity (Figure 2C). Regarding activation of naive T lymphocytes, we observed that conventional DCs but not cells treated with LPS or CpG induced proliferation of CD90-sorted T cells in an allogenic MLR (Figure 2D). In the murine system, TLR stimulation inhibited DC generation when given up to day 4 during the 9-day differentiation period (data not shown), but at later time points cells differentiated to DCs with an even more mature phenotype (MHC-class II expression, costimulatory molecules). Taken together, the data in the murine system confirm the inhibitory effect of TLR stimulation during the early DC generation period.
TLR stimulation inhibits murine dendritic cell development. Murine bone marrow cells (Balb/c) were differentiated for 9 days with GM-CSF in the absence or presence of 100 ng/mL LPS or 100 nM CpG-ODN. (A) Cells were analyzed for expression of CD11b, CD11c, MHC class II (n = 5), and F4/80 (n = 2) expression by FACS (mean+SD). (B) Cells were restimulated at day 9 with 1 ng/mL LPS and analyzed for secretion of IL-12p40 (n = 3) and TNF-α (n = 2) after overnight incubation (mean+SD). (C) Cells were analyzed for phagocytosis of FITC-labeled latex beads. Shown is ΔMFI (37°C-4°C) (mean+SD, n = 4). (D) Day-10 Balb/c DCs treated with LPS or CpG-ODN during the differentiation period were assayed for their capacity to induce proliferation of CD90-sorted T lymphocytes (C57BL/6, triplicates of 1 of 3 experiments).
TLR stimulation inhibits murine dendritic cell development. Murine bone marrow cells (Balb/c) were differentiated for 9 days with GM-CSF in the absence or presence of 100 ng/mL LPS or 100 nM CpG-ODN. (A) Cells were analyzed for expression of CD11b, CD11c, MHC class II (n = 5), and F4/80 (n = 2) expression by FACS (mean+SD). (B) Cells were restimulated at day 9 with 1 ng/mL LPS and analyzed for secretion of IL-12p40 (n = 3) and TNF-α (n = 2) after overnight incubation (mean+SD). (C) Cells were analyzed for phagocytosis of FITC-labeled latex beads. Shown is ΔMFI (37°C-4°C) (mean+SD, n = 4). (D) Day-10 Balb/c DCs treated with LPS or CpG-ODN during the differentiation period were assayed for their capacity to induce proliferation of CD90-sorted T lymphocytes (C57BL/6, triplicates of 1 of 3 experiments).
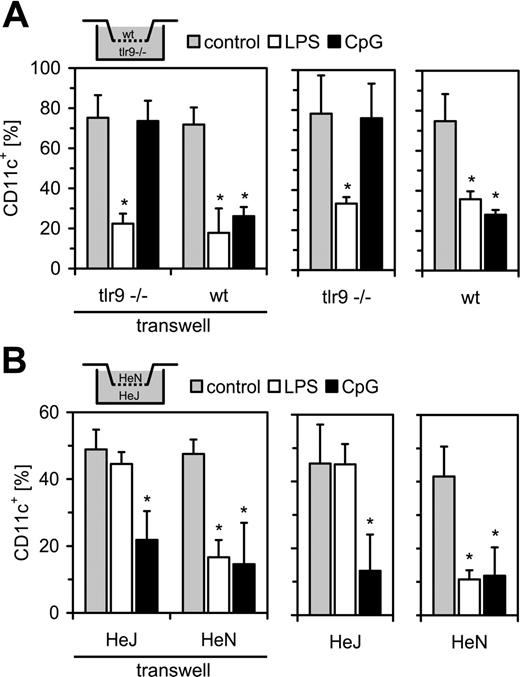
TLR stimulation inhibits DC generation in a direct manner
From the results obtained to this point, it was obvious that TLR stimulation during GM-CSF–mediated DC generation suppresses the development of functional DCs. This can be due either to a direct effect of TLR simulation within the precursor cells or to secreted, negative regulatory intermediates. Since TLR effects were operative in both the human and the murine system, we set up a transwell culture composed of murine BMCs from either TLR9–/– knockout mice or TLR4-defective C3H/HeJ mice together with the respective wt controls. In the wt/TLR9–/– culture, LPS inhibited DC generation in both cell compartments; in contrast, CpG-ODN administration led only to an inhibition of CD11c expression in wt cells (Figure 3A). In TLR9–/– cells, CpG-ODN failed to affect DC generation. The HeN/HeJ culture system confirmed these findings (Figure 3B). LPS was able to reduce numbers of CD11c+ cells in the HeN cells but had only minor effects in cells from HeJ mice. CpG-ODN was effective in blocking DC generation in both cell types. Collectively, these data ruled out a role of secreted intermediates, which should pass the transwell membrane.
TLR-stimulation results in SOCS induction
To further elucidate the mechanisms by which TLR stimulation interferes with DC development we examined the induction of SOCS family members CIS, SOCS-1, and SOCS-3 (Figure 4A-C). We observed basal expression of either SOCS in nonstimulated human monocytes, with SOCS-1 being expressed at only low levels. LPS induced expression of all of the tested SOCS mRNAs, but strength and kinetics differed. Upon LPS stimulation, SOCS-3 expression increased rapidly at 1 hour after stimulation and SOCS-1 was induced beginning after 2 hours. In contrast, CIS expression started to increase at 7 hours after stimulation. SOCS-3 showed peak expression at 7 hours (18-fold) whereas SOCS-1 was maximal at 24 hours after stimulation (120-fold). Transcription of SOCS-1, and to a lesser extent SOCS-3 and CIS, stayed elevated during the whole culture period. Other TLR ligands (Pam3CysSK4, Poly[(I:C)], and FSL) were also able to induce SOCS-1 and SOCS-3 (Figure 4E-F). We also analyzed expression of SOCS in the murine model after stimulation of BMCs with LPS (Figure 4D). All of the SOCS family members were induced 6 hours after stimulation but only SOCS-1 and SOCS-3 remained elevated longer than 20 hours. Since freshly prepared bone marrow is a mixture of different cells and since we used total RNAfrom this mixture it is difficult to deduce which cell types actually respond to TLR stimulation.
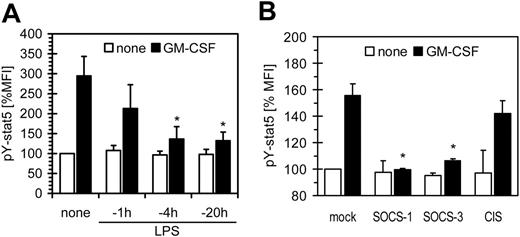
SOCS-1 and -3 are inhibitors of GM-CSF
Next we investigated whether LPS-induced SOCS proteins are able to inhibit GM-CSF signaling. The strong induction of SOCS upon LPS suggested that if inhibition of GM-CSF signaling is due to induction of SOCS, inhibition should be time dependent. To this we analyzed the phosphorylation of STAT-5 (pY-STAT5) induced by addition of GM-CSF to cells prestimulated with LPS. Pretreatment of monocytes prior to short stimulation with GM-CSF showed a significant loss of phosphorylation of pY-STAT5; these inhibitory effects increased within a time frame of 1 to 4 hours (Figure 5A), which paralleled SOCS-3 and -1 induction (Figure 4). Next we analyzed murine macrophages stably overexpressing either of the SOCS proteins for inhibition of GM-CSF (Figure 5B). We observed that in the murine system SOCS-1 and to a lesser extent SOCS-3 were able to reduce GM-CSF–mediated activation of STAT5.
TLR stimulation directly inhibits DC development. (A) Bone marrow cells from either TLR9–/– or wt (C57BL/6) mice were differentiated with GM-CSF in the absence or presence of LPS or CpG-ODN. Cells from the different mice were either combined in a transwell format (left) or incubated alone (right). Cells were analyzed at day 9 for CD11c expression by FACS. (B) Experiments were done as above with C3H/HeJ (LPS-unresponsive) and C3H/HeN (control) mice (mean + SD; n = 4, *P < .05 against the respective control stimulation).
TLR stimulation directly inhibits DC development. (A) Bone marrow cells from either TLR9–/– or wt (C57BL/6) mice were differentiated with GM-CSF in the absence or presence of LPS or CpG-ODN. Cells from the different mice were either combined in a transwell format (left) or incubated alone (right). Cells were analyzed at day 9 for CD11c expression by FACS. (B) Experiments were done as above with C3H/HeJ (LPS-unresponsive) and C3H/HeN (control) mice (mean + SD; n = 4, *P < .05 against the respective control stimulation).
TLR stimulation induces the expression of SOCS. Freshly isolated human CD14+ monocytes were stimulated with 10 ng/mL or 30 ng/mL LPS and analyzed for expression of (A) SOCS-1, (B) SOCS-3, and (C) CIS by RT-PCR at the indicated time points (mean of duplicate determinations from 1 of 3 typical donors). (D) Murine bone marrow cells (Balb/c) were stimulated with 100 ng/mL LPS immediately after preparation and were analyzed for expression of SOCS-1, SOCS-3, and CIS by RT-PCR at the indicated time points (n = 4, mean+SD). Expression of SOCS1 (E) or SOCS-3 (F) was determined in monocytes stimulated with 10 μg/mL Pam3CysSK4,1 μg/mL FSL, 50 μg/mL poly[(I:C)], or 10 ng/mL LPS for 7 hours (1 representative of 3 experiments).
TLR stimulation induces the expression of SOCS. Freshly isolated human CD14+ monocytes were stimulated with 10 ng/mL or 30 ng/mL LPS and analyzed for expression of (A) SOCS-1, (B) SOCS-3, and (C) CIS by RT-PCR at the indicated time points (mean of duplicate determinations from 1 of 3 typical donors). (D) Murine bone marrow cells (Balb/c) were stimulated with 100 ng/mL LPS immediately after preparation and were analyzed for expression of SOCS-1, SOCS-3, and CIS by RT-PCR at the indicated time points (n = 4, mean+SD). Expression of SOCS1 (E) or SOCS-3 (F) was determined in monocytes stimulated with 10 μg/mL Pam3CysSK4,1 μg/mL FSL, 50 μg/mL poly[(I:C)], or 10 ng/mL LPS for 7 hours (1 representative of 3 experiments).
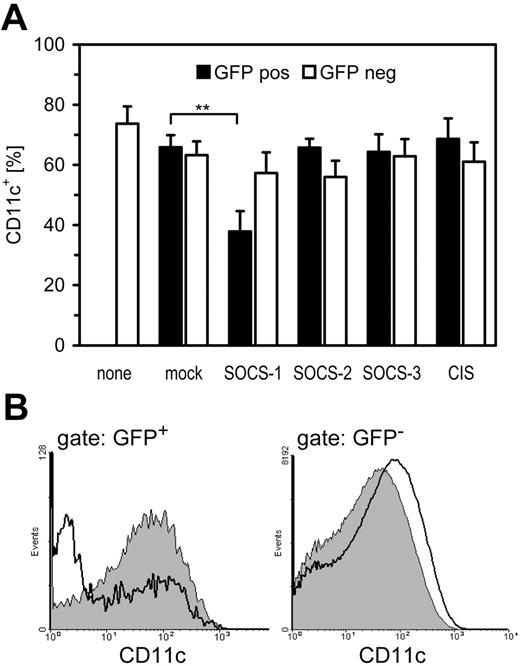
SOCS-1 inhibits DC differentiation in vitro
To further substantiate that SOCS proteins are operative in TLR-mediated inhibition of DC differentiation, we overexpressed SOCS in DC precursors by means of retroviral transfer. Only low virus titers could be applied during the differentiation period, otherwise there was a reduction in DC generation that was independent of the respective SOCS constructs and even independent of productive infection (as assessed by GFP expression; data not shown). It is therefore probable that at high concentrations the virus preparation itself has substantial TLR activity that inhibits DC generation. Using lower titers of the respective viruses it turned out that in the murine system cells that expressed SOCS-1 upon retroviral transfer (GFP-positive) failed to differentiate into CD11c+ DCs; that is, SOCS-1 was able to inhibit GM-CSF–mediated differentiation to DCs (Figure 6A). Noninfected cells (GFP-negative) in the same experiment showed equal expression of CD11c, ruling out soluble mediators or unspecific actions at the chosen conditions (Figure 6B). However, it cannot be ruled out that SOCS-3 also contributes to GM-CSF inhibition because we did not test combinations of different SOCS.
SOCS proteins inhibit GM-CSF signaling. (A) Human monocytes were preincubated with 100 ng/mL LPS for the indicated time. Subsequently, cells were stimulated with 500 U/mL GM-CSF for 30 minutes and were analyzed for phosphorylation of STAT-5 by FACS (mean fluorescence intensity of untreated cells was set as 100%). (B) Murine RAW264.7 macrophages stably overexpressing the indicated SOCS proteins were stimulated with 100 ng/mL GM-CSF for 30 minutes. Cells were analyzed for phosphorylation of STAT-5 by FACS. Data are mean + SD.
SOCS proteins inhibit GM-CSF signaling. (A) Human monocytes were preincubated with 100 ng/mL LPS for the indicated time. Subsequently, cells were stimulated with 500 U/mL GM-CSF for 30 minutes and were analyzed for phosphorylation of STAT-5 by FACS (mean fluorescence intensity of untreated cells was set as 100%). (B) Murine RAW264.7 macrophages stably overexpressing the indicated SOCS proteins were stimulated with 100 ng/mL GM-CSF for 30 minutes. Cells were analyzed for phosphorylation of STAT-5 by FACS. Data are mean + SD.
Discussion
Our experiments indicate that TLR stimulation during the generation of myeloid DCs from precursor cells inhibits differentiation to typical DCs as determined by phenotype and function. This outcome could be observed in human as well as murine model systems. Moreover, ligands for TLR-4, TLR-2, and TLR-9 (murine system) were effective to impede with differentiation of DCs. The capacious characterization of the nature of this DC “block” extends earlier findings8,9 and furthermore indicates that interfering with DC differentiation is a general property of TLR signaling. Human monocytes remained CD14+ in the presence of LPS and failed to express CD1a. In addition, they showed lower expression of costimulatory molecules and secreted less IL-12 and TNF upon restimulation. LPS-treated cells were less effective in inducing an allogenic MLR, yet only minorly hampered stimulation of an autologous reaction with SPEC. However, the capacity to phagocytose remained high, which indicates that the cells are not merely hyporesponsive as observed in the situation of LPS tolerance.25 Instead, these cells appear to be arrested in an early differentiation step. LPS-treated cells had a somehow macrophage-like phenotype and resembled the cells described by Palucka et al.8 These results were confirmed in a murine in vitro system, with the only exception being that restimulated TNF production was not affected. These observations might be of clinical interest since LPS-induced arrest was observed at LPS concentrations in the low nanogram range that can be found in blood during sepsis.
Regarding the mechanisms, we were able to show that LPS-induced inhibition of differentiation is confined to cells that could be stimulated directly by TLR ligands. The transwell experiments using combinations of different knockout mice provided clear evidence that secreted factors play no role, yet that interference with intracellular signaling pathways is operative. This is corroborated by reports that reduced DC migration or DC differentiation is not attributed to secreted cytokines.7,9
SOCS-1 inhibits DC differentiation. (A) Murine bone marrow cells were forced to overexpress SOCS-1, SOCS-2, SOCS-3, or CIS by means of retroviral transfer of SOCS/GFP bicistronic constructs on days 1, 2, and 3 of the differentiation period. Cells were differentiated with GM-CSF for a total of 9 days and then analyzed for expression of CD11c by FACS either in the GFP-positive, transfected population or in the GFP-negative control population (mean+SD, n = 6, **P < .01). (B) FACS analysis of CD11c expression of one typical experiment from panel A either on mock (filled) or SOCS-1–transfected cells (open). Shown are overlay graphs for GFP-positive, transfected cells and GFP-negative control cells within one experiment.
SOCS-1 inhibits DC differentiation. (A) Murine bone marrow cells were forced to overexpress SOCS-1, SOCS-2, SOCS-3, or CIS by means of retroviral transfer of SOCS/GFP bicistronic constructs on days 1, 2, and 3 of the differentiation period. Cells were differentiated with GM-CSF for a total of 9 days and then analyzed for expression of CD11c by FACS either in the GFP-positive, transfected population or in the GFP-negative control population (mean+SD, n = 6, **P < .01). (B) FACS analysis of CD11c expression of one typical experiment from panel A either on mock (filled) or SOCS-1–transfected cells (open). Shown are overlay graphs for GFP-positive, transfected cells and GFP-negative control cells within one experiment.
Our findings are compatible with a suggested role for intracellular SOCS proteins. Indeed, we observed that in human monocytes administration of TLR ligands results in an increase of SOCS-1, -3, and CIS transcription, which remained elevated throughout the culture period as in the case of SOCS-1. It has now been shown in multiple innate immune cells including monocytes, macrophages, and DCs that SOCS proteins can be induced upon TLR stimulation20-22,26 and that this is not mediated by soluble secondary factors.20,27 Accordingly, we observed that LPS prestimulation resulted in a time-dependent inhibition of GM-CSF signaling and this went along with the kinetics of SOCS induction.
Individual SOCS proteins are able to inhibit a variety of different cytokines28 and in general the induction of SOCS upon cytokine stimulation is indicative that the respective cytokine is regulated in a feedback mode. In a slightly different system it has been observed that SOCS-1 and SOCS-3 are induced in immature DCs by addition of IL-4 and GM-CSF, respectively.29 GM-CSF signaling, on the other hand, is subject to inhibition by SOCS-1. JAK2 is inhibited by SOCS-1 either via proteasomal degradation15 or by interference with JAK2 phosphorylation.14 In addition, IL-5, IL-3, and GM-CSF (which all use the beta-common chain and JAK2) are negatively regulated by SOCS-1. We show here that LPS-induced SOCS expression inhibits GM-CSF signaling and that overexpression of SOCS-1 and to a lesser extent of SOCS-3 in murine macrophages resulted in a marked reduction of GM-CSF–mediated STAT5 phosphorylation.
The fact that SOCS-1 knockout mice also display defective GM-CSF signaling further corroborates our conclusion. Despite the prominent role of SOCS-1 for IFN-γ signaling it has also been shown that hematopoetic progenitor cells of SOCS-1 knockout mice have an increased sensitivity to GM-CSF but not to M-CSF.18,19 It is noteworthy that the CSF-1 receptor belongs to the receptor-tyrosine kinase family, which in general is not a reported target of SOCS proteins. This could explain why TLR stimulation results in inhibition of GM-CSF–mediated DC formation but has no effects on the generation of a more macrophage-like phenotype, which possibly is due to effects of intrinsic CSF as a kind of default pathway. Indeed, it has been shown that SOCS proteins do not inhibit CSF-mediated macrophage differentiation whereas IL-6 signaling was sensitive to SOCS actions.30 Further confirmation for our hypothesis comes from the fact that overexpression of SOCS-1 in DC progenitors results in diminished DC formation. It cannot be ruled out at present that further SOCS proteins contribute to the observed inhibitory effects; however, SOCS-3 and CIS knockout mice have no reported defect in GM-CSF signaling,31 although CIS is able to interfere with JAK-STAT5 signaling.32 SOCS-1 has also been shown to exert further negative regulatory effects on the function of immature DCs as silencing by siRNA results in enhanced antigen presentation and stimulation of adaptive immunity.33
The results support a concept in which microbial ligands are able to skew the dichotomy of macrophage versus DC differentiation from common progenitors. In uninflamed tissues, GM-CSF induces the generation of immature DCs, preparing the host for the sensing of infectious danger. However, during infectious inflammation, TLR stimulation will drive incoming monocytes not to differentiate to DCs but to behave more like macrophages. This could be of help for the direct antimicrobial defense, which is more effectively mediated by macrophage-like cells with a high capacity to phagocytose. Pre-existing resident DCs are sufficient to perform the task of antigen sampling and transduction of information to the adaptive immune system. Thus, TLR stimulation would guide the innate immune system to assure a sufficient supply of phagocytic cells in inflamed tissues. Indeed, the observation that monocytes from trauma patients have a decreased capacity to differentiate to CD1a+ DCs due to decreased GM-CSF sensitivity34 further emphasizes this interpretation.
Taken together, our results indicate that TLR stimulation blocks generation of DCs from progenitor cells, yet retains macrophage functions. This is achieved by inhibition of GM-CSF signaling through the induction of SOCS-1.
Authorship
Contribution: H.B. performed experiments on human cells and gave scientific input; N.M.A. performed experiments on murine DC development; A.B. performed transfection; K.H. gave scientific input; and A.H.D. performed experiments on murine DC development and transfection and gave scientific input.
Conflict of interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 8, 2006; DOI 10.1182/blood-2006-03-008946.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Helene Bykow, Christine Barett, Aline Gierschke, and Adelina Dillmann for excellent technical support. A.B. is a PhD candidate at the University of Heidelberg, and this work is submitted in partial fulfillment of the requirement for the PhD.
This work was supported by grants from the Deutsche Forschungsgemeinschaft Da592/3 (A.H.D.) and SFB405/A12 (K.H.).

![Figure 1. TLR stimulation inhibits GM-CSF– and IL-4–induced development of DCs from human CD14+ monocytes. Human CD14+ monocytes were cultivated for 6 days with GM-CSF and IL-4 (indicated as GI) in either the presence or absence of 10 ng/mL LPS (indicated as GI+LPS). CD14+ cells left unstimulated for 6 days are indicated as “none.” (A) Cell size and granula, and expression of CD1a and CD14, were measured by flow cytometry (representatives of at least 20 experiments). (B) Histograms of indicated surface molecules from either control DCs (open) or LPS-treated cells (filled). Data are from one representative experiment out of 4 experiments. (C) Expression of CD1a and CD14 was measured in cultures that were treated with graded amounts of LPS as indicated (1 out of 4 experiments). (D) Monocytes from 3 different donors (marked by circles, rectangles, and triangles) were cultivated either with GM-CSF+IL-4 alone (GI) or in the presence of an additional 10 μg/mL Pam3 CysSK4, 1 μg/mL FSL, 50 μg/mL poly[(I:C)], 50 μg/mL zymosan, or 10 ng/mL LPS. Cells were analyzed for expression of CD1a at day 6. (E) Cell numbers from LPS-treated cultures were determined as mean values of triplicate counts (n = 8). (F) Cells were analyzed for phagocytosis of FITC-labeled latex beads. Shown is ΔMFI (37°C-4°C) of 1 of 4 experiments with similar results. (G) Cells were restimulated at day 6 with 50 ng/mL LPS (▦) or left unstimulated (□) and analyzed for secretion of IL-6, TNF, IL-12p40, and IL-10 after overnight incubation (displayed as mean and SD from 1 of 3 donors with similar results). (H) DCs either treated with LPS or not during the differentiation period and completely untreated CD14+ cells were assayed for their capacity to induce proliferation of allogenic CD3-sorted T lymphocytes (triplicates of 1 of 3 similar experiments). (I) Similarly, cells were assayed in an autologous MLR with CD3-sorted T lymphocytes (triplicates of 1 of 3 similar experiments). To induce proliferation, 0.1 ng/mL superantigen SPEC was added to autologous cocultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/13/10.1182_blood-2006-03-008946/7/m_zh80240604890001.jpeg?Expires=1763574706&Signature=ENf5PQXKkB~loJrUwaB7L5jQEMDBKgLNDYRwVx11gghrfSHUjQJWDWZ1beljawAk~LTB-UaBMdKM6zq58m1fk2YZ5tMbJCliSGfaYugbodWGvAbKaz9FgxztKTwBM64B77M1-ZjshzAgNtlja0EYQ-~Fwm3f54Xsbu9S~plOlp6Lc5ZulijNQYFyXVlXYtrFPHhvjQOWg8o3Z8cYTdeHlYso1Mk~i6aly-YiRFIpcjCJz2Gid-RDRJxEo8qVVJB6k3z6NBwSzzrpXT6FM4CQUrscjggli2xT-~MYrRVL-lpizuDkj5cXkjqL5zqhsqS8qG3L3EIXctBc6O~xZxOMBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. TLR stimulation induces the expression of SOCS. Freshly isolated human CD14+ monocytes were stimulated with 10 ng/mL or 30 ng/mL LPS and analyzed for expression of (A) SOCS-1, (B) SOCS-3, and (C) CIS by RT-PCR at the indicated time points (mean of duplicate determinations from 1 of 3 typical donors). (D) Murine bone marrow cells (Balb/c) were stimulated with 100 ng/mL LPS immediately after preparation and were analyzed for expression of SOCS-1, SOCS-3, and CIS by RT-PCR at the indicated time points (n = 4, mean+SD). Expression of SOCS1 (E) or SOCS-3 (F) was determined in monocytes stimulated with 10 μg/mL Pam3CysSK4,1 μg/mL FSL, 50 μg/mL poly[(I:C)], or 10 ng/mL LPS for 7 hours (1 representative of 3 experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/13/10.1182_blood-2006-03-008946/7/m_zh80240604890004.jpeg?Expires=1763574706&Signature=DS-VmH3D2aP30lXt0evoEypjC6ZETFzqv0NNJiaFyiwKxz6ZXyB~ES~PS9Pc1DQmg4AV6Bb5crFvaUsAxBt6i0ZCdnpCHQroWg6T~oD-E-QbG8RLg3Bpby5WJhRIlg62onD6EQ8LP9f-o9zoxzRq3REHb9GDuvoIjTeESz573aAJ0NxbU3AGNmNXZSv1iziQQLkdBsX737HARtSKoDnUoeIs5PiTucxAI3D2DgD8CSBUXJIfV9NlmKGr~xHTTs4Gkna-HDF6CPnJXU1D2cm0JmBJ4e9RieBIQHZkjzYq6s-03prSS5K2TCMptfh7KJvU7Bb29dYMeGK64KSXXaoqWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal