Abstract
Transforming growth factor–β1 (TGF-β1) and plasminogen activator inhibitor–type 1 (PAI-1) might play a role in the development of fibrosis and stenosis of hemodialysis vascular accesses. We studied polymorphisms in the TGFβ1 (869T>C; 915G>C), and PAI-1 (4G/5G) genes in 416 hemodialysis patients (107 access thrombosis cases, 309 controls), to determine if they are related to vascular access thrombosis. Three TGF-β1 production haplotypes (low, intermediate, and high) were defined according to the combination of polymorphisms found. The adjusted odds ratio (OR) and 95% confidence interval (CI) for access thrombosis in low TGF-β1 producers was 7.31 (2.15-24.88; P = .001). The interaction between low TGF-β1 production haplotype and the 4G/4G PAI-1 genotype was strongly associated with access thrombosis (adjusted OR 19.3; 95% CI 2.82-132.40; P = .003). Mean access thrombosis–free survival times in years (95% CI) were 14.65 (12.05-17.25), 11.96 (8.67-15.25), and 4.94 (3.06-6.83) in high, intermediate, and low TGF-β1 producers, respectively (P = .044). Analysis of the synergy index and the case-only cross-product supported the presence of an interaction. We concluded that low TGF-β1 production haplotype is a risk factor for hemodialysis access thrombosis and that in the presence of the 4G/4G PAI-1 genotype there is an additional increase in risk.
Introduction
The patency of vascular access (VA) is critical for the survival of patients undergoing hemodialysis. VA complications account for nearly one-fourth of all hospital admissions in hemodialysis patients, with important associated costs.1,2 Thrombosis is the most common cause of VA failure1 with several related factors. Current evidence indicates that thrombosis occurs in the setting of access stenosis,3 which arises in response to different stimuli, among which transforming growth factor–β1 (TGF-β1) could play an important role. It has been described that there is increased expression of TGF-β1, latent TGF-β1 binding protein-1, and TGF-β1 mRNA in occluded or narrowed fistulas.4,5 Single nucleotide polymorphisms (SNPs) in the signal sequence of the TGFβ1 gene are associated with an increased risk for cardiovascular diseases in dialysis patients,6 and lung and kidney fibrotic diseases.7,8 Different TGF-β1 production haplotypes can be defined according to the combination of SNPs9 and it has been suggested recently that the high TGF-β1 production haplotype is associated with an increase in the frequency of access failure.10 However, other factors might be involved in access failure and our group recently demonstrated that common thrombophilic traits result in an increased odds ratio (OR) for access thrombosis.11
Plasminogen activator inhibitor–type 1 (PAI-1) is a member of the SERPIN superfamily of protease inhibitors whose main function is to inhibit the tissue-type and urokinase-type plasminogen activators, thus decreasing fibrinolytic activity.12 PAI-1 is involved in the development of atherosclerosis and influences cell migration, angiogenesis, and tissue remodeling in cardiovascular and malignant diseases.13-15 It has been demonstrated that PAI-1 expression is increased in stenosed fistulas in dialysis patients,5 and some studies have suggested that the 4G/5G polymorphism in the PAI-1 gene is associated with thrombotic diseases such as myocardial infarction in dialysis patients16 and stroke in the general population,17 but it has not been studied in dialysis VA thrombosis. Since TGF-β1 is a well-known up-regulator of the PAI-1 gene,18 it is possible that polymorphisms affecting both genes might have an interaction.
Herein we report the results of a secondary analysis performed on hemodialysis patients from a previous large, case-control study.11 The main objective of the present study was to elucidate the effects of TGF-β1 production haplotypes on the occurrence of thrombosis of the VA. The secondary objective was to evaluate possible interactions of such haplotypes with SNPs occurring in the PAI-1 gene.
Patients, materials, and methods
Study design and participants
An unmatched case-control study was conducted in a network of 8 hemodialysis units caring for adult patients in Ottawa, Ontario, Canada. All participants provided written informed consent, in accordance with the Declaration of Helsinki, and the study was approved by the Ottawa Hospital Research Ethics Board. Inclusion and exclusion criteria and operational definitions have been previously reported.11 Patients were eligible to participate if they had a functioning permanent dialysis access. Functioning access was defined as successful cannulation with 2 needles and a minimum blood flow of 250 mL/min for at least 1 complete dialysis treatment. Patients were classified as cases if their first functioning dialysis access had a thrombotic occlusion; controls were those who never had a thrombotic occlusion. Thrombotic occlusion was defined as the absence of flow on clinical exam and inability to use the access for dialysis. The evaluation for thrombotic occlusion was determined by the clinical team, which was unaware of the genetic tests' results.
Laboratory tests
All participants had blood samples drawn before dialysis. DNA was isolated from leukocytes using standard methods. SNPs were identified using a capillary electrophoresis instrument (ABI PRISM 3100 Genetic Analyzer; Applied Biosystems, Foster City, CA), and analyzed with an automated software protocol (Genotyper Software, version 3.7; Applied Biosystems).19,20 SNPs in codons 10 (869T>C) and 25 (915G>C) of the TGFβ1 gene were determined as previously reported.10 TGF-β1 production haplotypes were defined as low (CC [codon 10]/CC [codon 25], CC/GC, TT/CC, and TC/CC), intermediate (CC/GG, TC/GC, and TT/GC), or high (TC/GG and TT/GG) as described by Perrey and coworkers.9 The PAI-1 4G/5G SNP was determined using previously described techniques.16 Primers for the SNP were designed and validated using recommended methods.20 Hardy-Weinberg equilibrium for the 3 polymorphisms was assessed in control subjects by the goodness-of-fit χ2 statistic with 1 degree of freedom using PowerMarker version 3.25.21 A 2-sided P value greater than .05 was considered indicative of Hardy-Weinberg equilibrium. In addition, the following thrombophilic factors were determined: factor V Leiden mutation, prothrombin gene mutation 20210G>A, methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism, factor VIII level, total homocysteine, lupus anticoagulant, anticardiolipin antibodies (IgG and IgM), and lipoprotein(a). Thrombophilia was defined using previously reported cutoff values11 as the presence of 1 or more of the aforementioned factors. The factor XIII Val34Leu polymorphism was also determined because it might modify the risk of thrombosis.22 Albumin, folate, and vitamin B12 concentrations were also measured. The techniques used are reported elsewhere.11 The laboratory staff that performed these tests was not aware of the patients' clinical status.
Statistical analysis
Because this was a secondary analysis, the sample size estimation (438 patients) was done as previously reported.11 Baseline continuous variables were compared using a Student t test or a Mann-Whitney U test, as appropriate. Dichotomous variables were compared using a χ2 statistic with Yates correction or a Fisher exact test as appropriate. P values less than .05 were considered significant. The primary analytical strategy was logistic regression. Using simple logistic regression we estimated the OR and 95% confidence intervals (CIs) for the occurrence of access thrombosis that were associated with each covariate. Those covariates reaching a significance of less than .2 in the simple logistic regression were included in the multiple logistic regression models, 3 of which were constructed forcing in the first step either the TGF-β1 production haplotype, the PAI-1 gene SNP, or both, and sequentially adjusting for all significant covariates. Covariates reaching a significance of less than .05 were retained in the final models. Finally, we included interaction terms between the PAI-1 gene polymorphisms and the TGF-β1 production haplotypes, and between the same covariates and lipoprotein(a). In addition, we conducted logistic regression stratified by the PAI-1 gene polymorphism (dichotomized as 4G/4G versus 4G/5G and 5G/5G) with the TGF-β1 production haplotypes as the explanatory variable. Goodness-of-fit was tested using the Hosmer-Lemeshow test and colinearity was evaluated by assessing variance inflation factors, condition indices, and variance proportions. Full details are available from the authors on request.
Thrombosis-free survival of the access was estimated using the method described by Kaplan and Meier.23 The cohort was grouped according to the TGF-β1 production haplotypes and stratified according to the PAI-1 SNP, and the log-rank test was used for comparison. A 2 × 4 table analysis for case-control designs was used to estimate individual and joint ORs and calculate the synergy index according to the method described by Khoury and Flanders.24 In addition, the case-only cross-product was calculated using a 2 × 2 table case-only approach as previously described.24-26 A sample size analysis for case-only designs was done using software developed at the University of Southern California (QUANTO Version 1.0; available at http://hydra.usc.edu/gxe)26 and under the following assumptions: 4G allele frequency of the PAI-1 gene, 0.494; low TGF-β1 production haplotype frequency, 0.208; baseline risk, 0.01; OR for the 4G/4G PAI-1 polymorphism, 0.81; OR for the low TGF-β1 production haplotype, 2.96; independence of the PAI-1 SNP and the TGF-β1 production haplotypes; and coinheritance of the SNPs defining the TGF-β1 production haplotype. All other calculations were done using Microsoft Excel 2002 (Microsoft, Redmond, WA) with the statistical add-in software package Analyse-It, release 1.71 (Analyse-It Software, Leeds, United Kingdom), and SPSS release 13.0 (SPSS, Chicago, IL).
Results
Baseline characteristics
Of the 419 patients included in the previous study,11 DNA was available for analysis in 416 (107 cases; 309 controls). Their characteristics are shown in Table 1. When compared with controls, the patients in the cases group were slightly younger (P = .023), had a lower proportion of arteriovenous fistulas (P < .001), higher proportion of accesses localized in the lower arm (P = .003), higher albumin (P = .022), lower urea reduction ratio (P < .001), higher proportion of thrombophilia (P = .004), and higher proportion of low TGF-β1 production haplotype (P = .006). There was no difference in the proportions of the PAI-1 genotypes (P = .552). All 3 polymorphisms were in Hardy-Weinberg equilibrium in control subjects. The median time elapsed between access surgery and thrombosis or end of observation was 548 days (interquartile range, 281-1179 days) in cases and 1037 days (interquartile range 597-1655 days) in controls.
Characteristics of study population
Characteristic . | Patients with access thrombosis . | Controls . | P . |
|---|---|---|---|
| N | 107 | 309 | |
| Age, y, mean ± SD | 60.6 ± 17.2 | 64.8 ± 16.0 | .023 |
| Female, no. (%) | 46 (43) | 112 (36.2) | .215 |
| Race, no. (%) | .176 | ||
| White | 90 (84.1) | 271 (87.7) | |
| Black | 10 (9.3) | 14 (4.5) | |
| Other | 7 (6.5) | 24 (7.8) | |
| Weight, kg, mean ± SD | 72.1 ± 18.9 | 72.4 ± 17.8 | .912 |
| Body mass index, mean ± SD | 25.5 ± 6.4 | 25.7 ± 5.6 | .856 |
| Medical history, no. (%) | |||
| Peripheral vascular disease | 29 (27.1) | 95 (30.7) | .478 |
| Coronary artery disease | 42 (39.3) | 114 (36.9) | .664 |
| Stroke or transient ischemic attack | 8 (7.5) | 45 (14.6) | .058 |
| Malignancy | 23 (21.5) | 51 (16.5) | .245 |
| Venous thromboembolism | 7 (6.5) | 12 (3.9) | .284 |
| Diabetes | 30 (28.0) | 104 (33.7) | .284 |
| Cause of end-stage renal disease, no. (%) | .379 | ||
| Diabetes | 18 (16.8) | 72 (23.3) | |
| Glomerulonephritis | 24 (22.4) | 62 (20.1) | |
| Hypertension | 12 (11.2) | 47 (15.2) | |
| Polycystic kidney disease | 8 (7.5) | 25 (8.1) | |
| Urinary obstruction/reflux | 9 (8.4) | 13 (4.2) | |
| Unknown | 16 (15.0) | 44 (14.2) | |
| Other | 20 (18.7) | 46 (14.9) | |
| Smoking tobacco use, no. (%) | .261 | ||
| Never | 34 (31.8) | 100 (32.4) | |
| Former | 48 (44.9) | 158 (51.1) | |
| Current | 25 (23.4) | 51 (16.5) | |
| Medications, no. (%) | |||
| Angiotensin converting enzyme inhibitor | 32 (29.9) | 123 (39.8) | .068 |
| Warfarin | 22 (20.6) | 41 (13.3) | .070 |
| Antiplatelet agent | 41 (38.3) | 130 (42.1) | .496 |
| Hormone replacement therapy or birth control pill | 8 (7.5) | 19 (6.1) | .631 |
| Vitamin B12, B6, folic acid, or multivitamin | 81 (75.7) | 257 (83.2) | .088 |
| Family history of venous thromboembolism, no. (%) | 4 (3.7) | 14 (4.5) | > .999 |
| Type of dialysis access, no. (%) | < .001 | ||
| Arteriovenous fistula | 81 (75.7) | 299 (96.8) | |
| Arteriovenous graft | 26 (24.3) | 10 (3.2) | |
| Location of dialysis access, no. (%) | .003 | ||
| Lower arm | 66 (61.7) | 151 (48.9) | |
| Upper arm | 38 (35.5) | 157 (50.8) | |
| Leg | 3 (2.8) | 1 (0.3) | |
| Median time from access surgery to first cannulation, d | 56.5 | 56.0 | .709 |
| Median time from access surgery to thrombosis or end of observation period, d | 548.0 | 1037.0 | < .001 |
| Dialysis access angiogram performed, no. (%) | 56 (52.3) | 185 (59.9) | .174 |
| Dialysis access angioplasty or surgical revision performed, no. (%) | 50 (46.7) | 130 (42.1) | .402 |
| Previous dialysis access created but never functioned, no. (%) | 29 (27.1) | 59 (19.1) | .080 |
| Hemoglobin level, g/L, mean ± SD | 116 ± 11 | 118 ± 11 | .084 |
| Erythropoietin dose, U/wk, mean ± SD | 12 104 ± 7567 | 12 353 ± 9107 | .800 |
| Albumin level, g/L, mean ± SD | 37 ± 5 | 36 ± 5 | .022 |
| Urea reduction ratio, mean ± SD | 73 ± 7 | 76 ± 6 | < .001 |
| Thrombophilia, no. (%) | |||
| Factor V Leiden | 7 (6.5) | 6 (1.9) | .046 |
| Prothrombin gene mutation 20210G > A | 3 (2.8) | 9 (2.9) | > .999 |
| Anticardiolipin antibodies (IgG) at least 30 GPL U/mL | 2 (1.9) | 5 (1.6) | > .999 |
| Anticardiolipin antibodies (IgM) at least 30 MPL U/mL | 2 (1.9) | 8 (2.6) | > .999 |
| Lupus anticoagulant | 2 (1.9) | 8 (2.6) | > .999 |
| Factor VIII level greater than 90th percentile* | 18 (16.8) | 30 (9.7) | .047 |
| Homocysteine at least at 85th percentile† | 22 (20.6) | 40 (12.9) | .057 |
| Lipoprotein(a) at least at 85th percentile‡ | 23 (21.5) | 41 (13.3) | .042 |
| Presence of any thrombophilia | 59 (55.1) | 121 (39.2) | .004 |
| TGF-β1 production haplotype, no. (%) | .006 | ||
| High producers | 69 (64.5) | 232 (75.1) | |
| Intermediate producers | 28 (26.2) | 69 (22.3) | |
| Low producers | 10 (9.3) | 8 (2.6) | |
| PAI-1 genotype, no. (%) | .552 | ||
| 4G/4G | 24 (22.4) | 75 (24.3) | |
| 4G/5G | 60 (56.1) | 155 (50.2) | |
| 5G/5G | 23 (21.5) | 79 (25.6) |
Characteristic . | Patients with access thrombosis . | Controls . | P . |
|---|---|---|---|
| N | 107 | 309 | |
| Age, y, mean ± SD | 60.6 ± 17.2 | 64.8 ± 16.0 | .023 |
| Female, no. (%) | 46 (43) | 112 (36.2) | .215 |
| Race, no. (%) | .176 | ||
| White | 90 (84.1) | 271 (87.7) | |
| Black | 10 (9.3) | 14 (4.5) | |
| Other | 7 (6.5) | 24 (7.8) | |
| Weight, kg, mean ± SD | 72.1 ± 18.9 | 72.4 ± 17.8 | .912 |
| Body mass index, mean ± SD | 25.5 ± 6.4 | 25.7 ± 5.6 | .856 |
| Medical history, no. (%) | |||
| Peripheral vascular disease | 29 (27.1) | 95 (30.7) | .478 |
| Coronary artery disease | 42 (39.3) | 114 (36.9) | .664 |
| Stroke or transient ischemic attack | 8 (7.5) | 45 (14.6) | .058 |
| Malignancy | 23 (21.5) | 51 (16.5) | .245 |
| Venous thromboembolism | 7 (6.5) | 12 (3.9) | .284 |
| Diabetes | 30 (28.0) | 104 (33.7) | .284 |
| Cause of end-stage renal disease, no. (%) | .379 | ||
| Diabetes | 18 (16.8) | 72 (23.3) | |
| Glomerulonephritis | 24 (22.4) | 62 (20.1) | |
| Hypertension | 12 (11.2) | 47 (15.2) | |
| Polycystic kidney disease | 8 (7.5) | 25 (8.1) | |
| Urinary obstruction/reflux | 9 (8.4) | 13 (4.2) | |
| Unknown | 16 (15.0) | 44 (14.2) | |
| Other | 20 (18.7) | 46 (14.9) | |
| Smoking tobacco use, no. (%) | .261 | ||
| Never | 34 (31.8) | 100 (32.4) | |
| Former | 48 (44.9) | 158 (51.1) | |
| Current | 25 (23.4) | 51 (16.5) | |
| Medications, no. (%) | |||
| Angiotensin converting enzyme inhibitor | 32 (29.9) | 123 (39.8) | .068 |
| Warfarin | 22 (20.6) | 41 (13.3) | .070 |
| Antiplatelet agent | 41 (38.3) | 130 (42.1) | .496 |
| Hormone replacement therapy or birth control pill | 8 (7.5) | 19 (6.1) | .631 |
| Vitamin B12, B6, folic acid, or multivitamin | 81 (75.7) | 257 (83.2) | .088 |
| Family history of venous thromboembolism, no. (%) | 4 (3.7) | 14 (4.5) | > .999 |
| Type of dialysis access, no. (%) | < .001 | ||
| Arteriovenous fistula | 81 (75.7) | 299 (96.8) | |
| Arteriovenous graft | 26 (24.3) | 10 (3.2) | |
| Location of dialysis access, no. (%) | .003 | ||
| Lower arm | 66 (61.7) | 151 (48.9) | |
| Upper arm | 38 (35.5) | 157 (50.8) | |
| Leg | 3 (2.8) | 1 (0.3) | |
| Median time from access surgery to first cannulation, d | 56.5 | 56.0 | .709 |
| Median time from access surgery to thrombosis or end of observation period, d | 548.0 | 1037.0 | < .001 |
| Dialysis access angiogram performed, no. (%) | 56 (52.3) | 185 (59.9) | .174 |
| Dialysis access angioplasty or surgical revision performed, no. (%) | 50 (46.7) | 130 (42.1) | .402 |
| Previous dialysis access created but never functioned, no. (%) | 29 (27.1) | 59 (19.1) | .080 |
| Hemoglobin level, g/L, mean ± SD | 116 ± 11 | 118 ± 11 | .084 |
| Erythropoietin dose, U/wk, mean ± SD | 12 104 ± 7567 | 12 353 ± 9107 | .800 |
| Albumin level, g/L, mean ± SD | 37 ± 5 | 36 ± 5 | .022 |
| Urea reduction ratio, mean ± SD | 73 ± 7 | 76 ± 6 | < .001 |
| Thrombophilia, no. (%) | |||
| Factor V Leiden | 7 (6.5) | 6 (1.9) | .046 |
| Prothrombin gene mutation 20210G > A | 3 (2.8) | 9 (2.9) | > .999 |
| Anticardiolipin antibodies (IgG) at least 30 GPL U/mL | 2 (1.9) | 5 (1.6) | > .999 |
| Anticardiolipin antibodies (IgM) at least 30 MPL U/mL | 2 (1.9) | 8 (2.6) | > .999 |
| Lupus anticoagulant | 2 (1.9) | 8 (2.6) | > .999 |
| Factor VIII level greater than 90th percentile* | 18 (16.8) | 30 (9.7) | .047 |
| Homocysteine at least at 85th percentile† | 22 (20.6) | 40 (12.9) | .057 |
| Lipoprotein(a) at least at 85th percentile‡ | 23 (21.5) | 41 (13.3) | .042 |
| Presence of any thrombophilia | 59 (55.1) | 121 (39.2) | .004 |
| TGF-β1 production haplotype, no. (%) | .006 | ||
| High producers | 69 (64.5) | 232 (75.1) | |
| Intermediate producers | 28 (26.2) | 69 (22.3) | |
| Low producers | 10 (9.3) | 8 (2.6) | |
| PAI-1 genotype, no. (%) | .552 | ||
| 4G/4G | 24 (22.4) | 75 (24.3) | |
| 4G/5G | 60 (56.1) | 155 (50.2) | |
| 5G/5G | 23 (21.5) | 79 (25.6) |
TGF-β1 indicates transforming growth factor-β1; PAI-1, plasminogen activator inhibitor type 1; and SD, standard deviation.
Equivalent to > 2.37 × 103 IU/dL.
Equivalent to ≥ 28.9 μM.
Equivalent to ≥ 1.642 μM (46.0 mg/dL).
Influence of the TGF-β1 production haplotype on the occurrence of access thrombosis
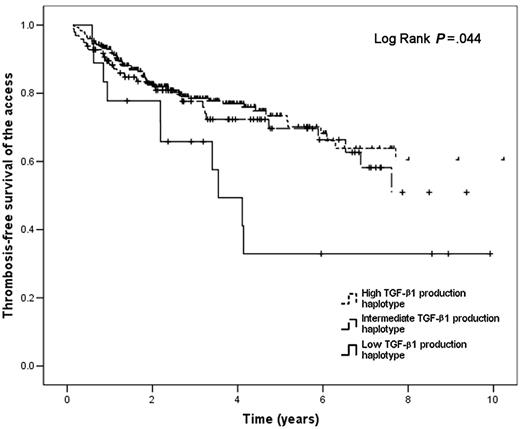
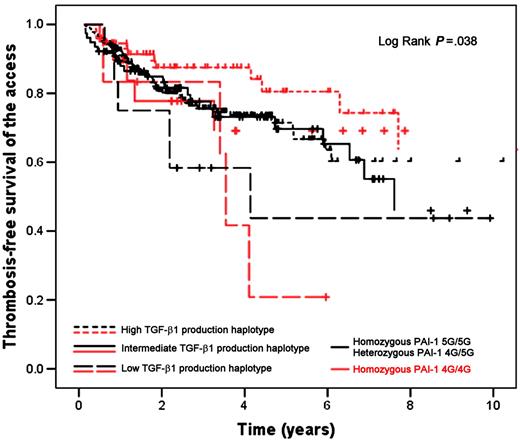
The presence of a low TGF-β1 production haplotype was associated with a higher risk for developing thrombosis of the access (OR 5.11; 95% CI 1.93-13.55) (Table 2). This risk remained significant after adjusting for thrombophilia, type of access (fistula versus graft), time of follow-up of the access, location of the access (lower arm, upper arm, or legs), average albumin, average urea reduction ratio, and average arterial pressure during the 8 weeks preceding the event (OR 7.31; 95% CI 2.15-24.88) (Table 2). The rest of the covariates did not reach the predefined statistical significance for inclusion in the final logistic regression models. The stratified logistic regression analysis showed that the low TGF-β1 production haplotype was associated with a higher risk for developing thrombosis of the access in patients with the 4G/4G PAI-1 genotype (crude OR 9.83; 95% CI 1.61-59.92; P = .013; adjusted OR 33.72; 95% CI 3.25-350.28; P = .003) and also in patients with either one of the 4G/5G or 5G/5G genotypes (crude OR 3.86; 95% CI 1.19-12.56; P = .025; adjusted OR 5.56; 95% CI 1.01-30.69; P = .049). Thrombosis-free survival of the access was shorter in patients with the low TGF-β1 production haplotype (mean 4.94 years; 95% CI 3.06-6.83) when compared with patients with the intermediate (mean 11.96 years; 95% CI 8.67-15.25) and high (mean 14.65 years; 95% CI 12.05-17.25) TGF-β1 production haplotypes (Figure 1), and the difference was statistically significant (P = .044). In the survival analysis stratified according to the PAI-1 SNP (Figure 2) we found a shorter thrombosis-free survival of the access among patients with the low TGF-β1 production haplotype independently of the type of PAI-1 genotype (P = .038).
Logistic regression models examining the odds of access thrombosis associated with the TGF-β1 production haplotypes, PAI-1 genotype, and their interaction in hemodialysis patients
Model, by TGF-β1 production haplotype/PAI-1 genotype . | TGF-β1 production haplotype . | . | PAI-1 genotype . | . | Interaction between TGF-β1 production haplotype and PAI-1 genotype . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |||
| Unadjusted model | |||||||||
| High/5G/5G | Reference | Reference | Reference | Reference | — | — | |||
| Intermediate/4G/5G | 1.30 (0.74-2.29) | .355 | 1.63 (0.87-3.03) | .127 | — | — | |||
| Low/4G/4G | 5.11 (1.93-13.55) | .001 | 1.37 (0.66-2.81) | .399 | 8.23 (1.47-45.97) | .016 | |||
| Model 1 adjusted for thrombophilia | |||||||||
| Intermediate/4G/5G | 1.22 (0.69-2.16) | .501 | 1.77 (0.94-3.34) | .078 | — | — | |||
| Low/4G/4G | 6.16 (2.26-16.79) | < .001 | 1.44 (0.69-2.99) | .334 | 9.63 (1.67-55.61) | .011 | |||
| Model 1 adjusted for thrombophilia and access type | |||||||||
| Intermediate/4G/5G | 1.05 (0.57-1.91) | .881 | 1.52 (0.79-2.91) | .211 | — | — | |||
| Low/4G/4G | 5.00 (1.74-14.42) | .003 | 1.21 (0.57-2.59) | .620 | 9.75 (1.57-60.59) | .015 | |||
| Model 1 adjusted for several factors* | |||||||||
| Intermediate/4G/5G | 1.39 (0.70-2.75) | .347 | 1.59 (0.77-3.30) | .214 | — | — | |||
| Low/4G/4G | 7.31 (2.15-24.88) | .001 | 1.56 (0.67-3.65) | .303 | 19.32 (2.82-132.40) | .003 | |||
Model, by TGF-β1 production haplotype/PAI-1 genotype . | TGF-β1 production haplotype . | . | PAI-1 genotype . | . | Interaction between TGF-β1 production haplotype and PAI-1 genotype . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |||
| Unadjusted model | |||||||||
| High/5G/5G | Reference | Reference | Reference | Reference | — | — | |||
| Intermediate/4G/5G | 1.30 (0.74-2.29) | .355 | 1.63 (0.87-3.03) | .127 | — | — | |||
| Low/4G/4G | 5.11 (1.93-13.55) | .001 | 1.37 (0.66-2.81) | .399 | 8.23 (1.47-45.97) | .016 | |||
| Model 1 adjusted for thrombophilia | |||||||||
| Intermediate/4G/5G | 1.22 (0.69-2.16) | .501 | 1.77 (0.94-3.34) | .078 | — | — | |||
| Low/4G/4G | 6.16 (2.26-16.79) | < .001 | 1.44 (0.69-2.99) | .334 | 9.63 (1.67-55.61) | .011 | |||
| Model 1 adjusted for thrombophilia and access type | |||||||||
| Intermediate/4G/5G | 1.05 (0.57-1.91) | .881 | 1.52 (0.79-2.91) | .211 | — | — | |||
| Low/4G/4G | 5.00 (1.74-14.42) | .003 | 1.21 (0.57-2.59) | .620 | 9.75 (1.57-60.59) | .015 | |||
| Model 1 adjusted for several factors* | |||||||||
| Intermediate/4G/5G | 1.39 (0.70-2.75) | .347 | 1.59 (0.77-3.30) | .214 | — | — | |||
| Low/4G/4G | 7.31 (2.15-24.88) | .001 | 1.56 (0.67-3.65) | .303 | 19.32 (2.82-132.40) | .003 | |||
TGF-β1 indicates transforming growth factor-β1; PAI-1, plasminogen activator inhibitor type 1; OR, odds ratio; CI, confidence interval; —, not significant.
Factors include thrombophilia, access type, time of follow-up of the access, location of the access, average albumin, average urea reduction ratio, and average arterial pressure before event.
Influence of the PAI-1 gene polymorphism on the occurrence of access thrombosis
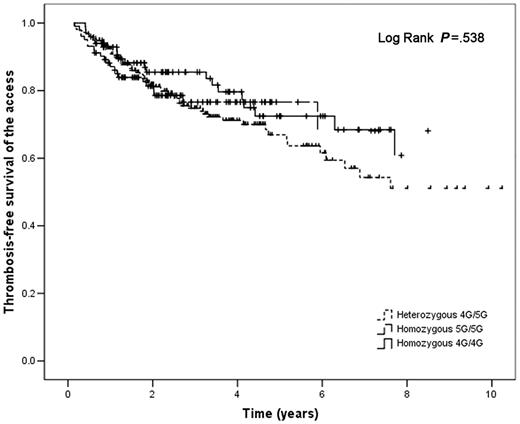
The PAI-1 4G/4G genotype was not associated with an increased risk for developing access thrombosis even after adjusting for significant covariates (Table 2). Survival analysis according to the type of PAI-1 genotype (Figure 3) did not show any difference between groups (P = .538). Survival analysis stratified by type of TGF-β1 production haplotype was consistent with this these results (data not shown).
Influence of the interaction between the TGF-β1 production haplotype and the PAI-1 gene polymorphism on the occurrence of access thrombosis
We found a significant interaction between the low TGF-β1 production haplotype and the 4G/4G genotype of the PAI-1 gene (OR 8.23; 95% CI 1.47-45.97; P = .016) which further increased after adjusting for significant covariates (OR 19.32; 95% CI 2.82-132.40; P = .003) (Table 2). The analysis of the interaction between the TGF-β1 production haplotypes, the PAI-1 gene polymorphism, and the level of lipoprotein(a) resulted in an adjusted OR of 1.134 (95% CI 1.010-1.272; P = .033) in the patients with low TGF-β1 production haplotype and PAI-1 4G/4G genotype. The analysis of a 2 × 4 table for case-control designs showed that the individual OR for access thrombosis was 2.96 (95% CI 0.76-11.39) for the low TGF-β1 production haplotype and 0.81 (95% CI 0.44-1.45) for the 4G/4G PAI-1 genotype. The joint OR was 5.92 (95% CI 0.82-66.21) and the synergy index was 2.47, indicating a departure from multiplicativity of the joint OR. The case-only cross-product calculated using a 2 × 2 case-only table was 2.57 (95% CI 0.48-11.94), again indicating a departure from multiplicativity. The sample size analysis for the case-only design showed that in order to demonstrate a joint effect of 5 with an α equal to 0.05 (2-sided), and under the conditions that we assumed, we would require 84 cases for a β equal to 0.2 and 113 cases for a β equal to 0.1. If the true joint effect were 6 then the number of cases required to demonstrate it with an α equal to 0.01 (2-sided) would be 95 for a β equal to 0.2 and 121 for a β equal to 0.1.
Discussion
Thrombosis is the leading cause of VA failure in hemodialysis patients.3 Several studies have tried to identify genetic or acquired markers potentially predictive of access thrombosis,10,11,27-29 with variable results. In the present study we demonstrated that (1) a low TGF-β1 production haplotype is associated with a high risk for developing VA thrombosis in hemodialysis patients independently of the PAI-1 polymorphism, and (2) the presence of the 4G/4G PAI-1 genotype further increases this risk. Another relevant finding was that in patients with the low TGF-β1 production haplotype and the 4G/4G PAI-1 genotype the odds of access thrombosis increase 13.4% for each .01 g/L (1 mg/dL) increase in lipoprotein(a).
Thrombosis-free survival of the vascular access in hemodialysis patients according to TGF-β1 production haplotype. Solid line indicates low TGF-β1 production haplotype; dashed line, intermediate TGF-β1 production haplotype; dotted line, high TGF-β1 production haplotype.
Thrombosis-free survival of the vascular access in hemodialysis patients according to TGF-β1 production haplotype. Solid line indicates low TGF-β1 production haplotype; dashed line, intermediate TGF-β1 production haplotype; dotted line, high TGF-β1 production haplotype.
A potential limitation of our study is that it was not initially designed to evaluate TGF-β1 haplotypes and therefore the sample size might have been inadequate to determine the presence of a gene-gene interaction using logistic regression. To overcome this risk we used 2 additional analytical strategies. Using a 2 × 4 table analysis for case-control studies we determined the synergy index24 and using a case-only approach we determined the case-only cross-product.24,25 Both showed values greater than 1, indicating a departure from multiplicativity of effects, thus suggesting an interaction between the low TGF-β1 production haplotype and the 4G/4G PAI-1 genotype. An important characteristic of the case-only approach is that although it does not allow estimating individual effects, it is very efficient to demonstrate interactions requiring smaller sample sizes.26 We therefore conducted a case-only sample size analysis, which showed that our sample was adequate to detect a joint effect of 5 at the .05 level of significance; thus we are confident that our results are correct. In addition, the effect modification is also suggested by the survival analyses stratified according to the PAI-1 polymorphism showing that patients with the low TGF-β1 production haplotype had a steeper thrombosis-free survival curve if they also had the 4G/4G PAI-1 genotype.
Thrombosis-free survival of the vascular access in hemodialysis patients according to TGF-β1 production haplotype and stratified by PAI-1 polymorphism. Black lines denote patients with homozygous 5G/5G or heterozygous 4G/5G PAI-1 genotypes; red lines, patients with homozygous 4G/4G PAI-1 genotype; dashed lines, low TGF-β1 production haplotype; solid lines, intermediate TGF-β1 production haplotype; dotted lines, high TGF-β1 production haplotype.
Thrombosis-free survival of the vascular access in hemodialysis patients according to TGF-β1 production haplotype and stratified by PAI-1 polymorphism. Black lines denote patients with homozygous 5G/5G or heterozygous 4G/5G PAI-1 genotypes; red lines, patients with homozygous 4G/4G PAI-1 genotype; dashed lines, low TGF-β1 production haplotype; solid lines, intermediate TGF-β1 production haplotype; dotted lines, high TGF-β1 production haplotype.
Thrombosis-free survival of the vascular access in hemodialysis patients according to PAI-1 genotypes. Solid line indicates homozygous 4G/4G PAI-1 polymorphism; dashed line, homozygous 5G/5G PAI-1 polymorphism; dotted line, heterozygous 4G/5G PAI-1 polymorphism.
Thrombosis-free survival of the vascular access in hemodialysis patients according to PAI-1 genotypes. Solid line indicates homozygous 4G/4G PAI-1 polymorphism; dashed line, homozygous 5G/5G PAI-1 polymorphism; dotted line, heterozygous 4G/5G PAI-1 polymorphism.
Our results contrast with those of Heine and coworkers,10 who described an association of access failure with the high TGF-β1 production haplotype. We believe that this discrepancy is likely due to the fact that they excluded patients with a low TGF-β1 production haplotype from the analysis and because they defined access failure differently than we did. Their definition (eg, the need for any angioplastic or surgical intervention to correct or replace a poorly functioning or nonfunctioning fistula) most likely included patients with stenosed fistulas but no thrombosis. We do not think that population characteristics accounted for the discrepancy because we found similar proportions of patients with each TGF-β1 production haplotype in both studies (χ2 = 1.27; P = .529).
TGF-β1 is a profibrogenic cytokine that regulates the expression of PAI-1 and has been extensively studied in atherosclerosis. Woodward and coworkers18 demonstrated that TGF-β1 directly stimulates the PAI-1 promoter activity and it is conceivable that polymorphisms occurring in the PAI-1 gene might influence the extent of TGF-β1 stimulation or the activity of other unknown stimulatory signals. Even though early studies suggested that TGF-β1 stimulates vascular smooth muscle cell (VSMC) proliferation,30,31 later evidence showed that it indeed inhibits VSMC proliferation32 and migration.33 It is thought that high levels of TGF-β protect from atherosclerosis by favoring the maintenance of small amounts of components of the extracellular matrix (ECM) and that a reduction in TGF-β irreversibly promotes proliferation and migration of VSMC. Further changes in TGF-β activity would result in the formation of both stable and unstable atherosclerotic lesions.34 It has been proposed that stenotic arteriovenous fistulas can be regarded as a model for accelerated atherosclerosis,4 and studies in hemodialysis patients have shown that lipoprotein(a)35 and low TGF-β1 serum levels36 are risk factors for atherosclerosis. It is also known that lipoprotein(a)37 and low shear stress38 might suppress TGF-β activity and that low shear stress also promotes intimal hyperplasia.39 After the initial rise in the shear-stress rate ensuing the creation of a Brescia-Cimino dialysis access, the vessel remodeling process and the adaptive changes finally result in a low pressure–low shear stress environment in the venous segment of the access40,41 and the low blood viscosity in patients with end-stage renal disease further lowers the shear stress.42 Thus, the expression of TGF-β1 within the access could be influenced by timing and anatomical location and the study of the TGF-β1 production haplotypes becomes relevant, especially because a shear response element has been identified in the TGFB1 gene promoter.43
Taking together the previous information and the data of our study, it is possible to speculate on a theoretical model of thrombogenesis in dialysis accesses. The presence of a low TGF-β1 production haplotype facilitates the inhibitory effect of the altered hemodynamic forces within the access, resulting in a disruption of the ECM. A 4G/4G PAI-1 genotype impairs the response of the fibrinolytic system, further enhancing this process. Both alterations would promote fibrosis and the subsequent stenosis of the access, and lipoprotein(a) might further enhance this process. The impairment of the fibrinolytic system by the 4G/4G PAI-1 genotype and possibly the lipoprotein(a) would result in a procoagulant phenotype within the access. A further decrease of TGF-β1 would result in an unstable ECM with endothelial disruption, infiltration by leukocytes, and activation of the coagulation system, finally leading to thrombosis. An appealing characteristic of this model is that it is dynamic and therefore it might help to explain the discrepancies found in the literature regarding the role of TGF-β1 in VA thrombosis. It also raises interesting questions regarding the role of these genes in the pathogenesis of atherosclerosis and thrombosis in other territories. We are currently conducting studies in other populations.
In conclusion, the results of our study demonstrate that the low TGF-β1 production haplotype is an important risk factor for thrombosis of the dialysis access and that this risk is increased by the presence of a PAI-1 gene 4G/4G genotype. Identification of this high-risk population might help determine the need for a more intensive vigilance or prophylaxis and in the future to develop interventions modulating the inflammatory response and/or the vascular remodeling process.
Authorship
Contribution: A.L.-L. designed and conducted research, analyzed data, and wrote the paper; G.A.K. designed and conducted research and collected and analyzed data; P.S.W. designed research, analyzed data, and wrote the paper; N.C. collected and analyzed data; M.A.R. designed and conducted research, collected and analyzed data, and wrote the paper. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 24, 2006; DOI 10.1182/blood-2006-06-028902.
Presented in part at the 38th American Society of Nephrology Renal Week Meeting, Philadelphia, PA, November 10, 2005, and at the 47th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 10, 2005.44
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank the staff and patients from the dialysis units that participated in the study as well as Rebecca Grimwood and Angela Shypipka for laboratory assistance.
This work was supported by the Heart and Stroke Foundation of Ontario (grant no. NA4709). A.L.-L. is the recipient of a Graduate Scholarship from Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico, and is supported in part by an International Fellowship awarded by the University of Ottawa and by Program Grant PRG 5513 of the Heart and Stroke Foundation of Ontario. M.A.R. is supported by the Maureen Andrew New Investigator award from the Heart and Stroke Foundation of Ontario. P.S.W. is supported by the Canada Research Chairs Program.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal