Abstract
We have established an in vivo model for genetic analysis of the inflammatory response by generating a transgenic zebrafish line that expresses GFP under the neutrophil-specific myeloperoxidase promoter. We show that inflammation is induced after transection of the tail of zebrafish larvae and that this inflammation subsequently resolves over a similar time course to mammalian systems. Quantitative data can be generated from this model by counting of fluorescent cells or by digital image analysis. In addition, we show that the resolution of experimentally induced inflammation can be inhibited by the addition of a pancaspase inhibitor, zVD.fmk, demonstrating that experimental manipulation of the resolution of inflammation is possible in this model.
Introduction
Neutrophilic inflammation is essential for the maintenance of health and life, but failure to resolve the response in a timely manner can cause irreparable tissue damage because of the release of toxic granule contents of persisting neutrophils.1 An understanding of the genetic basis of inflammation resolution would undoubtedly provide an important basis for the development of approaches to limiting neutrophil-mediated tissue injury. To facilitate such a genetic analysis, an animal model in which the cellular components of inflammation can be readily visualized in wild-type and genetically manipulated individuals is required.
Zebrafish larvae are transparent, allowing excellent visualization of fluorescent proteins in cellular processes in vivo. Zebrafish neutrophils (heterophils) are identifiable from approximately 48 hours after fertilization,2 and the innate immune system exists in isolation from any adaptive system, which does not arise until approximately 4 weeks after fertilization.3 A range of tools is available for the genetic manipulation of the zebrafish, as are extensive genomics resources, including a draft sequence of the entire genome. We therefore selected this species as an ideal organism for the generation of a simplified, genetically tractable model using fluorescent neutrophils to track the inflammatory response. Here, we report the generation of a transgenic zebrafish line for use in such experiments and describe the onset and resolution of inflammation in this model. We show that inflammation proceeds with kinetics similar to those in mammalian systems and that experimental manipulation of inflammation in this system is achievable and quantifiable.
Materials and methods
Zebrafish were maintained according to standard protocols.4 Reagents were from Sigma (Poole, United Kingdom) unless otherwise specified. zVD.fmk was from Bachem (Weil am Rhein, Germany).
MPO::GFP line
BAC (zC91B8) was modified by the use of a red recombinase system in EL250 cells (gift of Dr Neal Copeland, National Cancer Institute, Frederick, MD).5 EGFP with an SV40 polyadenylation site (Clontech, Palo Alto, CA) was inserted at the mpo (also called mpx) ATG start site. This BAC, linearized with PI-Sce1, was used to generate stable transgenic lines according to published protocols.4
Tail transection
Three- to 5-day-postfertilization (dpf) larvae were anesthetized by immersion in E3 with 4.2% tricaine, and complete transection of the tail was performed with a sterile scalpel, in accordance with United Kingdom Home Office–approved procedures.
Images were acquired with a TE-2000U microscope (Nikon, Tokyo, Japan) and a Hamamatsu (Shizuoka, Japan) Orca-AG camera using IPlab for Windows 3.5 (Scanalytics, Fairfax, VA) or an FV1000 confocal on a BX61 microscope (Olympus, Tokyo, Japan) with FV10-ASWv1.4 software at 20°C to 22°C. Excitation was performed using lasers at 488 and 543 nm. Analysis was performed using IPlab and ImageJ.6 Immersion oil was from Cargille Laboratories (Cedar Grove, NJ).
Myeloperoxidase staining
Larvae fixed in 4% paraformaldehyde/4% sucrose were stained for 10 minutes at 20°C to 22°C in 0.5 mg/mL diaminobenzidine and 0.0003% hydrogen peroxide in PBS/0.1% Triton-X100. In situ hybridizations were developed with Fast Red substrate (Roche, Basel, Switzerland) and were GFP detected with rabbit anti-GFP (Torrey Pines Biolabs, Houston, TX). Data were analyzed (Prism 4.0; GraphPad Software, San Diego, CA) using t tests for paired data and ANOVA (with appropriate posttest adjustment) for other data.
Results and discussion
To study the resolution of neutrophilic inflammation in zebrafish, we initially used a histochemical approach, staining the enzymatic action of a neutrophil-restricted granule protein, myeloperoxidase (MPO). This approach has a number of limitations: it is time consuming, does not allow analysis of the inflammatory response in a single larva over time, and does not permit in vivo analysis. Therefore, we sought to establish a stable transgenic zebrafish line containing fluorescent neutrophils. Existing myeloid transgenics (Pu.17,8 and fli-19 ) are not neutrophil specific; however, in larval zebrafish, expression of the mpo gene is restricted to neutrophil granulocytes and their precursors.2,10 The mpo promoter has been studied in mammalian systems,11,12 but we are unaware of any sequence that directs expression uniquely to MPO-expressing cells in vivo. For this reason, we sought to identify a BAC likely to contain the entire mpo regulatory region. We established 2 transgenic lines, Tg(BACmpo:gfp)i113 and Tg(BACmpo:gfp)i114, from a BAC construct that has GFP with more than 130 kb mpo upstream sequence. GFP expression was detectable by fluorescence microscopy at 30 hours after fertilization around the anterior yolk sac of transgenic embryos. Initially, the signal was faint, but in subsequent hours expression became more clearly visible in cells over the yolk sac and within the embryo (Figure 1A-B). Expression was restricted to motile cells with dynamic morphology typical of polymorphonuclear cells (Video S1, available on the Blood website; see the Supplemental Video link at the top of the online article). GFP expression colocalized with mpo mRNA but not with L-plastin mRNA (Figure 1C-D) and with histochemical staining for myeloperoxidase activity but not with neutral red staining for macrophages (data not shown). Expression extended over subsequent days, such that the total numbers of fluorescent cells seen at 48, 72, and 96 hours were 60 ± 6, 111 ± 10, and 164 ± 10, respectively (mean ± SEM, n = 15), and persisted into adulthood. In addition, immotile GFP-expressing cells likely to be immature myeloid precursors were present in the posterior blood island.
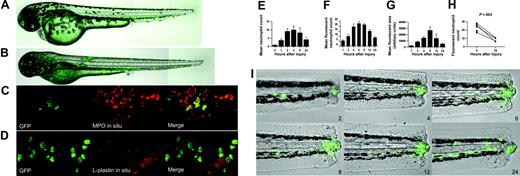
The MPO::GFP construct drives fluorescent protein expression in neutrophils, which participate in a spontaneously resolving inflammatory response to injury. Zebrafish larvae were anesthetized by the addition of 4.2% tricaine to the medium in which they were maintained (E3). GFP was visualized by excitation at 492 nm. All photomicrographs were taken under air immersion using either 10×/0.3 NA or 20× ELWD/0.45 NA Plan Fluor objectives. No nonlinear normalization was performed. Confocal images (C, D) were taken using a 60×/1.40 NA oil immersion Plan Apo objective. (A-B) Photomicrographs of larvae at 56 and 96 hours show expression of GFP over the first days of zebrafish myelopoiesis. (C) Summed Z stacks of consecutive images through the intermediate cell mass (ICM) of a day 3 transgenic zebrafish stained by in situ hybridization for MPO and by immunohistochemistry for GFP. Images taken through the Alexa-488 and TRITC channels are shown, followed by a merged image. In all GFP-positive cells (green), some MPO in situ signal (red) can be seen. Examination of individual sections confirms colocalization of these markers. (D) Summed Z stacks of consecutive images through the ICM of a day 3 transgenic zebrafish stained by in situ hybridization for L-plastin and by immunohistochemistry for GFP. Images taken through the Alexa-488 and TRITC channels are shown, followed by a merged image. GFP-positive cells (green) do not coexpress L-plastin (red). Examination of individual sections confirms absence of colocalization of these markers. (E) Tailfins of anesthetized wild-type AB zebrafish were transected at 4 days after fertilization. At the time points indicated, fish were anesthetized, then fixed and stained as described in “Materials and methods.” The number of neutrophils participating in the inflammatory response (excluding cells in the posterior blood island) was assessed. Data shown are mean ± SEM, n = 3, with 5 replicates per experiment. (F) Tailfins of anesthetized transgenic zebrafish were transected at 4 days after fertilization. At the time points indicated, individual fish were anesthetized and imaged as described. The number of fluorescent neutrophils participating in the inflammatory response was assessed. Data shown are mean ± SEM (n = 5). (G) Images taken for the counts shown in panel E were processed (IPlab) to quantify the total area of fluorescence. For each image, a series of fluorescence images in Z were projected into a single image using the maximum intensity at each point. These images were normalized, and a region of interest was defined corresponding to the area of inflammation quantitated. Segmentation of this region was performed in IPlab, and the area of fluorescence was exported to Excel. The procedure was automated by use of an IPlab script. (H) Fluorescence counts of individual larvae at 6 and 24 hours after injury (hpi) are shown. The individual pattern of resolution is evident for each fish. (I) Images showing a single transgenic zebrafish larva at the time points indicated (hpi). The accumulation of fluorescent cells and their subsequent removal can be clearly seen.
The MPO::GFP construct drives fluorescent protein expression in neutrophils, which participate in a spontaneously resolving inflammatory response to injury. Zebrafish larvae were anesthetized by the addition of 4.2% tricaine to the medium in which they were maintained (E3). GFP was visualized by excitation at 492 nm. All photomicrographs were taken under air immersion using either 10×/0.3 NA or 20× ELWD/0.45 NA Plan Fluor objectives. No nonlinear normalization was performed. Confocal images (C, D) were taken using a 60×/1.40 NA oil immersion Plan Apo objective. (A-B) Photomicrographs of larvae at 56 and 96 hours show expression of GFP over the first days of zebrafish myelopoiesis. (C) Summed Z stacks of consecutive images through the intermediate cell mass (ICM) of a day 3 transgenic zebrafish stained by in situ hybridization for MPO and by immunohistochemistry for GFP. Images taken through the Alexa-488 and TRITC channels are shown, followed by a merged image. In all GFP-positive cells (green), some MPO in situ signal (red) can be seen. Examination of individual sections confirms colocalization of these markers. (D) Summed Z stacks of consecutive images through the ICM of a day 3 transgenic zebrafish stained by in situ hybridization for L-plastin and by immunohistochemistry for GFP. Images taken through the Alexa-488 and TRITC channels are shown, followed by a merged image. GFP-positive cells (green) do not coexpress L-plastin (red). Examination of individual sections confirms absence of colocalization of these markers. (E) Tailfins of anesthetized wild-type AB zebrafish were transected at 4 days after fertilization. At the time points indicated, fish were anesthetized, then fixed and stained as described in “Materials and methods.” The number of neutrophils participating in the inflammatory response (excluding cells in the posterior blood island) was assessed. Data shown are mean ± SEM, n = 3, with 5 replicates per experiment. (F) Tailfins of anesthetized transgenic zebrafish were transected at 4 days after fertilization. At the time points indicated, individual fish were anesthetized and imaged as described. The number of fluorescent neutrophils participating in the inflammatory response was assessed. Data shown are mean ± SEM (n = 5). (G) Images taken for the counts shown in panel E were processed (IPlab) to quantify the total area of fluorescence. For each image, a series of fluorescence images in Z were projected into a single image using the maximum intensity at each point. These images were normalized, and a region of interest was defined corresponding to the area of inflammation quantitated. Segmentation of this region was performed in IPlab, and the area of fluorescence was exported to Excel. The procedure was automated by use of an IPlab script. (H) Fluorescence counts of individual larvae at 6 and 24 hours after injury (hpi) are shown. The individual pattern of resolution is evident for each fish. (I) Images showing a single transgenic zebrafish larva at the time points indicated (hpi). The accumulation of fluorescent cells and their subsequent removal can be clearly seen.
To assess the response of fluorescent neutrophils to an inflammatory stimulus, we performed a tail transection under tricaine anesthesia that caused GFP cells to be recruited to the site of injury. By time-lapse video microscopy (Video S1), these cells are seen to be exiting the vasculature and entering the tailfin, suggesting that although GFP-positive cells were present in the tissues of uninjured fish, the principal route of delivering inflammatory cells to a site of injury is through the circulation.
The time course of inflammation after tail transection of wild-type zebrafish can be studied with the use of MPO staining and by counting neutrophils at various time points in parallel groups of fish. MPO-positive cells accumulated 6 to 12 hours after injury, followed by a phase of resolution that was largely complete by 24 hours (Figure 1E). GFP-labeled neutrophils enabled such analyses to be performed on individual living fish. Counting fluorescent cells participating in the inflammatory response confirmed that a wave of neutrophil influx followed injury and resolved within the next 24 hours (Figure 1F), demonstrating the capacity of this system to generate quantitative data on the degree of inflammation at any chosen time. Two further advantages of the transgenic approach are apparent. First, it permitted automated assessment of the fluorescent area, which correlated well with manual counting (Figure 1G). Second, the progress of inflammation within a single larva could be assessed (Figure 1H-I).
Resolution of inflammation is thought to depend on the death of inflammatory neutrophils by apoptosis.1 Indirect evidence has suggested that the caspase inhibitor zVD.fmk13 should delay neutrophil apoptosis and, hence, normal resolution of inflammation.14,15 We tested this hypothesis with our transgenic model. The extent of resolution that occurred between 6 and 24 hours after injury was significantly reduced by treatment with zVD.fmk (Figure 2A-B). This effect was clearly demonstrated when the response was assessed in individual larvae (Figure 2C). One explanation for this is that caspase-dependent apoptosis is important for determining the lifespan of the neutrophil during inflammation. If the hypothesis is true, this would be the first direct evidence linking the inhibition of neutrophil apoptosis with a delay in the resolution of inflammation. To assess whether the lifespan of circulating neutrophils is altered by caspase inhibition, we assessed total neutrophil counts at 3 and 4 days in uninjured control and zVD.fmk-treated larvae. The mean increase in neutrophil count in that 24-hour period was 99.8 ± 10.5 in control larvae and 132.8 ± 14.6 in zVD.fmk-treated larvae (mean ± SEM; n = 10; P < .05). This increase in total neutrophil numbers was consistent with a role for caspase activity in determining neutrophil lifespan.
Resolution of inflammation is modulated by inhibition of the caspase family of apoptotic proteases. Tailfins of transgenic zebrafish were transected at 4 days after fertilization. At 4 hours after injury, either control medium (E3 only) or 100 μM zVD.fmk was added to the culture medium. At 6 and 24 hours after injury, each fish was taken individually and anesthetized with tricaine, and the number of fluorescent cells visible in the tail was assessed by fluorescence microscopy as described for Figure 1. Larvae treated with DMSO vehicle at equivalent concentrations were indistinguishable from controls (data not shown). (A) The region to the right of the black line corresponds to the region included in the analysis shown here. The number of cells to the right of this line was reduced from 6 to 24 hours in control fish but remained the same in zVD.fmk-treated fish. For each image, a series of fluorescence images in Z was projected to a single image using the maximum intensity at each point. These images were normalized and superimposed in the best in-focus image from a bright-field stack or an extended depth-of-field image generated from the best in-focus information from each image in the Z series. (B) Fluorescent cells at the site of injury were counted for the conditions shown. The reduction in neutrophil numbers was not seen for zVD.fmk-treated larvae (n = 16-19; P < .05 [control 24 hours after injury vs all other groups]). (C) The reduction in neutrophil numbers seen in each individual fish in the control group was not seen in the zVD.fmk-treated group (P values shown).
Resolution of inflammation is modulated by inhibition of the caspase family of apoptotic proteases. Tailfins of transgenic zebrafish were transected at 4 days after fertilization. At 4 hours after injury, either control medium (E3 only) or 100 μM zVD.fmk was added to the culture medium. At 6 and 24 hours after injury, each fish was taken individually and anesthetized with tricaine, and the number of fluorescent cells visible in the tail was assessed by fluorescence microscopy as described for Figure 1. Larvae treated with DMSO vehicle at equivalent concentrations were indistinguishable from controls (data not shown). (A) The region to the right of the black line corresponds to the region included in the analysis shown here. The number of cells to the right of this line was reduced from 6 to 24 hours in control fish but remained the same in zVD.fmk-treated fish. For each image, a series of fluorescence images in Z was projected to a single image using the maximum intensity at each point. These images were normalized and superimposed in the best in-focus image from a bright-field stack or an extended depth-of-field image generated from the best in-focus information from each image in the Z series. (B) Fluorescent cells at the site of injury were counted for the conditions shown. The reduction in neutrophil numbers was not seen for zVD.fmk-treated larvae (n = 16-19; P < .05 [control 24 hours after injury vs all other groups]). (C) The reduction in neutrophil numbers seen in each individual fish in the control group was not seen in the zVD.fmk-treated group (P values shown).
Authorship
Contribution: S.A.R., M.K.B.W., and P.W.I. designed the study and wrote the manuscript. S.A.R., C.A.L., D.M.I.T., and S.E. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 22, 2006; DOI 10.1182/blood-2006-05-024075.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by an MRC Clinician Scientist Fellowship (reference number G108/595) (S.A.R.) and by an MRC Centre Development Grant and an MRC Programme Grant (reference numbers G0400100 and G0100151) (P.W.I.).
The authors thank the MRC CDBG aquarium staff, especially Lisa Gleadall, for their expert care of the zebrafish, and all members of the Ingham laboratory who provided guidance and help in establishing this model.

![Figure 2. Resolution of inflammation is modulated by inhibition of the caspase family of apoptotic proteases. Tailfins of transgenic zebrafish were transected at 4 days after fertilization. At 4 hours after injury, either control medium (E3 only) or 100 μM zVD.fmk was added to the culture medium. At 6 and 24 hours after injury, each fish was taken individually and anesthetized with tricaine, and the number of fluorescent cells visible in the tail was assessed by fluorescence microscopy as described for Figure 1. Larvae treated with DMSO vehicle at equivalent concentrations were indistinguishable from controls (data not shown). (A) The region to the right of the black line corresponds to the region included in the analysis shown here. The number of cells to the right of this line was reduced from 6 to 24 hours in control fish but remained the same in zVD.fmk-treated fish. For each image, a series of fluorescence images in Z was projected to a single image using the maximum intensity at each point. These images were normalized and superimposed in the best in-focus image from a bright-field stack or an extended depth-of-field image generated from the best in-focus information from each image in the Z series. (B) Fluorescent cells at the site of injury were counted for the conditions shown. The reduction in neutrophil numbers was not seen for zVD.fmk-treated larvae (n = 16-19; P < .05 [control 24 hours after injury vs all other groups]). (C) The reduction in neutrophil numbers seen in each individual fish in the control group was not seen in the zVD.fmk-treated group (P values shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/13/10.1182_blood-2006-05-024075/8/m_zh80240605230002.jpeg?Expires=1766514146&Signature=clNRCwda-AkJWFwy~GtwJmiDSQVVBKTawrvIasz7MKccBtfxRvUFtkdKNqptqqHP8ZfHRVkEyywXrDQpLqbyF-3Vwquy0iAPK4Loeb8dT~3I782-B4W3f1C2Q~g9TSTrQ-bUN5F7kw43w3tC9ng8rw2rf07xCStShZTHsDFpO6HIysNObLr4bhiA4~SaTrK~q5X70fAL~TOaZ0qftJOKpfnTIzPRsyxANFLxv9rqjywccYiKs2Ja5GvLs9MR9nU7T993gK~zXd8cEt9aV9QdVZ0cpsIGlufbabSxJg1dTCBjWib1OtL7-TMbQ4sBHoce2nyJ~fOOBFHxfYH2I1a9xg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal