Comment on Hoffmann et al, page 4260
Human CD45RA+ Treg cells can proliferate in vitro without losing Treg-cell phenotype, suppressive activity, or the expression of lymph node–homing molecules.
The interest in regulatory T (Treg) cells has been renewed since Sakaguchi et al1 reported that Treg cells could be identified based on the expression CD25. Further studies demonstrate that Treg cells are critical in maintaining self-tolerance. Adoptive transfer of Treg cells is able to prevent and to treat a variety of autoimmune diseases. Moreover, Treg cells have the ability to control both transplant rejection and graft-versus-host disease. Despite the dramatic advances in our understanding of Treg cells and the great potentials of these cells in treating a wide vaiety of diseases, progress in clinical applications of Treg cells has been slow. One of the difficulties is the low number of Treg cells that can be harvested. Human adoptive Treg-cell therapy will likely require ex vivo expansion. CD4 and CD25 have been used to isolate Treg cells for ex vivo expansion. However, CD4+CD25+ T cells are not homogenous and contain both Treg cells and conventional T cells. Current expansion protocols, which usually use natural or artificial antigen-presenting cells and interleukin-2, activate not only Treg cells but also conventional T cells. Because it takes a longer time for Treg cells to enter the S phase, conventional T cells may even outgrow Treg cells. Treg cells can also lose suppressive activity after repetitive stimulation in vitro. Another technical challenge is how to expand Treg cells without losing Treg-cell phenotype and suppressive activity.FIG1
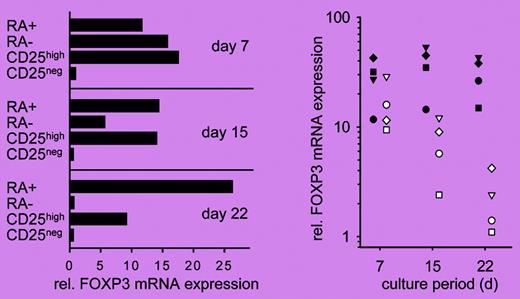
Only the CD45RA+ subset of CD4+CD25high human Treg cells maintains the expression of FOXP3 after expansion. See the complete figure in the article beginning on page 4260.
Only the CD45RA+ subset of CD4+CD25high human Treg cells maintains the expression of FOXP3 after expansion. See the complete figure in the article beginning on page 4260.
In this issue of Blood, Hoffmann and colleagues describe a novel strategy for ex vivo expansion of Treg cells without losing Treg-cell phenotype and suppressive activity. The authors demonstrate that Treg-cell phenotype, FOXP3 expression, and suppressive activity are maintained only in Treg cells that coexpress CD62L and CCR7, 2 lymph node–homing molecules, after expansion. Further analyses demonstrate that only the CD45RA+ but not the CD45RA– or CD62L+CCR7+ subsets of CD4+CD25high T cells maintain Treg-cell phenotype (see figure) and function, as well as the expression of CD62L and CCR7 throughout the culture. This strategy will be able to produce Treg cells with improved purity. The ability of this strategy to maintain the expression of CD62L and CCR7 may also be important for the function of Treg cells in vivo because recent data suggest that the CD62L+ subset is much better than the CD62L– subset of Treg cells in treating both autoimmunity2 and graft-versus-host disease3,4 in animal models.
One of the surprising findings is that naive Treg cells are able to maintain the expression of CD62L and CCR7. This is unique because the expression of CD62L is usually down-regulated in conventional T cells after activation. More work is needed to understand the underlying mechanisms. It also remains to be determined whether this strategy can be successfully used to expand antigen-specific Treg cells, which are more effective and specific in controlling immune responses. In addition, these new findings may suggest that the expansion of Treg cells will be challenging in elderly individuals since the number of naive T cells, including naive Treg cells, declines with age. Nevertheless, if these results can be reproduced in a large-scale setting, the strategy described in this paper would allow the production of large numbers of relatively pure Treg cells for adoptive transfer.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal