Abstract
Although the graft-versus-leukemia effect of allogeneic bone marrow transplantation (BMT) is of paramount importance in the maintenance of disease remission, the role played by the autologous T-cell response in antitumor immune surveillance is less defined. We evaluated the emergence of antileukemia cytotoxic T-lymphocyte precursors (CTLp's) and the correlation of this phenomenon with maintenance of hematologic remission in 16 children with acute myeloid leukemia (AML), treated with either chemotherapy alone (5 patients) or with autologous BMT (A-BMT, 11 patients). Antileukemia CTLp's were detectable in 8 patients in remission after induction chemotherapy; none of them subsequently had a relapse. Of the 8 patients who did not show detectable CTLp frequency while in remission after induction chemotherapy, 7 subsequently experienced leukemia relapse. In patients undergoing A-BMT, molecular fingerprinting of the TCR-Vβ repertoire, performed on antileukemia lines, demonstrated that selected antileukemia T-cell clonotypes, detectable in bone marrow before transplantation, survived ex vivo pharmacologic purging and were found in the recipient after A-BMT. These data provide evidence for an active role of autologous T cells in the maintenance of hematologic remission and also suggest that quantification of antileukemia CTLp frequency may be a useful tool to identify patients at high risk for relapse, thus potentially benefiting from an allogeneic antitumor effect.
Introduction
T lymphocytes specific for tumor-associated antigens (TAAs) are believed to play an important role of immune surveillance in patients with hematologic malignances who are able to control the disease. Evidence supporting this hypothesis stems mainly from the demonstration that cytotoxic T lymphocytes (CTLs) specific for the peptide PR1, derived from proteinase 3, are detectable in patients with Philadelphia-positive chronic myeloid leukemia (CML) in complete cytogenetic remission after treatment with interferon-α2b (IFN-α), suggesting a role for this cytokine in stimulating an otherwise silent, endogenous antileukemia T-cell response.1 Bone marrow (BM)–derived effector T cells specific for preneoplastic plasma cells are also detectable in patients with monoclonal gammopathy of undetermined significance (MGUS), but not in patients with a clinical diagnosis of multiple myeloma, thus suggesting a tumor-specific immune recognition in human preneoplasia, which influences the early growth of transformed cells.2 Moreover, BM of patients affected by either hematologic or solid cancer may contain memory T cells specific for neoplastic cells,3-5 and studies in mice indicate that BM and lymph nodes are privileged sites where potentially lethal tumor cells are controlled in a dormant state by the immune system.6,7
A relevant amount of data proving the key role played by the T-cell response against neoplastic cells also derives from clinical and experimental studies on patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) for hematologic malignancies, in whom the donor immune system plays a critical role for prevention or control of tumor relapse.1,8-12 Widely distributed minor histocompatibility antigens account for the graft-versus-leukemia (GVL) effect associated with graft-versus-host disease (GVHD), whereas tissue-restricted or leukemia-specific antigens can elicit a specific, T cell–mediated GVL reaction.1,8-12 CTLs directed against leukemia-specific antigens are likely to develop following encounter with residual leukemia blasts (LBs), escaping pretransplantation chemotherapy or radiotherapy, and are believed to significantly contribute to maintenance of a state of hematologic remission.11,12
Despite this large body of evidence on the presence of antitumor CTLs in some hematologic malignancies, such as Philadelphia-positive CML and MGUS, and on the allogeneic, T cell–mediated, GVL effect, it remains to be proven that antileukemia T cells spontaneously emerge and exert an efficient immune surveillance also in patients treated for acute myeloid leukemia (AML) with chemotherapy alone or followed by autologous bone marrow transplantation (A-BMT).
To address this issue, in children with AML, either treated with chemotherapy alone or also undergoing A-BMT, we evaluated the frequency of antileukemia CTL precursors (CTLp's) in the perspective of finding a correlation between their generation and persistence over time and a sustained hematologic remission. We also investigated, in patients undergoing A-BMT: (1) the frequency of antileukemia CTLp's in BM collected at time of harvesting and treated ex vivo with pharmacologic purging using mafosfamide; and (2) through the T-cell receptor Vβ (TCR-Vβ) molecular fingerprint method,13 whether these antileukemia CTLp's can be transferred with the graft and expand in vivo after transplantation, becoming detectable in patients' peripheral blood.
Patients, materials, and methods
The Institutional Review Board of Pediatric Hematology and Oncology, Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy, approved the design of this study.
Patients
This study includes consecutive patients with AML treated with chemotherapy alone or undergoing A-BMT who: (1) were younger than 18 years old at time of diagnosis; (2) were diagnosed between January 1999 and June 2004 at the Pediatric Hematology Unit of IRCCS Policlinico San Matteo, Pavia, Italy; (3) had at least 50 × 106 LBs collected and cryopreserved at time of diagnosis; (4) lacked an HLA-identical sibling; and (5) were in morphologic complete remission (CR) at the time of CTLp frequency evaluation.
Sixteen children with AML were enrolled consecutively in this study. Clinical characteristics of the children enrolled in the study are detailed in Tables 1 and 2. The initial diagnosis of AML with its subtype was established according to the French-American-British (FAB) classification.14 Eight additional children with AML diagnosed in the same time period were not included in the study either because they received allogeneic BM transplants as consolidation therapy (5 cases) or did not achieve morphologic CR (1 case), or did not have a sufficient number of cryopreserved blast cells (2 cases).
Characteristics of patients given chemotherapy alone
Patient no. . | FAB classification . | Sex . | Age at diagnosis, y . | Cytogenetic/genetic anomalies . | WBC count at diagnosis, × 109/L . | First-line chemotherapy . | Outcome (+ mo elapsed from diagnosis) . |
|---|---|---|---|---|---|---|---|
| 1 | M3 | M | 8 | t(15; 17) | 1.8 | *ATRA + IDA; ARA-C + IDA; MTZ + VP-16 ARA-C + IDA + 6-TG | A&W (+74) |
| 2 | M4 | F | 3 | inv(16) | 105 | ICE (3 + 5 + 10) × 2; AVE; HAM | Relapsed (+5). Died after unrelated donor BMT |
| 3 | M3 | F | 6 | PML/RARα fusion transcript | 2.7 | *ATRA + IDA; ARA-C + IDA; MTZ + VP-16 ARA-C + IDA + 6-TG | Relapsed (+13). A&W (+26) after unrelated donor BMT |
| 4 | M2 | M | 13 | t(8;21) | 12.8 | ICE (3 + 5 + 10) × 2; AVE; HAM; HD-ARA-C | Relapsed (+17). A&W (+7) after unrelated donor BMT |
| 5 | M2 | M | 11 | t(8;21) | 15 | ICE (3 + 5 + 10) × 2; AVE; HAM; HD-ARA-C | A&W (+38) |
Patient no. . | FAB classification . | Sex . | Age at diagnosis, y . | Cytogenetic/genetic anomalies . | WBC count at diagnosis, × 109/L . | First-line chemotherapy . | Outcome (+ mo elapsed from diagnosis) . |
|---|---|---|---|---|---|---|---|
| 1 | M3 | M | 8 | t(15; 17) | 1.8 | *ATRA + IDA; ARA-C + IDA; MTZ + VP-16 ARA-C + IDA + 6-TG | A&W (+74) |
| 2 | M4 | F | 3 | inv(16) | 105 | ICE (3 + 5 + 10) × 2; AVE; HAM | Relapsed (+5). Died after unrelated donor BMT |
| 3 | M3 | F | 6 | PML/RARα fusion transcript | 2.7 | *ATRA + IDA; ARA-C + IDA; MTZ + VP-16 ARA-C + IDA + 6-TG | Relapsed (+13). A&W (+26) after unrelated donor BMT |
| 4 | M2 | M | 13 | t(8;21) | 12.8 | ICE (3 + 5 + 10) × 2; AVE; HAM; HD-ARA-C | Relapsed (+17). A&W (+7) after unrelated donor BMT |
| 5 | M2 | M | 11 | t(8;21) | 15 | ICE (3 + 5 + 10) × 2; AVE; HAM; HD-ARA-C | A&W (+38) |
FAB indicates French-American-British classification; WBC, white blood cell; A&W, alive and well; ICE, idarubicin 12 mg/m2/d 60-minute infusion on days 1, 3, 5; etoposide 100 mg/m2/d 1-hour intravenous (IV) infusion on days 1-5; cytarabine 25 mg/m2/d IV push on day 1 followed by 100 mg/m2/d continuous IV infusion on days 1-10; AVE, high-dose cytarabine 3 g/m2 3-hour infusion every 12 hours and etoposide 125 mg/m2/d 1-hour IV infusion for 4 days; HAM, high-dose cytarabine 3 g/m2 3-hour infusion every 12 hours for 3 days and mitoxantrone 10 mg/m2 60-minute infusion for 2 days; HD-ARA-C, high-dose cytarabine 3 g/m2 3-hour infusion every 12 hours for 3 days.
Induction therapy consisted of oral all-trans-retinoic acid (ATRA) 25 mg/m2/d, divided into 2 doses administered every 12 hours, up to hematologic remission and for a maximum of 90 days, and 4 brief intravenous infusions of idarubicin (IDA) 12 mg/m2 on days 2, 4, 6, and 8. Patients in hematologic complete remission (HCR) received 3 monthly consolidation courses consisting of cytosine arabinoside (ARA-C) 1000 mg/m2/d by IV infusion over 6 hours on days 1, 2, 3, and 4 and IDA 5 mg/m2/d by brief IV infusion on days 1, 2, 3, and 4, 3 hours after the end of the ARA-C infusion (course 1); mitoxantrone (MTZ) 10 mg/m2/d by brief IV infusion on days 1, 2, 3, 4, and 5 and etoposide (VP-16) 100 mg/m2/d by IV infusion lasting 45-60 minutes on days 1, 2, 3, 4, and 5 (12 hours after the start of MTZ) (course 2); IDA 12 mg/m2 IV infusion on day 1, ARA-C 150 mg/m2 every 8 hours given subcutaneously on days 1, 2, 3, 4, and 5, and 6-thioguanine (6-TG) 70 mg/m2 every 8 hours on days 1, 2, 3, 4, and 5 (course 3).
Characteristics of patients undergoing A-BMT
Patient no. . | FAB classification . | Sex . | Age at diagnosis, y . | Cytogenetic data . | WBC count at diagnosis, × 109/L . | First-line chemotherapy (complete remission after induction therapy: yes/no) . | Conditioning regimen . | Interval between diagnosis and A-BMT, mo . | No. of cells infused, × 108/kg . | Outcome (+mo elapsed from A-BMT) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | M2 | F | 2.4 | t(6;9) | 66 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 1.5 | A&W (+64) |
| 7 | M5a | M | 14 | Normal karyotype | 4.6 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 1.0 | A&W (+47) |
| 8 | M5a | M | 9 | (45,XY)-7 | 2 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.4 | A&W (+42) |
| 9 | M6 | F | 7.5 | Normal karyotype | 70 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.0 | Relapsed (+5); died due to disease progression |
| 10 | M4 | M | 15.2 | Normal karyotype | 192 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 5 | 1.3 | Relapsed (+6); died after unrelated donor BMT |
| 11 | M5a | M | 1.2 | Normal karyotype | 132 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | BAVC | 4 | 3.7 | Relapsed (+9); died after haploidentical BMT |
| 12 | M4 | M | 1.7 | Normal karyotype | 78 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | BU/CY/L-PAM | 5 | 4.4 | A&W (+31) |
| 13 | M1 | M | 14 | Normal karyotype | 23 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 1.2 | A&W (+30) |
| 14 | M2 | F | 7 | Normal karyotype | 2.7 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.5 | Relapsed (+9); A&W after unrelated donor BMT |
| 15 | M5a | M | 16 | t(9;11) | 99 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.3 | A&W (+21) |
| 16 | M5a | F | 1 | Normal karyotype | 38 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 7 | 4.5 | A&W (+68) |
Patient no. . | FAB classification . | Sex . | Age at diagnosis, y . | Cytogenetic data . | WBC count at diagnosis, × 109/L . | First-line chemotherapy (complete remission after induction therapy: yes/no) . | Conditioning regimen . | Interval between diagnosis and A-BMT, mo . | No. of cells infused, × 108/kg . | Outcome (+mo elapsed from A-BMT) . |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | M2 | F | 2.4 | t(6;9) | 66 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 1.5 | A&W (+64) |
| 7 | M5a | M | 14 | Normal karyotype | 4.6 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 1.0 | A&W (+47) |
| 8 | M5a | M | 9 | (45,XY)-7 | 2 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.4 | A&W (+42) |
| 9 | M6 | F | 7.5 | Normal karyotype | 70 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.0 | Relapsed (+5); died due to disease progression |
| 10 | M4 | M | 15.2 | Normal karyotype | 192 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 5 | 1.3 | Relapsed (+6); died after unrelated donor BMT |
| 11 | M5a | M | 1.2 | Normal karyotype | 132 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | BAVC | 4 | 3.7 | Relapsed (+9); died after haploidentical BMT |
| 12 | M4 | M | 1.7 | Normal karyotype | 78 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | BU/CY/L-PAM | 5 | 4.4 | A&W (+31) |
| 13 | M1 | M | 14 | Normal karyotype | 23 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 1.2 | A&W (+30) |
| 14 | M2 | F | 7 | Normal karyotype | 2.7 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.5 | Relapsed (+9); A&W after unrelated donor BMT |
| 15 | M5a | M | 16 | t(9;11) | 99 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 4 | 2.3 | A&W (+21) |
| 16 | M5a | F | 1 | Normal karyotype | 38 | ICE (3 + 5 + 10) × 2; AVE; HAM (yes) | TBI + L-PAM | 7 | 4.5 | A&W (+68) |
ICE indicates idarubicin 12 mg/m2/d 60-minute infusion on days 1, 3, 5; etoposide 100 mg/m2/d 1-hour IV infusion on days 1-5; cytarabine 25 mg/m2/d IV push on day 1 followed by 100 mg/m2/d continuous IV infusion on days 1-10; AVE, high-dose cytarabine 3 g/m2 3-hour infusion every 12 hours for 3 days and etoposide 125 mg/m2/d 1-hour IV infusion for 4 days; HAM, high-dose cytarabine 3 g/m2 3-hour infusion every 12 hours for 3 days and mitoxantrone 10 mg/m2 60-minute infusion for 2 days; TBI, total body irradiation (990-1200 cGy); L-PAM, melphalan (140 mg/m2); BU, busulfan (16 mg/kg); CY, cyclophosphamide (120 mg/kg); BAVC, BCNU (800 mg/m2), amsacrine (450 mg/m2), etoposide (450 mg/m2), and cytarabine (900 mg/m2).
Five patients received chemotherapy alone, and 11 patients were also given A-BM transplants in first CR after a fully myeloablative conditioning regimen, which, in all patients but one, included either fractionated total body irradiation (TBI; 990-1200 cGy in 3-6 fractions) or busulfan (16 mg/kg; Table 2). The choice of chemotherapy alone versus A-BMT as consolidation therapy was based on the presence of specific genetic anomalies, namely AML1-ETO fusion transcript, anomalies of CBF-β and either translocations t(15;17) or PML/RARα fusion transcript. In fact, several studies have demonstrated that, as compared to other patients with AML, individuals with one of these favorable genetic anomalies have a better probability of survival and may benefit from less intensive postremission therapies, not including transplantation.15-17 All patients given chemotherapy alone as postremission therapy showed one of these favorable genetic anomalies (see Table 1 for details).
All patients, except those with acute promyelocytic leukemia, received 2 cycles of a 10-day induction therapy including idarubicin, cytarabine, and etoposide (ICE 3 + 5 + 10).18 After achieving CR, these patients received 2 courses of consolidation therapy. These regimens included mainly high-dose cytarabine, which was given at different dosages together with other drugs in combination (ie, anthracyclines and epipodophyllotoxins). Then, patients were given either a further course of consolidation therapy based on the use of high-dose cytarabine or A-BMT. Patients with acute promyelocytic leukemia were treated according to a recently published strategy including chemotherapy and all-trans retinoic acid.17
In all patients undergoing A-BMT, pharmacologic purging of BM cells was performed with standard mafosfamide dose according to a previously described method.19
All patients included in the study, during the follow-up, were routinely evaluated and both those given chemotherapy and those undergoing A-BMT did not experience any relevant infection.
Written informed consent in accordance with the Declaration of Helsinki from patients' parents to collect LBs, BM, and peripheral blood (PB), as well as to analyze antileukemia T cells, was obtained in all cases.
Cell preparation
The frequency of antileukemia CTLp's was evaluated by limiting dilution analysis (LDA) in PB of the 5 AML pediatric patients treated with chemotherapy alone, at various time points after achieving CR, and in both PB and BM of the 11 patients undergoing A-BMT. In particular, BM cells were cryopreserved after in vitro purging with mafosfamide, while PB mononuclear cells (PBMCs) were collected in patients undergoing A-BMT 2 and 6 months after the procedure. In 4 of these 11 patients, BM cells were also collected 2 months after transplantation.
LBs were obtained from the patients at time of diagnosis. PBMCs, BM cells, and LBs were isolated by Ficoll-Hypaque density gradient, cryopreserved in fetal calf serum (FCS), 10% DMSO and stored in liquid nitrogen for later use. After thawing, LBs displayed the same phenotype observed at the diagnosis and their viability was in the range of 70% to 90%. The medium used for the cultures was RPMI 1640 (Gibco BRL, Life Technologies, Paisley, Scotland), supplemented with 2 mM l-glutamine, 50 μg/mL gentamicin, and 5% pooled human serum (HS).
Evaluation of leukemia-specific CTLp frequencies by LDA
The medium used was RPMI-HS supplemented with 100 U/mL recombinant human interleukin 2 (rIL-2). Patient's PBMCs or BM cells were used as responder cells, and autologous LBs were used as stimulators. Decreasing numbers of responder cells (8 × 104, 4 × 104, 2 × 104, 104, 5 × 103, 2.5 × 103, 1.25 × 103) were seeded in 96-round bottom well microplates with 2 × 104 irradiated (30 Gy) LBs, in a total volume of 200 μL. Control wells contained irradiated autologous LBs, without effector cells. The cultures were incubated 37°C in 5% CO2 for 10 days.
Cytotoxicity assay
On day 10, cultures were assayed for cytolytic activity against 51Cr-labeled targets that included patient LBs and patient BM remission cells (BMRCs), which were used as controls according to a previously described method.11 Assay wells were accurately resuspended and 2 aliquots of 70 μL were transferred from each well in other microplates for simultaneous evaluation of CTLp frequency against LBs and BMRCs in the cytotoxicity assay; about 30 μL for each well was used for growing antileukemia T-cell lines. For assessment of antileukemia CTLp frequency, assay wells were defined as positive when 51Cr release exceeded the average +3 SDs of control wells; the frequency of responding cells was determined by maximum likelihood estimation using a statistical program and the variance by use of 95% confidence limits.22
In cytotoxicity assays, 3 wells of total release and 12 wells of spontaneous release were established to confirm the reliability of the test. The mean spontaneous release ranged between 15% and 22% of total release when target cells were patient's LB and from 15% and 20% when target cells were BMRCs or PHA-blasts.
Antileukemia T-cell lines
On the basis of the results of the cytotoxicity assay, antileukemia T-cell lines were obtained pooling positive LDA wells and subsequently cryopreserved for molecular analysis. Surface phenotype of effector cells was performed by flow cytometry analysis as previously described.20
PCR and carrier-heteroduplex analysis
The polymerase chain reaction (PCR) and heteroduplex analysis were performed according to published protocols.23,24 Briefly, total RNA was extracted from 5 × 105 to 1 × 106 cells of the T-cell lines, reverse transcribed into cDNA, and amplified by PCR using a panel of oligonucleotides specific for the 24 Vβ families together with a Cβ downstream oligonucleotide. Vβ-Cβ PCR products were subjected to heteroduplex formation in the presence of 5 μg of the clonal carrier PCR.24 The heteroduplexes were separated on 12% acrylamide gel, electroblotted onto Hybond filters (Amersham Pharmacia Biotech, Piscataway, NJ), and hybridized with Cβ-specific oligonucleotides annealing to a Cβ region present only in the carrier DNA.
DNA extraction and purification from polyacrylamide gel
To sequence the TCR-Vβ clonotypes of interest, the selected DNA heteroduplex bands were excised from polyacrylamide gels and extracted using the “crush-and-soak” method described by Sambrook et al.25 Purified DNA was either directly sequenced using a Cβ internal primer (dwCβ-seq. 5′TGCTTCTGATGGCTCAA3′), if the amount of DNA was sufficient, or reamplified and purified on acrylamide gel, using the crush-and-soak method.
TCR-Vβ N-region hybridization
For each TCR-Vβ sequence, we generated an oligonucleotide overlapping the V-D-J sequence (N-region–specific probes; see underlined sequences in Figure 5A). Filters containing the Vβ9, Vβ13.2, and Vβ24 heteroduplexes have been stripped from previous Cβ probes, according to Amersham manufacturing protocols, exposed to film to exclude any contamination from the previous hybridization probe, and rehybridized with the following oligonucleotides: N-reg-Vβ9a 5′-AGCCAAGTTAGGCAGATGAAC-3′, N-reg-Vβ9b 5′-CAAGTGCGAGGGCGGTACAAT-3′, N-reg-Vβ13.2 5′-AGCAGCGGACTCAATGAGCA-3′, N-reg.Vβ24 5′-AGCAGATCAGGGGAGCTGTT-3′ in 6 × SSC and 1% Blotto at 42°C overnight, after hybridization filters were washed in 6 × SSC and 0.1% SDS for 1 to 2 hours at 42°C, and exposed to film for 1 to 3 hours at –80°C.
Results
Analysis of antileukemia CTLp frequency in patients treated with chemotherapy
The frequency of CTLp's directed against autologous LBs was evaluated in PB of 5 patients; PBMCs were obtained from patients after obtaining CR, at various time points during postremission treatment (Figure 1).
In patients 1 and 5, a high frequency of antileukemia CTLp's was already detected 3 months after the initial diagnosis of AML and persisted for at least 12 months. In patient 1, the frequency declined (15 CTLp/106 responder cells) 30 months after diagnosis (23 months after treatment discontinuation). Both patients 1 and 5 are still in remission with a follow-up of 74 and 38 months, respectively. Patients 2, 3, and 4, displayed very low values of antileukemia CTLp's at all time points investigated; these patients had relapses 5, 13, and 17 months after the diagnosis, respectively (Table 1 and Figure 1 provide further details).
Antileukemia CTLp frequency in AML patients treated with chemotherapy. Antileukemia CTLp frequencies were evaluated in PBMCs obtained approximately 3 months (▦), 6 months (▩), and 12 months (▪) after diagnosis. In patient 1 antileukemia CTLp frequency was evaluated also 30 months after the diagnosis (□). Results are expressed as number of CTLp's for 106 cells. Patients 2, 3, and 4 experienced leukemia relapse (see Table 1 and “Results”).
Antileukemia CTLp frequency in AML patients treated with chemotherapy. Antileukemia CTLp frequencies were evaluated in PBMCs obtained approximately 3 months (▦), 6 months (▩), and 12 months (▪) after diagnosis. In patient 1 antileukemia CTLp frequency was evaluated also 30 months after the diagnosis (□). Results are expressed as number of CTLp's for 106 cells. Patients 2, 3, and 4 experienced leukemia relapse (see Table 1 and “Results”).
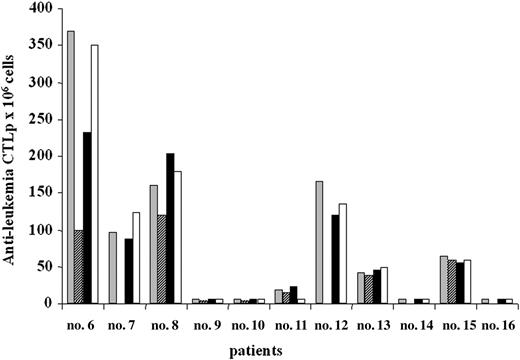
Antileukemia CTLp frequency in AML patients undergoing A-BMT. Antileukemia CTLp frequencies were evaluated in pretransplantation PBMCs (▦), postpurging BM cells (▨), PBMCs 2 months after transplantation (▪), and PBMCs 6 months after transplantation (□). Results are expressed as number of CTLp's for 106 cells. In patients 7, 12, 14, and 16 pretransplantation BM cells for evaluation of CTLp frequency were not available. Patients 9, 10, 11, and 14 had relapses after A-BMT (see Table 2 and “Results”).
Antileukemia CTLp frequency in AML patients undergoing A-BMT. Antileukemia CTLp frequencies were evaluated in pretransplantation PBMCs (▦), postpurging BM cells (▨), PBMCs 2 months after transplantation (▪), and PBMCs 6 months after transplantation (□). Results are expressed as number of CTLp's for 106 cells. In patients 7, 12, 14, and 16 pretransplantation BM cells for evaluation of CTLp frequency were not available. Patients 9, 10, 11, and 14 had relapses after A-BMT (see Table 2 and “Results”).
Analysis of antileukemia CTLp frequency in patients undergoing A-BMT
The frequency of CTLp's directed against autologous LBs was evaluated in PB of 11 patients; PBMCs were obtained after chemotherapy and before A-BMT, as well as 2 and 6 months after transplantation. In 6 patients (6, 7, 8, 12, 13, and 15) a sizeable frequency of antileukemia CTLp's (range, 38-370 CTLp's/106 responder cells) was detected both before and at the 2 different time points after transplantation (Figure 2). These 6 patients remain in CR, with a follow-up time ranging from 21 to 64 months after A-BMT (Table 2).
Patient 11 displayed low, but measurable, frequency of CTLp's both before and 2 months after transplantation (19 and 22 CTLp/106 responder cells, respectively); thereafter, the CTLp frequency declined, reaching undetectable levels 6 months after A-BMT and the patient had a relapse 2 months later. Patients 9, 10, and 14 showed either extremely low (< 5 CTLp/106 responder cells) or undetectable antileukemia CTLp frequencies at all observation time points and all of them had relapses between 3 and 9 months after transplantation. Patient 16, displaying a very low (< 5 CTLp/106 responder cells) frequency of antileukemia CTLp's at all time points, is still in remission with a follow-up of 68 months.
Antileukemia CTLp frequency was also evaluated in BM of 8 of the 11 AML patients who underwent A-BMT; samples were collected at time of harvesting and analyzed before and after in vitro purging with mafosfamide. For patients 7, 12, 14, and 16, BM samples for LDA were not available. Results showed that antileukemia CTLp's were detectable also in mafosfamide-purged BM of those patients who had a sizeable CTLp frequency in PB. In these patients comparable values of CTLp were detected in the BM samples before purging (data not shown). Surface phenotype analysis of antileukemia T-cell lines obtained from patients who underwent A-BMT confirmed our previously reported data, in those undergoing allogeneic HSCT,11 showing that more than 85% of effector cells were CD3+/CD8+ T lymphocytes.
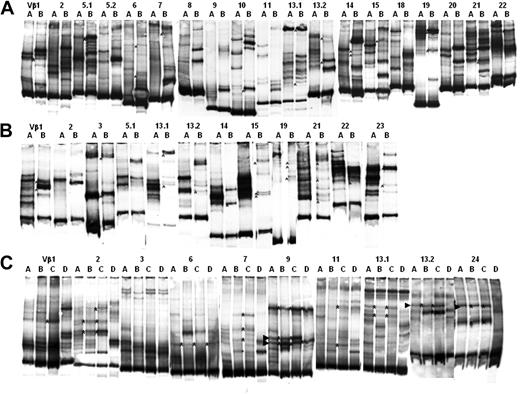
The TCR repertoire of BM resident T cells is highly heterogeneous. cDNA was prepared from patient total BM cells and amplified with Vβ-specific oligonucleotides. Amplified PCR products were subjected to heteroduplex analysis as described in “PCR and carrier-heteroduplex analysis.” (A-B) The heteroduplex analysis performed on patients 7 and 8. Numbers on each line indicate the amplified TCR-Vβ family.
The TCR repertoire of BM resident T cells is highly heterogeneous. cDNA was prepared from patient total BM cells and amplified with Vβ-specific oligonucleotides. Amplified PCR products were subjected to heteroduplex analysis as described in “PCR and carrier-heteroduplex analysis.” (A-B) The heteroduplex analysis performed on patients 7 and 8. Numbers on each line indicate the amplified TCR-Vβ family.
In LDA experiments, simultaneous evaluation of CTLp frequency against LBs, BMRCs, or PHA-blasts, demonstrated very low (< 10 CTLp/106 responder cells) frequency of CTLp's against autologous nonmalignant cells, even in samples obtained from patients displaying sizeable antileukemia CTLp frequency.
Tracking the fate of antileukemia TCR clonotypes transferred with the autologous graft
After demonstrating the presence of antileukemia CTLp's in BM, we investigated whether these cells could be transferred and detected in the recipient after A-BMT. Because marking of somatic cells with reporter genes is not allowed in Italy, we tested this hypothesis by comparing molecular fingerprints of TCR clonotypes expressed by antileukemia T-cell lines derived from BM and PB both before and after A-BMT.
Shared TCR clonotype in antileukemic T-cell lines derived from BM cells and PBMCs. The carrier-heteroduplex analysis was performed as described in “PCR and carrier-heteroduplex analysis.” Carrier-heteroduplex analysis performed on LDA generated from PBMCs obtained after A-BMT (lines A) and BM cells (lines B) from patient 7 (A) and patient 8 (B). Asterisks indicate shared heteroduplex bands. (C) Shows carrier-heteroduplex analysis performed on LDA generated from PBMCs (lines A and B) and BM cells (lines C and D), respectively, before (lines A and C) and after (lines B and D) A-BMT from patient 12. Asterisks indicate shared clonotypes; arrowheads indicate 4 shared heteroduplex bands selected for further molecular characterization.
Shared TCR clonotype in antileukemic T-cell lines derived from BM cells and PBMCs. The carrier-heteroduplex analysis was performed as described in “PCR and carrier-heteroduplex analysis.” Carrier-heteroduplex analysis performed on LDA generated from PBMCs obtained after A-BMT (lines A) and BM cells (lines B) from patient 7 (A) and patient 8 (B). Asterisks indicate shared heteroduplex bands. (C) Shows carrier-heteroduplex analysis performed on LDA generated from PBMCs (lines A and B) and BM cells (lines C and D), respectively, before (lines A and C) and after (lines B and D) A-BMT from patient 12. Asterisks indicate shared clonotypes; arrowheads indicate 4 shared heteroduplex bands selected for further molecular characterization.
The molecular fingerprint was determined by the PCR carrier-heteroduplex technique, which allows exploration of the complexity of the TCR repertoire, and specifically traces the presence of selected Vβ clonotypes in heterogeneous T-cell populations. The heteroduplex analysis provides an immediate qualitative estimate of TCR-Vβ clonotypes shared among T-cell populations, and gives a comprehensive picture of the clonal composition in different compartments.
We first amplified the cDNA obtained from the BM of 4 patients (6, 7, 8, and 12) at the time of harvesting using a panel of oligonucleotides specific for TCR-Vβ. The purpose of this experiment was to investigate the primary Vβ repertoire and clonal distribution in the BM of some representative patients, before the generation of the antileukemia T-cell lines. As shown in Figure 3, in 2 representative patients, there are some expanded T-cell clones, represented by emerging bands present in: Vβ1, 5.1, 5.2, 9, 10, 21 in panel A, and Vβ5.2, 8, 9, 11, 18, 19, 22 in panel B. The other Vβ show an homogeneous smear (Vβ2, 3, 6, 7, 13.1, 13.2, 14, 19, 20, 22, 24 in panel A, and Vβ1, 2, 3, 6, 7, 13.1, 13.2, 14, 16, 18, 20 in panel B) suggesting that the primary TCR-Vβ repertoire present in the BM is heterogeneous and is made of a large number of different T-cell clones. We then determined the TCR clonal heterogeneity of BM-derived antileukemia T-cell lines, and verified whether some TCR Vβ clonotypes expressed by these antileukemia T-cell lines had been transferred into the recipient with the graft. For this purpose, PCR analysis of the TCR Vβ repertoire, combined with the carrier-heteroduplex analysis, was performed on antileukemia T-cell lines obtained from LDA+ wells derived from both pretransplantation and posttransplantation BM cells and PBMCs of 3 of the 6 patients showing an antileukemia precursor frequency. Figure 4 shows the heteroduplex analysis performed on antileukemia T-cell lines generated from BM of patient 7 (A) and 8 (B) at time of harvesting, and from PB 2 months after A-BMT. Heteroduplex bands formed by the carrier cDNA with Vβ clonotypes having the same V-D-J sequence in different samples will have the same electrophoretic mobility in polyacrylamide gel.23,24 The direct comparison of the carrier-heteroduplex patterns identifies some shared clonotypes (indicated by asterisks), which likely correspond to identical TCR Vβ sequences, among many additional bands representing different T-cell clones that are not shared by the 2 compartments. Figure 4C shows the heteroduplex analysis performed on antileukemia T-cell lines generated from PB and BM before and after A-BMT in patient 12. The analysis shows comigrating bands (asterisks), which are shared mainly between pretransplantation BM and posttransplantation PB (lines B and C, respectively). Interestingly, we observed a shift of tumor-specific T-cell clones from the BM, where they were present before transplantation, to the PB after the transplantation, consistent with the already observed capacity of T-cell clones to circulate from the BM to the periphery.13 To give a quantitative estimate of the complexity of the repertoire, we determined the total number of all detectable heteroduplex bands, as well as the total number of shared bands, in BM before transplantation and PBMCs after transplantation in the 3 patients analyzed at the molecular level. As shown in Table 3, the 2 compartments contain a number of either total or shared TCR-Vb clonotypes in comparable ranges.
Number of heteroduplex bands in BM and PBMCs
Patient no. . | Total discrete heteroduplex bands in pretransplantation BM . | Total discrete heteroduplex bands in posttransplantation PBMCs . | Shared heteroduplex bands between BM before and after transplantation of PBMCs . |
|---|---|---|---|
| 7 | 138 | 131 | 17 |
| 8 | 87 | 60 | 22 |
| 12 | 82 | 63 | 14 |
Patient no. . | Total discrete heteroduplex bands in pretransplantation BM . | Total discrete heteroduplex bands in posttransplantation PBMCs . | Shared heteroduplex bands between BM before and after transplantation of PBMCs . |
|---|---|---|---|
| 7 | 138 | 131 | 17 |
| 8 | 87 | 60 | 22 |
| 12 | 82 | 63 | 14 |
Discrete heteroduplex bands were counted by visual inspection of heteroduplex gels.
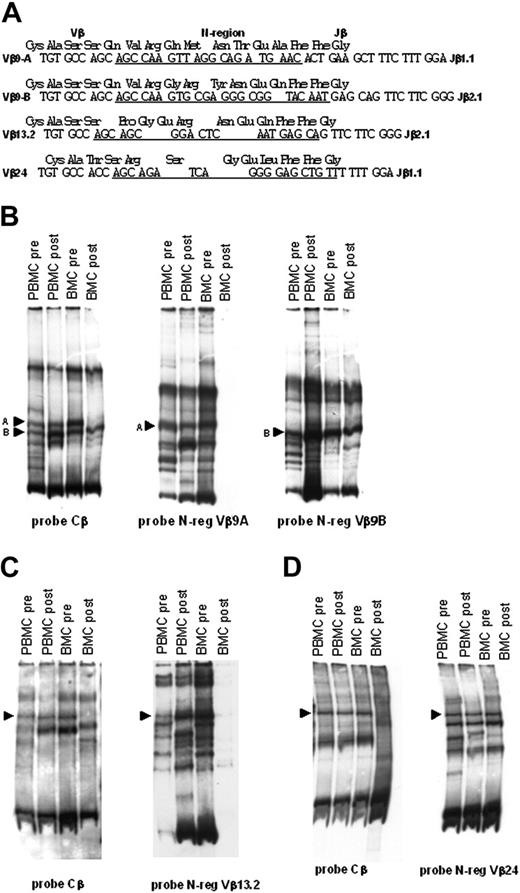
To get an insight into the dynamics of antileukemia CTL response at various time points in patient 12, we purified and sequenced 4 emerging heteroduplex bands from the gels, indicated by arrowheads in Figure 4C. N-region–specific oligonucleotides (sequences underlined in Figure 5A) were then used as probes to hybridize heteroduplexes obtained from antileukemia T-cell lines generated from BM cells and PBMCs, before and after autologous transplantation. As shown in Figure 5B-D, 2 TCR-Vβ clonotypes (Vβ9A and Vβ24) were present in the antileukemia T-cell lines generated from both BM cells and PBMCs before A-BMT, and could be identified in the antileukemia T-cell lines generated from PBMCs after transplantation. The other 2 clonotypes analyzed (Vβ9B and Vβ13.2) were detected in the antileukemia T-cell lines generated from both BM cells and PBMCs before and after A-BMT.
Altogether, these findings support the interpretation that the shared selected antileukemia T-cell clonotypes are present in BM and PBMCs of the patient before A-BMT, survive in vitro pharmacologic purging with mafosfamide, are transferred with the graft, and expand and recirculate in different anatomic sites, contributing to antileukemia immune surveillance.
Discussion
This study provides evidence for the emergence of a naturally elicited antileukemia CTL response in pediatric patients with AML in CR, which, we believe, may play a critical, protective role against leukemia relapse. A sizeable frequency of antileukemia CTLp's was found in all 9 patients, but one, who achieved, and maintained, sustained CR after either chemotherapy alone or A-BMT, whereas either very low or undetectable frequency of CTLp's was found in the 7 patients experiencing leukemia recurrence (P = .002 using the χ2 test).
Molecular evidence of the transfer of antileukemia T cells with BM. The 4 selected shared Vβ clonotypes shown in Figure 4 were sequenced to generate clonotype-specific probes. These probes were used to visualize the presence of the antileukemia Vβ clonotypes in BM cells and PBMCs of patient 12 and to track their transfer into PBMCs following A-BMT. (A) Sequences of TCR Vβ-D-Jβ regions of the clonotypes indicated by the arrowheads in Figure 4. Heteroduplex bands were excised from acrylamide gels and directly sequenced. Underlined are the sequences corresponding to the N-region probes used to track these clonotypes in the T-cell lines generated from PBMCs and BM cells. (B) Filter containing the carrier-heteroduplex analysis of the Vβ9 PCR products amplified from LDA derived from PBMCs and BM cells before and after BMT, hybridized with either Cβ-specific probe or Vβ9A or Vβ9B N-regions–specific probes, as indicated at the bottom of the filters. (C) Filter containing the carrier-heteroduplex analysis of Vβ13.2 PCR products amplified from LDA derived from PBMCs and BMCs before and after BMT, hybridized with either Cβ-specific probe or Vβ13.2 N-region–specific probes, as indicated at the bottom of the filters. (D) Filter containing the heteroduplex analysis of Vβ24 PCR products amplified from LDA derived from PBMCs and BM cells before and after BMT, hybridized with either Cβ-specific probe or Vβ24 N-region–specific probe as indicated at the bottom of the filters. Arrowheads indicate the heteroduplex bands formed by the TCR clonotypes of interest and the carrier. The other bands present in filters, that hybridize with the clonotype-specific probes, are due to heteroduplexes formed by the selected clonotypes and other amplified products in the sample.
Molecular evidence of the transfer of antileukemia T cells with BM. The 4 selected shared Vβ clonotypes shown in Figure 4 were sequenced to generate clonotype-specific probes. These probes were used to visualize the presence of the antileukemia Vβ clonotypes in BM cells and PBMCs of patient 12 and to track their transfer into PBMCs following A-BMT. (A) Sequences of TCR Vβ-D-Jβ regions of the clonotypes indicated by the arrowheads in Figure 4. Heteroduplex bands were excised from acrylamide gels and directly sequenced. Underlined are the sequences corresponding to the N-region probes used to track these clonotypes in the T-cell lines generated from PBMCs and BM cells. (B) Filter containing the carrier-heteroduplex analysis of the Vβ9 PCR products amplified from LDA derived from PBMCs and BM cells before and after BMT, hybridized with either Cβ-specific probe or Vβ9A or Vβ9B N-regions–specific probes, as indicated at the bottom of the filters. (C) Filter containing the carrier-heteroduplex analysis of Vβ13.2 PCR products amplified from LDA derived from PBMCs and BMCs before and after BMT, hybridized with either Cβ-specific probe or Vβ13.2 N-region–specific probes, as indicated at the bottom of the filters. (D) Filter containing the heteroduplex analysis of Vβ24 PCR products amplified from LDA derived from PBMCs and BM cells before and after BMT, hybridized with either Cβ-specific probe or Vβ24 N-region–specific probe as indicated at the bottom of the filters. Arrowheads indicate the heteroduplex bands formed by the TCR clonotypes of interest and the carrier. The other bands present in filters, that hybridize with the clonotype-specific probes, are due to heteroduplexes formed by the selected clonotypes and other amplified products in the sample.
In 4 patients unable to develop measurable levels of antileukemia CTLp's, sizeable levels of EBV-specific or alloantigen-specific CTLs were present (data not shown), thus suggesting a selective inability of these patients to mount a leukemia-directed CTL response.
Because these CTLs did not react against autologous nonmalignant targets (ie, BMRCs, PHA-blasts), the emergence of an antileukemia T-cell response argues for an active antileukemia effect of autologous T cells, naturally elicited in vivo by the encounter with LB-associated peptide antigens. The decline of antileukemia CTLp frequency observed in patient 1, 23 months after discontinuing chemotherapy, is in keeping with data previously reported in allogeneic BM transplant recipients, documenting the decrease of antitumor CTLp frequency about 18 to 24 after transplantation in children maintaining a stable remission.11
The heteroduplex analysis of TCR-Vβ repertoire demonstrates that at least some selected antileukemia T-cell clonotypes already present in BM of the patients before transplantation are detectable in the recipient after A-BMT, possibly contributing to the early antileukemia immune response. One possible interpretation of this finding is that antileukemia T cells may have escaped the lytic effect displayed by the preparative regimen (although in any case it was fully myeloablative) and have re-expanded after transplantation. In fact, previously published studies in patients given T cell–depleted allogeneic BM transplants or cord blood transplants proved that a few, patient-derived T cells may also contribute to posttransplantation immune recovery.26,27 An alternative hypothesis, favored by molecular results of the analysis of TCR-Vβ clonotypes, together with the rapidity of antileukemia CTLp recovery, is that at least a fraction of antileukemia T cells present in the BM survive ex vivo pharmacologic purging with mafosfamide and are transferred with the graft into the recipient. Support for this interpretation is also provided by the comparable frequencies of CTLp's before and after in vitro purging with mafosfamide detected in the 5 patients who were investigated. These data on antileukemia CD8+ T cells are in agreement with our previous findings obtained in both allogeneic and autologous BM transplant recipients proving that tetanus toxoid (TT)–specific CD4+ T cells are located also in the BM and can be transferred in the recipient with the graft.13,24 Although it is widely accepted that the success of allogeneic HSCT is largely based on immunologic mechanisms, namely, the GVL effect, mediated by donor T cells that are thought to actively contribute to tumor eradication,9,10,19 the efficacy of A-BMT has been attributed mainly to the use of high-dose chemo/radiotherapy during the preparative regimen. The demonstration of the presence of antileukemia CTLs in A-BM transplant recipients raises the intriguing hypothesis that also A-BMT is endowed with immunologic effects, resulting in effective reactivation of leukemia-reactive autologous T cells, either transferred with the graft or deriving from residual antileukemia T cells surviving the preparative regimen. Because a rapid expansion of antileukemia CTLp's, reaching frequencies comparable to those detected before transplantation as early as 2 months after A-BMT, was observed in our patients, it is conceivable to hypothesize that the profound lymphocyte depletion induced by the pretransplantation conditioning regimen might have facilitated the peripheral re-expansion of antileukemia CTLs activated by the encounter with malignant cells possibly escaping conditioning regimen. This interpretation is supported by recent results showing that immune-depleting chemotherapy can be used as a strategy to favor the expansion and persistence of tumor-reactive T cells adoptively transferred in patients with metastatic melanoma.28,29 It is reasonable to speculate that also the repeated courses of intensive chemotherapy may have facilitated the emergence and persistence of leukemia-reactive CTLs in our children given chemotherapy alone as postremission therapy and that also in these children an immunologic effect played by antileukemia CTLs favors the maintenance of a state of remission.
Previously published data obtained in adults with Philadelphia-positive CML showed a strong correlation between the presence of PR1-specific T cells, identified using the PR1/HLA-A2 tetramer, and the achievement of cytogenetic remission after treatment with IFN-α.1 The results of our study extend these findings also to patients with AML, documenting an autologous antileukemia CTL response in children with AML experiencing sustained remission after either chemotherapy alone or A-BMT. Distinct mechanisms appear, however, to account for the induction of autologous antileukemia CTLs in these 2 diseases. In adult CML, IFN-α treatment, but not chemotherapy alone, is able to induce CTLs capable of eradicating BCR/ABL+ cells; by contrast, our data suggest that the maintenance of hematologic remission in pediatric AML patients could require the synergistic contribution of both cytotoxic therapy and emergence of naturally elicited autologous antileukemia CTLs.
Although we did not identify the target antigens recognized by antileukemia CTLs, the correlation observed between a sizeable in vitro antileukemia CTLp frequency and the persistence of a remission state suggests that the CTLs identified with our experimental approach are actively involved in antileukemia immune surveillance. Moreover, our approach, based on the use of whole tumor cells, both as stimulator and target cells, offers the advantage of documenting the emergence of antileukemia CTLs, without the need for identifying the leukemia-specific antigens and irrespective of patient's HLA typing, provided a sufficient number of LBs to be cryopreserved at diagnosis.
The advantage of allogeneic HSCT from an HLA-identical sibling over the other 2 forms of consolidation therapy, namely, chemotherapy and autologous transplantation, has been consistently found in randomized studies on childhood AML.30-32 By contrast, the critical question of whether A-BMT is a better consolidation treatment for childhood AML in first CR than chemotherapy is still debated. In the 4 randomized studies addressing this question in pediatric patients, only one,33 in which autologous transplantation was compared to no further therapy, showed an advantage in terms of leukemia-free survival for patients given transplants, whereas the remaining 3 reported comparable outcomes.30-32
Because AML is a heterogeneous disease with distinct cytogenetics/molecular and morphologic subtypes and a variable response to therapy, we decided to tailor the postremission treatment reserving chemotherapy alone to patients with specific genetic anomalies, namely, AML1-ETO fusion transcript, anomalies of CBF-β, and either translocations t(15;17) or in the presence of PML/RARα fusion transcript. Our results indicate that both repeated courses of myelosuppressive chemotherapy and A-BMT neither impair the possibility of naturally developing an antileukemia T-cell response nor promote the emergence of antileukemia CTLs in patients naturally unable to develop them. Irrespective of the type of postremission treatment used, in view of the relationship between the absence of a spontaneous antileukemia T-cell response and the inability to maintain a stable remission for childhood AML, what seems to predict a favorable outcome is the detection of sizeable antileukemia CTLp frequency. Quantification of the frequency of antileukemia CTLp's may, therefore, be a useful tool for the identification of patients at high risk of relapse who should be considered for allogeneic BMT from either a related or even an unrelated donor. Our data also provide support to the rationale of implementing strategies able to augment the autologous antileukemia immune response inAML patients, such as adoptive transfer of ex vivo–generated antileukemia CTL lines.20
Authorship
D.M. and G.C. were responsible for study conception and design, data analysis, and writing the manuscript; R.M. contributed to study conception; P. D. contributed to study conception and writing the manuscript; F.L. provided patients, clinical data analysis, and assistance in study design and writing the manuscript; E.M., L.D., and I.T. were responsible for sample collection and cryopreservation, establishment and analysis of antileukemia T cell lines, and cytotoxicity assays; S.P. and D.L. contributed to molecular analysis; C.G. contributed to heteroduplex analysis; and F.B. provided patients and collected clinical data.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, July 25, 2006; DOI 10.1182/blood-2006-05-021535.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work has been partly supported by grants from the European Community (FP6 Program, Allostem; F.L.), CNR (Consiglio Nazionaledelle Ricerche; F.L.), MIUR (Ministero dell'Istruzione, dell'Universitàe della Ricerca; F.L. and D.M.), Ricerca Finalizzata (F.L., R.M., G.C. and P.D.), Ricerca Corrente IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) Policlinico San Matteo (F.L. and R.M.), andAIRC (Associazione Italiana Ricerca sul Cancro; D.M., F.L., R.M., G.C., and P.D.). L.D. is on leave from the Hospital de Clinicas de Porto Alegre-Brazil and is supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) Brazil and by a private Italian grant in memory of Sofia Luce Rebuffat.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal