Abstract
The stages of human natural killer (NK) cell differentiation are not well established. Culturing CD34+ progenitors with interleukin 7 (IL-7), IL-15, stem cell factor (SCF), FLT-3L, and murine fetal liver cell line (EL08.1D2), we identified 2 nonoverlapping subsets of differentiating CD56+ cells based on CD117 and CD94 (CD117highCD94– and CD117low/–CD94+ cells). Both populations expressed CD161 and NKp44, but differed with respect to NKp30, NKp46, NKG2A, NKG2C, NKG2D, CD8, CD16, and KIR. Only the CD117low/– CD94+ population displayed cytotoxicity and interferon-γ production. Both populations arose from a single CD34+CD38– Lin– cell and their percentages changed over time in a reciprocal fashion, with CD117highCD94– cells predominating early and decreasing due to an increase of the CD117low/–CD94+ population. These 2 subsets represent distinct stages of NKcell differentiation, since purified CD117high CD94– cells give rise to CD117low/–CD94+ cells. The stromal cell line (EL08.1D2) facilitated the transition from CD117highCD94– to CD117low/–CD94+ via an intermediate phenotype (CD117lowCD94low/–). EL08.1D2 also maintained the mature phenotype, preventing the reversion of CD117low/–CD94+ cells to the intermediate (CD117lowCD94low/–) phenotype. An analogous population of CD56+CD117highCD94– cells was found in cord blood. The identified stages of NK-cell differentiation provide evidence for coordinated acquisition of HLA-specific inhibitory receptors (ie, CD94/NKG2A) and function in developing human NK cells.
Introduction
Natural killer (NK) cells are CD56+CD3– innate immune effector cells that recognize target cells that have undergone cellular stress, such as malignant transformation or viral infection.1 Individual NK cells display a diverse repertoire of activating and inhibitory receptors, including the killer immunoglobulin-like receptors (KIRs), natural cytotoxicity receptors (NKp30, NKp44, and NKp46), and c-lectin receptors (NKG2A/CD94, NKG2C/CD94, NKG2D, and CD161). The summation of activating signals, when not opposed by inhibitory signaling, leads to the release of granzymes and perforin and target cytolysis.2 NK-cell activation also results in the elaboration of proinflammatory cytokines, including interferon γ (IFN-γ),3,4 stimulating other compartments of the immune system.
The stages of human NK-cell development are not well characterized. Since human NK cells can be differentiated from CD34+ hematopoietic progenitor cells (HPCs) in vitro, such stages of differentiation may be elucidated. Early studies showed that interleukin 2 (IL-2) could induce the differentiation of NK cells from CD34+ HPCs.5,6 More recently, IL-15 has been found to be critical for NK-cell development since IL-15–/– and IL-15Rα–/– mice show a near-complete absence of NK cells.7,8 Accordingly, culture of human HPCs with IL-15 gives rise to NK cells,9 but other cytokines, such as IL-3, IL-7, stem cell factor (SCF), and FLT-3L increase the efficiency of in vitro NK-cell differentiation.10-12 Both SCF and FLT-3L induce the expression of IL-15Rα mRNA in developing progenitors, inducing IL-15 responsiveness.11 CD34+ cells cultured with cytokines and stromal cells give higher yields of NK cells that more accurately reflect peripheral blood NK cells with respect to KIR expression.12-15
To prevent auto-aggression, NK cells express inhibitory receptors that recognize self major histocompatibility complex (MHC) class I. The current paradigm of NK-cell tolerance suggests that every NK cell must express at least one self MHC-specific inhibitory receptor, since this has been demonstrated at both the clonal and polyclonal NK-cell levels.16,17 Despite this paradigm, NK cells lacking self MHC class I–specific receptors can be detected in mice. These cells are not fully functional, suggesting that inhibitory receptor signaling is involved in acquisition of function by developing NK cells.18,19 Likewise, in humans with virtually no MHC class I expression (due to TAP2 deficiency) NK-cell function is reduced.20
Discrete stages of Tand B-cell differentiation have been described based on surface receptor expression.21,22 We hypothesized that as NK cells develop from HPCs they acquire NK-cell receptors (NKRs) and that the expression could define NK-cell developmental stages. To investigate this, umbilical cord blood (UCB)–derived HPCs were cultured with a cytokine combination (IL-3, IL-7, IL-15, SCF, and FLT-3L) previously shown to be optimal for NK-cell development on a murine fetal liver cell line.12 Here we demonstrate that CD56+ NK cells derived from UCB CD34+ HPCs in vitro progress through a developmental stage characterized by a CD56+CD117highCD94– phenotype. At this point they lack expression of most NK-cell activating receptors, all MHC-specific inhibitory receptors, and have no capacity for either cytotoxicity or IFN-γ production. These CD56+CD117highCD94– cells advance to the next discrete developmental stage, CD56+CD117low/–CD94+. We show that this progression is associated with the coordinated acquisition of activating and inhibitory receptors, cytotoxicity, and IFN-γ production. These results demonstrate that during human NK-cell differentiation, the acquisition of cytotoxicity and self MHC-specific inhibitory receptor expression are closely linked.
Materials and methods
Isolation of CD34+ and CD56+ cells from UCB
CD34+ progenitor cells or CD56+ NK cells were isolated from UCB using magnetic bead selection (Miltenyi Biotech, Auburn, CA). Selected cells were routinely more than 97% pure. CD34+ contained no CD56+ cells (not shown). Where specified, enriched CD34+ cells were sorted using fluorescence activated cell sorting (FACS) into CD34+CD38–Lin– (lineage markers were CD3, CD5, CD7, CD8, CD10, CD14, and CD19). For single cell-culture experiments, single CD34+CD38–Lin– cells were sorted directly into 96-well plates (BD Biosciences, San Jose, CA). In some experiments, CD56+CD117highCD94– cells were FACS purified from the CD56+ fraction of UCB.
Culture of the stromal cell line EL08.1D2
The embryonic liver cell line EL08.1D2 was cultured on gelatinized plates at 32°C in 40.5% α–minimum essential medium (α-MEM; Invitrogen, Carlsbad, CA), 50% myelocult (M5300; Stem Cell Technologies, Vancouver, BC, Canada), 7.5% fetal bovine serum (FBS), with β-mercaptoethanol (50 μM/L), glutamax (2 mM), penicillin (100 U/mL)/streptomycin (100 U/mL), and hydrocortisone (10–6 M). Prior to coculture with progenitor cells, EL08.1D2 cells were irradiated (3000 rads).
NK-cell differentiation cultures
CD34+ selected cells (500 per well) were plated in a 24-well plate on an irradiated confluent monolayer of EL08.1D2 cells in Ham F12 plus Dulbecco modified Eagle medium (DMEM; 1:2 ratio) with 20% male, human AB– sera (Sera Care Life Sciences, Oceanside, CA), ethanolamine (50 μM), ascorbic acid (20 mg/L), 5 μg/L sodium selenite (Na2SeO3), β-mercaptoethanol (24 μM), and penicillin (100 U/mL)/streptomycin (100 U/mL). At the start of cultures, IL-3 (5 ng/mL), IL-7 (20 ng/mL), IL-15 (10 ng/mL), SCF (20 ng/mL), and FLT-3L (10 ng/mL) were added. Weekly thereafter, cultures were refed by demi-depletion (50% volume change) supplemented with the previously mentioned cytokines except IL-3. Where specified, cells were harvested after 3 weeks of culture and enriched for CD56+ cells using magnetic beads (MACS, Miltenyi Biotech). The resulting populations were FACS sorted into CD56+CD117highCD94–, CD56+CD117lowCD94low/–, and CD56+CD117low/–CD94+ fractions. These cells were then cultured (in triplicate) either in the absence or presence of EL08.1D2. In some experiments, cells were separated from direct contact with EL08.1D2 using transwell inserts (Corning, Corning, NY). Cells were analyzed by FACS using polystyrene beads for enumeration (Polybead; Polysciences, Worrington, PA).
FACS staining and monoclonal antibodies
The following antibodies were used: CD3-FITC, CD5-FITC, CD7-FITC, CD8-FITC, CD10-FITC, CD14-FITC, CD16 (FITC or PE), CD19-FITC, CD34 (Percp, PE, and APC), CD38-PE, CD56-APC, CD94-FITC, CD117 (PE or PercpCy5.5), CD161 (FITC or PE), CD158a-PE, CD158b-PE, and FasL-PE, (all from BD Biosciences, San Jose, CA). Additional antibodies included NKG2A-PE, NKG2C-PE, NKG2D-PE (R&D Systems, Minneapolis, MN), NKp30-PE, NKp44-PE, and NKp46-PE (Beckman Coulter, Hialeah, FL). Intracellular staining for granzyme-B–FITC, perforin-PE, IFN-γ–PE, and Ki-67–FITC were performed using cytofix/cytoperm (BD Biosciences). IFN-γ staining was performed after 16 hours of stimulation with IL-12 (10 ng/mL) and IL-18 (100 ng/mL). Brefeldin A was added for the last 4 hours.
FACS analysis and FACS sorting
Threeand 4-color FACS was performed on a FACS Calibur (BD Biosciences). Data were analyzed using WinMDI software (John Trotter, Scripps Research Institute, La Jolla, CA). FACS sorting was performed on either a FACS Vantage or FACS Aria (BD Biosciences). Postsort reanalysis showed greater than 99.8% purity (not shown).
Polymerase chain reaction (PCR)
Total RNA was isolated immediately after FACS sorting CD117highCD94– and CD117low/–CD94+ populations using the RNeasy Mini Kit (QIAGEN, Santa Clarita, CA) and reverse transcribed to cDNA (iScript; Bio-Rad, Hercules, CA). cDNA (2 ng) was amplified using recombinant Taq Polymerase (Gibco BRL, Carlsbad, CA) for 30 cycles. The following primers were used: DAP10, forward CATCTGGGTTCACATCCTCTT, reverse CAGAAGTCAAAGGTCCAAGC; DAP12. forward CCGCAAAGACCTGTACGCCA, reverse TGGACTTGGGAGAGGACTGG; FcϵR1γ, forward ATGATTCCAGCAGTGGTCTTG, reverse GTGCTCAGGCCCGTGTAAA; CD3ζ, forward GCACAGTTGCCGATTACAGA, reverse GGTTCTTCCTTCTCGGCTTT; granzyme B, forward GGGGGAGCTCCATAAATGTCACCT, reverse TACACACAAGAGGGCCTCCAGAGT; perforin, forward CGGCTCACACTCACAGG, reverse CTGCCGTGGATGCCTATG; FasL, forward CAAGTCCAACTCAAGGTCCATGCC, reverse, CAGAGAGAGCTCAGATACGTTGAC; TRAIL, forward CCAGAGGAAGAAGCAACACA, reverse TTCCCCCTTGATAGATGGAAT; and actin, forward CCGCAAAGACCTGTACGCCA, reverse TGGACTTGGGAGAGGACTGG. PCR products were resolved on 2% agarose gels and bands were visualized with ethidium bromide.
51Cr release assay
K562 targets were labeled with 51Cr (Dupont-NEN, Boston, MA) by incubating 1 × 106 cells in 11.1 MBq (300 μCi) 51Cr for 2 hours at 37°C, 5% CO2. The cells were washed with phosphate-buffered saline (PBS), resuspended in RPMI with 10% FBS, and plated in 96-well plates at 1 × 104 cells/well in triplicate. Effector cells were added at specified ratios (from 10:1 to 0.625:1) and incubated for 4 hours at 37°C, 5% CO2. Supernatants were collected and counted (Wizard 1470; Perkin-Elmer, Shelton, CT). Specific 51Cr lysis was calculated using the equation: % specific lysis = 100 × (test release–spontaneous release)/(maximal release–spontaneous release).
Results
Kinetics of NK-cell development and receptor acquisition in NK-cell differentiation cultures
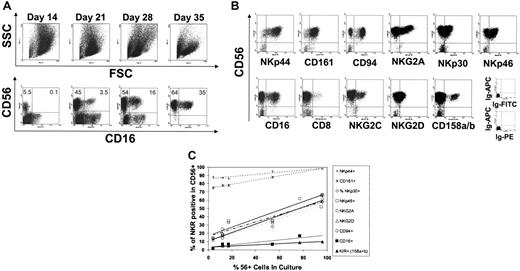
To investigate human NK-cell development using an in vitro model system, UCB-derived CD34+ cells were plated on an irradiated murine fetal liver cell line (EL08.1D2)23 with IL-3, IL-7, IL-15, SCF, and FLT3-L. Within 2 weeks of culture there was tremendous proliferation (2858± 1201-fold expansion, n = 21), resulting in a population of CD56– large cells, with monocytoid appearance by forward and side scatter (Figure 1A). Also during the first 2 weeks of culture, CD34 expression was lost and by day 14, virtually all cells were CD34– (not shown). Starting on days 10 to 14, small numbers of CD56+-staining cells were identified in the lymphocyte gate (Figure 1A). The overall number of cells within the lymphocyte gate increased over the culture period and concurrent with this, the percentage of CD56+ cells increased. After 4 to 5 weeks, the majority of cells (> 90%) were CD56+ and CD3– (Figure 1A and not shown). At day +28 a proportion of the CD56+ cells were also CD16+ (Figure 1A), demonstrating that mature NK cells resembling those found in peripheral blood could be generated.
Phenotype and NKR expression of CD34+-derived NK cells. (A) Forward and side scatter appearance of cells at days 14, 21, 28, and 35 of culture (top panels). Early in culture, large cells with high side and forward scatter are seen. Over time these cells decrease and there is an increase in cells within the lymphocyte gate. Concurrent with the increasing percentages of lymphocytes, both CD56+ and CD16+ cells can be detected (bottom panels, gated on viable cells using forward and side scatter). By day 35 of culture, the vast majority of cells are CD56+ with a significant proportion coexpressing CD16. Results were representative of more than 20 healthy UCB donors. (B) Expression of NK-cell receptors on in vitro–generated CD56+ cells at day 28. Shown are data from cells falling within the lymphoid gate. Results representative of more than 20 donors. (C) The fraction of CD56+ cells expressing an individual NKR as a function of the percentage of CD56+ cells in culture. Shown are the percentage of NK cells in culture (x-axis) and the percentage of receptor-expressing cells (y-axis). At all time points studied (days 14, 17, 19, 21, 25, 28), the majority of CD56+ cells (> 75%) expressed NKp44 and CD161. There was progressive acquisition of NKp30, NKp46, NKG2A, CD94, and NKG2D as the percentage of NK cells increased in the culture. A third group of receptors (CD16 and KIR) was acquired more gradually. Results were representative of more than 20 donors.
Phenotype and NKR expression of CD34+-derived NK cells. (A) Forward and side scatter appearance of cells at days 14, 21, 28, and 35 of culture (top panels). Early in culture, large cells with high side and forward scatter are seen. Over time these cells decrease and there is an increase in cells within the lymphocyte gate. Concurrent with the increasing percentages of lymphocytes, both CD56+ and CD16+ cells can be detected (bottom panels, gated on viable cells using forward and side scatter). By day 35 of culture, the vast majority of cells are CD56+ with a significant proportion coexpressing CD16. Results were representative of more than 20 healthy UCB donors. (B) Expression of NK-cell receptors on in vitro–generated CD56+ cells at day 28. Shown are data from cells falling within the lymphoid gate. Results representative of more than 20 donors. (C) The fraction of CD56+ cells expressing an individual NKR as a function of the percentage of CD56+ cells in culture. Shown are the percentage of NK cells in culture (x-axis) and the percentage of receptor-expressing cells (y-axis). At all time points studied (days 14, 17, 19, 21, 25, 28), the majority of CD56+ cells (> 75%) expressed NKp44 and CD161. There was progressive acquisition of NKp30, NKp46, NKG2A, CD94, and NKG2D as the percentage of NK cells increased in the culture. A third group of receptors (CD16 and KIR) was acquired more gradually. Results were representative of more than 20 donors.
The expression of NK-cell receptors (NKRs) was studied at various time points. The kinetics of receptor acquisition varied considerably depending on the receptor studied. Receptor acquisition fell into 3 distinct patterns: (1) receptors that were present on virtually all CD56+ cells at all time points tested (NKp44 and CD161); (2) receptors that were progressively acquired by the majority of cells over time (NKp30, NKp46, NKG2A, CD94, and NKG2D); and (3) receptors that were expressed by a smaller fraction of CD56+ cells later in culture (NKG2C, KIR, CD16, and CD8) (Figure 1B-C). Collectively, these results demonstrate that after 4 to 5 weeks in differentiation cultures, CD56+ NK cells acquire all NKRs studied, although to a variable extent. The 3 distinct patterns of receptor acquisition suggest a step-wise pattern of receptor expression, which may define distinct stages of NK-cell development.
CD117 and CD94 define 2 differentiating NK-cell subpopulations
In NK-cell differentiation cultures, the majority of CD56+ cells express CD117 but 2 populations, CD56+CD117high and CD56+CD117low/–, were present at each time point tested (Figure 2A). As CD117high expression defined early stages of T and DC development,24 we hypothesized that the same may be true for NK-cell differentiation. We investigated whether the CD56+CD117high and CD56+CD117low/– subsets differed with respect to inhibitory and activating NKR expression. When tested at 3 to 4 weeks of culture, both the CD56+CD117high and CD56+CD117low/– cells expressed similar levels of CD161 and NKp44 (Figure 2B-C). These 2 populations differed considerably since the vast majority of CD56+CD117low/– cells (but not CD56+CD117high cells) expressed NKp30, NKp46, NKG2D, CD94, and NKG2A. Other receptors including NKG2C, CD16, KIR (CD158a/h and CD158b/j), and CD8 were present exclusively on a fraction of CD56+CD117low/– cells (Figure 2B-C). Thus, CD117 defined 2 populations of developing NK cells with differential expression of NKRs. Notably, this relates to the observations of NKR acquisition over time in culture (Figure 1C), since the first grouping of receptors (CD161 and NKp44) was expressed on both subsets and the second grouping of receptors (NKp30, NKp46, NKG2D, CD94, and NKG2A) was found mainly on the CD56+CD117low/– cells, whereas the third group of receptors (NKG2C, KIR, CD16, and CD8) was present exclusively on a fraction of CD56+CD117low/– cells. This prompted us to better characterize the 2 subsets and the relationship between them. To do this, a clear distinction between the 2 populations was required. Although the CD56+CD117high and CD56+CD117low/– cells appeared as separate populations, there was some overlap (Figure 2A). We therefore took advantage of the fact that CD94 was expressed exclusively on CD56+CD117low/– cells and that this was stable over time. The combination of CD117 and CD94 distinguished 2 discrete subsets of differentiating CD56+ NK cells into CD117highCD94– and CD117low/–CD94+ (Figure 2D).
Expression of activating receptor adapter proteins in CD117highCD94– and CD117low/–CD94+ cells
Inhibitory NK-cell receptors, such as NKG2A/CD94 and KIR, contain signaling motifs that directly transmit intracellular signals upon receptor ligation.25,26 The activating receptors NKp30, NKp44, NKp46, and NKG2D require the association of adapter proteins to transmit intracellular signals including: CD3-ζ, FcϵRIγ, DAP10, and DAP12 (reviewed in Moretta et al2 and Lanier27 ). Since the CD117highCD94– and CD117low/–CD94+ populations differed with respect to NKp30, NKp46, and NKG2D, we investigated whether there was differential expression of adapter proteins associated with these receptors. To do this we performed reverse transcriptase (RT)–PCR on FACS-sorted (Figure 2D) CD117highCD94– and CD117low/–CD94+ populations of CD56+ cells. CD117low/–CD94+ cells expressed message for CD3-ζ, FcϵRIγ, DAP10, and DAP12, whereas CD117highCD94– cells expressed DAP12 but showed minimal CD3-ζ, FcϵRIγ, and DAP10 message (Figure 2E). Both populations expressed NKp44 (Figure 2C) as well as mRNA for its adapter protein, DAP12 (Figure 2E). In contrast, the 2 developing NK-cell populations differed with respect to NKp30, NKp46, and NKG2D (Figure 2C). Likewise, the adapter proteins that associate with these receptors were differentially expressed (Figure 2E). Thus, the described subsets differ with respect to the phenotype of activating NKRs and this correlates well with adapter protein expression.
CD117 and CD94 define 2 separate populations of differentiating NK cells. (A) CD117 defines 2 populations of CD56+ cells. FACS staining with CD56-APC versus CD117-PE shows 2 populations: CD56+CD117high and CD56+CD117low/–. (B) Expression of inhibitory receptors on CD56+CD117high and CD56+CD117low/– cell populations. Cells were stained with a monoclonal antibodies (mAbs) directed against CD56, CD117 in combination with CD161, KIR (CD158a and CD158b), NKG2A, and CD94 at day 28 of culture. Shown are histograms generated by gating on either the CD56+CD117high or CD56+CD117low/– populations. Results are representative of more than 20 donors. (C) Expression of activating receptors on CD56+CD117high and CD56+CD117low/– cells. FACS staining for NKp44, NKp46, NKp30, NKG2D, NKG2C, CD16, and CD8 after gating on either CD56+CD117high or CD56+CD117low/– cells. Results were representative of more than 20 donors. (D) CD117 and CD94 define 2 discrete populations of differentiating NK cells. FACS staining for CD117 and CD94 after gating on the CD56+ fraction. Results were representative of more than 20 experiments. (E) Adaptor protein expression in CD94–CD117high and CD94+CD117low/– populations. Cells were FACS sorted into CD56+CD94–CD117high and CD56+CD94+CD117low/– fractions at days 21 to 28 of culture. RT-PCR was performed using primers specific for DAP12, DAP10, CD3ζ, FcϵRIγ, and actin. Results were representative of 3 or more experiments.
CD117 and CD94 define 2 separate populations of differentiating NK cells. (A) CD117 defines 2 populations of CD56+ cells. FACS staining with CD56-APC versus CD117-PE shows 2 populations: CD56+CD117high and CD56+CD117low/–. (B) Expression of inhibitory receptors on CD56+CD117high and CD56+CD117low/– cell populations. Cells were stained with a monoclonal antibodies (mAbs) directed against CD56, CD117 in combination with CD161, KIR (CD158a and CD158b), NKG2A, and CD94 at day 28 of culture. Shown are histograms generated by gating on either the CD56+CD117high or CD56+CD117low/– populations. Results are representative of more than 20 donors. (C) Expression of activating receptors on CD56+CD117high and CD56+CD117low/– cells. FACS staining for NKp44, NKp46, NKp30, NKG2D, NKG2C, CD16, and CD8 after gating on either CD56+CD117high or CD56+CD117low/– cells. Results were representative of more than 20 donors. (D) CD117 and CD94 define 2 discrete populations of differentiating NK cells. FACS staining for CD117 and CD94 after gating on the CD56+ fraction. Results were representative of more than 20 experiments. (E) Adaptor protein expression in CD94–CD117high and CD94+CD117low/– populations. Cells were FACS sorted into CD56+CD94–CD117high and CD56+CD94+CD117low/– fractions at days 21 to 28 of culture. RT-PCR was performed using primers specific for DAP12, DAP10, CD3ζ, FcϵRIγ, and actin. Results were representative of 3 or more experiments.
Functional characteristics of the CD117highCD94– and CD117low/–CD94+ populations
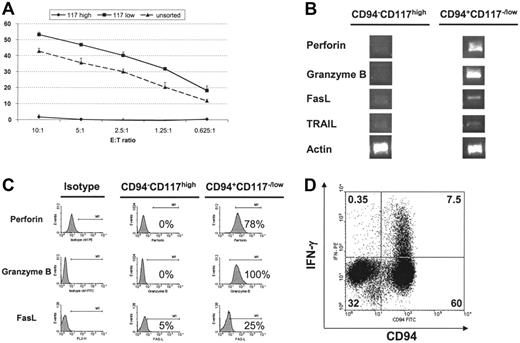
To investigate the contribution of the 2 cell populations, CD56+CD117highCD94– and CD56+CD117low/–CD94+ cells were FACS sorted and tested for cytotoxicity against K562 cells. As shown in Figure 3A, CD117highCD94– cells were not cytotoxic, whereas the CD117low/–CD94+ population effectively killed K562 cells. Further analysis of these 2 populations demonstrated that only CD117low/–CD94+ cells expressed significant amounts of message for perforin and granzyme B (Figure 3B). Intracellular staining for perforin and granzyme B confirmed these results (Figure 3C). IL-12 is known to enhance NK-cell cytotoxicity through the up-regulation of perforin and granzyme expression.17,28 Culture with IL-12 increased intracellular perforin and granzyme B expression in the CD117low/–CD94+ population but both remained negative in the CD117highCD94– fraction (not shown). Fas ligand (FasL) and TRAIL mRNA could also be amplified from the CD117low/–CD94+ population, whereas it was nearly absent in the CD117highCD94– cells (Figure 3B). FasL was also detected by FACS on a higher percentage of CD117low/–CD94+ cells (Figure 3C). IFN-γ production after monokine challenge is another hallmark of NK-cell function. The ability to produce IFN-γ was tested by IL-12 and IL-18 stimulation.29 As shown in Figure 3D, IFN-γ production was confined to CD94+ cells (also CD117low/–, not shown). Taken together, these results demonstrate 2 distinct subpopulations of CD56+ cells in NK-cell differentiation cultures defined by CD117 and CD94, such that the CD117highCD94– population either lacks or has low-level expression of the majority of NKRs, adapter molecules, and effector proteins, and is unable to mediate cytotoxicity and IFN-γ secretion. In contrast, the CD117low/– CD94+ subset expressed all studied NK-cell activating receptors and their adapter molecules, as well as cytotoxic effector molecules. Only the latter population expressed HLA-specific inhibitory receptors (CD94/NKG2A and KIR on a fraction of cells), and displayed cytotoxicity and IFN-γ secretion.
Functional differences between CD94–CD117high and CD94+CD117low/– NK cells. (A) CD56+CD94–CD117high and CD56+CD94+CD117low/– populations were FACS sorted at days 21 to 28 of culture, rested for 24 hours, and then used in a 51Cr release assay with K562 cells as targets. Error bars depict standard deviation. Results are representative of 3 or more experiments. (B) Expression of cytotoxic molecules by RT-PCR in developing NK cells. mRNA was isolated from the FACS-sorted cell populations and was used for RT-PCR with primers specific for perforin, granzyme B, Fas ligand, TRAIL, and actin. Results were representative of 3 or more experiments. (C) Differential expression of perforin, granzyme B, and FasL in CD56+CD94–CD117high and CD56+CD94+CD117low/– cell populations. At days 21 to 28, cultures were analyzed for the expression of perforin and granzyme B using intercellular staining. FasL was assayed using surface staining. High levels of perforin, granzyme B, and FasL could be detected in the CD56+CD94–CD117low/– population, whereas these proteins were either absent or present at minimal amounts in the CD56+CD94–CD117high population. Results were representative of 3 or more experiments. (D) Interferon-γ secretion. HPC-derived NK cells were challenged with IL-12 (10 μg/mL) and IL-18 (100 μg/mL) for 18 hours, and cultures were assayed for intracellular interferon-γ. In the gated CD56+ population, intracellular interferon-γ was detected only in CD94+ cells representing the CD56+CD94+CD117low/– population. Results were representative of 3 or more experiments.
Functional differences between CD94–CD117high and CD94+CD117low/– NK cells. (A) CD56+CD94–CD117high and CD56+CD94+CD117low/– populations were FACS sorted at days 21 to 28 of culture, rested for 24 hours, and then used in a 51Cr release assay with K562 cells as targets. Error bars depict standard deviation. Results are representative of 3 or more experiments. (B) Expression of cytotoxic molecules by RT-PCR in developing NK cells. mRNA was isolated from the FACS-sorted cell populations and was used for RT-PCR with primers specific for perforin, granzyme B, Fas ligand, TRAIL, and actin. Results were representative of 3 or more experiments. (C) Differential expression of perforin, granzyme B, and FasL in CD56+CD94–CD117high and CD56+CD94+CD117low/– cell populations. At days 21 to 28, cultures were analyzed for the expression of perforin and granzyme B using intercellular staining. FasL was assayed using surface staining. High levels of perforin, granzyme B, and FasL could be detected in the CD56+CD94–CD117low/– population, whereas these proteins were either absent or present at minimal amounts in the CD56+CD94–CD117high population. Results were representative of 3 or more experiments. (D) Interferon-γ secretion. HPC-derived NK cells were challenged with IL-12 (10 μg/mL) and IL-18 (100 μg/mL) for 18 hours, and cultures were assayed for intracellular interferon-γ. In the gated CD56+ population, intracellular interferon-γ was detected only in CD94+ cells representing the CD56+CD94+CD117low/– population. Results were representative of 3 or more experiments.
CD117highCD94– cells are precursors to CD117low/–CD94+ cells
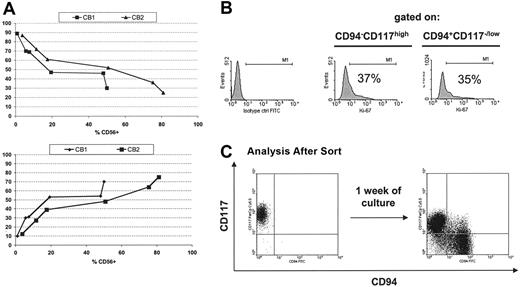
As shown, the CD117highCD94– cells lacked properties of mature NK cells, suggesting that they may represent an earlier stage of NK-cell development. For the CD117highCD94– cells to be a precursor to the CD117low/–CD94+ population, a number of observations should be fulfilled. First, both should arise from a single primitive progenitor cell (to exclude that these 2 populations have distinct precursors). To test this, FACS-purified, UCB-derived CD34+CD38–Lin– cells were sorted and a single cell was deposited per well and cultured in the presence of the stromal cell line (EL08.1D2) and cytokines. All cultures giving rise to CD56+ cells (40.75%, n = 2 donors) contained both the CD117highCD94– and CD117low/–CD94+ cell populations. Second, if the CD117highCD94– cells represent an immature step in NK-cell differentiation, then these cells should appear earlier in culture and decrease over time. This was examined by evaluating cultures at various times for the proportions of CD117highCD94– versus CD117low/–CD94+ cells. The emergence of CD56+ cells occurs at approximately day 14 (Figure 1A), and at this point, the vast majority of CD56+ cells were CD117highCD94– (Figure 4A, top). Over the following 3 weeks, concomitant with the increasing percentage of CD56+ cells, the fraction of CD117low/–CD94+ cells increased (Figure 4A, bottom) with a reciprocal decrease in the CD117highCD94– population (Figure 4A, top). Interestingly, at day +28 to day 35 there was not a complete disappearance of CD117highCD94– cells (18.4% ± 8.8%, n = 18). Since these results could be explained by more rapid proliferation of the CD117low/–CD94+ cells, cultures were evaluated for proliferation using Ki-67. As shown in Figure 4B, the fraction of Ki-67–expressing cells was nearly identical for both populations of cells. Finally, if these 2 populations are developmentally related to one another, then purified CD117highCD94– cells should give rise to the CD117low/–CD94+ population. To test this, CD117highCD94– cells were FACS sorted and cultured on EL08.1D2 in the presence of cytokines. After 1 week a clear population of CD117low/–CD94+ cells was detected (Figure 4C). Moreover, this newly arising population had the phenotypic and functional characteristics of CD117low/–CD94+ cells (ie, expression of NKp30, NKp46, KIR, CD16, perforin, and granzyme B, and cytotoxicity against K562 cells [not shown]). Collectively, these results demonstrate that CD117highCD94– cells are precursors giving rise to CD117low/–CD94+ cells.
The transition from the CD56+CD117highCD94– to the CD56+CD117low/–CD94+ phenotype is supported by the stromal cell line EL08.1D2
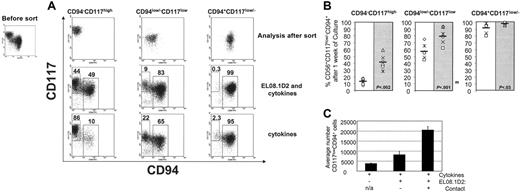
Apart from the 2 populations described, a discrete intermediate population of cells (CD117lowCD94low/–) can be observed (Figure 5A). These CD117lowCD94low/– intermediate cells may represent a transition from CD117highCD94– to CD117low/– CD94+. To investigate this theory and the role of the stromal cell line (EL08.1D2) in the process, all 3 populations (CD117highCD94–, CD117lowCD94low/–, and CD117low/–CD94+) were FACS sorted and cultured in media containing cytokines (IL-7, IL-15, SCF, FLT-3L) either with or without the support of the stromal cell line. After 1 week, a significant percentage of CD117highCD94– cells differentiated into CD117low/–CD94+ cells when cultured on the EL08.1D2 (Figure 5A). In contrast, significantly fewer CD117highCD94– cells differentiated into CD117low/–CD94+ cells in the absence of the feeder cell line (ie, after culture with cytokines alone) (41.5% ± 11.2% vs 12.8% ± 4.1%; P < .002; n = 6) (Figure 5A-B). The intermediate CD117lowCD94low/– population had less strict requirements for the stromal cell line since the majority of cells transitioned in its absence. However, the stromal cell line facilitated this process significantly (80.3% ± 12.4% vs 57.7% ± 11%; P < .001; n = 6) (Figure 5A-B). This provides evidence that this intermediate population represents cells that have already initiated the transition from CD117highCD94– to CD117low/– CD94+. The vast majority of FACS-sorted CD117low/–CD94+ cells maintained their phenotype after culture both in the presence and absence of EL08.1D2 cells (Figure 5A-B). However, a minimal fraction of cells reverted to the intermediate phenotype (CD117lowCD94low/–) and this occurred more frequently in the absence of the feeder cell line (98.1% ± 2.2% vs 91.3% ± 7.3%; P < .03; n = 6). To further exclude that these observations were not due to contaminating cells (postsort reanalysis showed greater than 99.8% purity), sorted cells were plated at 100, 1000, and 10 000 cells per well (96-well plate) and the findings were consistent (not shown).
CD56+CD94–CD117high cells are a precursor population to CD56+CD94–CD117low/– cells. (A) The percentage of CD94–CD117high cells (top) and CD94+CD117low/– cells (bottom) within the CD56+ population as a function of the percentage of CD56+ cells in culture. CD34+Lin– cells were isolated by FACS and cultured as described in “Materials and methods.” The plots demonstrate that at early time points when the fraction of CD56+ cells is low the majority of CD56+ cells are CD94–CD117high. As the percentage of CD56+ cell increases, the fraction of CD56+CD94–CD117high cells decreases and the fraction of CD56+CD94+CD117low/– cells increases (bottom). Results of 2 individual donors tested at days 14, 17, 19, 21, 25, and 28 are shown. (B) Proliferation rate of CD56+CD94–CD117high and CD56+CD94+CD117low/– cells by Ki67 staining. To determine whether there was a differential rate of proliferation in developing NK-cell subsets, cells at days 21 to 28 of culture were permeabilized (as described in “Materials and methods”) and stained for the nuclear antigen Ki67. The results were representative of 3 experiments. (C) CD56+CD94–CD117high cells are precursors to CD56+CD94+CD117low/– cells. At day +21 (±3) of culture, CD56+CD94–CD117high cells were FACS sorted and cultured with cytokines and the feeder cell line (EL08.1D2). Cells were reanalyzed 1 week later for the presence of CD56+CD94–CD117high and CD56+CD94+CD117low/– populations. Shown are FACS plots after gating on the viable fraction. Results were representative of more than 3 individual experiments.
CD56+CD94–CD117high cells are a precursor population to CD56+CD94–CD117low/– cells. (A) The percentage of CD94–CD117high cells (top) and CD94+CD117low/– cells (bottom) within the CD56+ population as a function of the percentage of CD56+ cells in culture. CD34+Lin– cells were isolated by FACS and cultured as described in “Materials and methods.” The plots demonstrate that at early time points when the fraction of CD56+ cells is low the majority of CD56+ cells are CD94–CD117high. As the percentage of CD56+ cell increases, the fraction of CD56+CD94–CD117high cells decreases and the fraction of CD56+CD94+CD117low/– cells increases (bottom). Results of 2 individual donors tested at days 14, 17, 19, 21, 25, and 28 are shown. (B) Proliferation rate of CD56+CD94–CD117high and CD56+CD94+CD117low/– cells by Ki67 staining. To determine whether there was a differential rate of proliferation in developing NK-cell subsets, cells at days 21 to 28 of culture were permeabilized (as described in “Materials and methods”) and stained for the nuclear antigen Ki67. The results were representative of 3 experiments. (C) CD56+CD94–CD117high cells are precursors to CD56+CD94+CD117low/– cells. At day +21 (±3) of culture, CD56+CD94–CD117high cells were FACS sorted and cultured with cytokines and the feeder cell line (EL08.1D2). Cells were reanalyzed 1 week later for the presence of CD56+CD94–CD117high and CD56+CD94+CD117low/– populations. Shown are FACS plots after gating on the viable fraction. Results were representative of more than 3 individual experiments.
The influence of the feeder cell line EL08.1D2 on the transition from CD56+CD94–CD117high to CD56+CD94+CD117low/–. (A) At day 21 (±3) of culture, CD56+CD94–CD117high, CD56+CD94low/–CD117low/–, and CD56+CD94+CD117low/– populations were FACS sorted and cultured for 1 week on either the fetal liver cell line (EL08.1D2) and cytokines (IL-7, IL-15, SCF, and FLT3L) or in media containing the cytokines alone. After 1 week in culture, the sorted populations were analyzed by FACS. Shown are the results after gating on the CD56+ fraction. Results were representative of more than 3 experiments. (B) The percentage of CD56+CD94+CD117low/– cells detected after 1 week of culture either in the presence or absence of EL08.1D2. Shown are the results from 3 consecutive experiments (2 donors/experiment) where cells were sorted as in panel A and then cultured in cytokine containing media either without (white) or with (gray) EL08.1D2. Horizontal bar represents average and P is the result of a paired t test. (C) FACS-sorted CD56+CD9–CD117high cells (20 000) were cultured in triplicate with cytokines alone (left) or with cytokines and the stromal cell line EL08.1D2. Contact with the stromal cell line was either prevented with a transwell (middle) or allowed (right). Values represent the average number (+SD) of CD56+CD94+CD117low/– cells detected after 1 week of culture by FACS. Shown are the results from a single donor, representative of 6 individual donors.
The influence of the feeder cell line EL08.1D2 on the transition from CD56+CD94–CD117high to CD56+CD94+CD117low/–. (A) At day 21 (±3) of culture, CD56+CD94–CD117high, CD56+CD94low/–CD117low/–, and CD56+CD94+CD117low/– populations were FACS sorted and cultured for 1 week on either the fetal liver cell line (EL08.1D2) and cytokines (IL-7, IL-15, SCF, and FLT3L) or in media containing the cytokines alone. After 1 week in culture, the sorted populations were analyzed by FACS. Shown are the results after gating on the CD56+ fraction. Results were representative of more than 3 experiments. (B) The percentage of CD56+CD94+CD117low/– cells detected after 1 week of culture either in the presence or absence of EL08.1D2. Shown are the results from 3 consecutive experiments (2 donors/experiment) where cells were sorted as in panel A and then cultured in cytokine containing media either without (white) or with (gray) EL08.1D2. Horizontal bar represents average and P is the result of a paired t test. (C) FACS-sorted CD56+CD9–CD117high cells (20 000) were cultured in triplicate with cytokines alone (left) or with cytokines and the stromal cell line EL08.1D2. Contact with the stromal cell line was either prevented with a transwell (middle) or allowed (right). Values represent the average number (+SD) of CD56+CD94+CD117low/– cells detected after 1 week of culture by FACS. Shown are the results from a single donor, representative of 6 individual donors.
To investigate the mechanism of action of the stromal cell line, transwell experiments were performed. Purified CD117highCD94– cells were cultured with cytokines either in the absence or in the presence of the stromal cell line with direct contact prohibited (transwell), or contact was allowed. As shown in Figure 5C, the number of cells making the transition to the CD117low/–CD94+ stage was significantly enhanced when contact was allowed (P < .001). However, there was also a significant contribution of diffusible molecules since more CD117low/–CD94+ cells developed in the transwell cultures compared with cultures without the stromal cell line (P < .001). Collectively, these results demonstrate that the proximity of CD117highCD94– cells to EL08.1D2 facilitates the transition to the CD117low/–CD94+ stage. This likely underscores the role of direct cell contact in this process. However, we cannot rule out the contribution of EL08.1D2-derived soluble factors present at higher concentration in their direct proximity mediating this effect. In the absence of EL08.1D2 cells, the transition from CD117highCD94– to CD117low/– CD94+ is inefficient and a small fraction of the CD117low/–CD94+ cells reverts to a CD117lowCD94low/– phenotype.
Umbilical cord blood contains a small fraction of NK-cell progenitors with the CD117highCD94– phenotype
A number of different NK-cell progenitor populations has been described in umbilical cord blood.30-32 We therefore investigated whether the CD56+CD117highCD94– population was present in UCB. Of note, in freshly isolated UCB CD56+ cells the overall CD117 staining intensity was lower compared with in vitro–derived NK cells, yet we could consistently identify a population of CD56+CD117+CD94– cells with higher CD117 density than their CD94+ counterparts (Figure 6A). After 1 week of culture with cytokines used in differentiation media (IL-7, IL-15, SCF, and FLT-3L), the CD117 staining intensity matched that of in vitro–derived NK cells (Figure 6A). Similar to our findings with in vitro–derived NK cells, the percentage of CD56+CD117highCD94– cells was lower when UCB NK cells were cultured with the EL08.1D2 stromal cell line as compared with culture without EL08.1D2 (2.7% ± 1.8% vs 8.9% ± 7.1%; n = 6; P < .03).
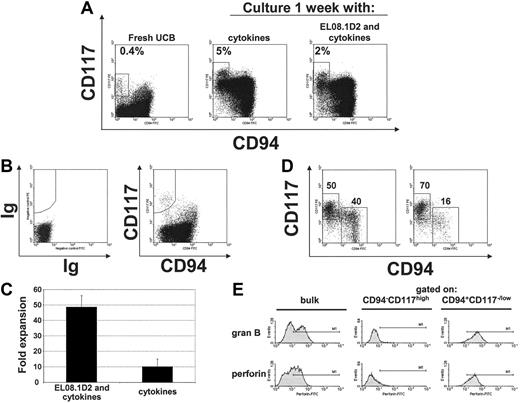
CD56+CD94–CD117high cells are present in UCB. (A) Small numbers of CD56+CD94–CD117high cells can be detected in UCB. FACS staining for CD117 and CD94 (after gating on CD56) of UCB-derived NK cells (left). FACS analysis after 1 week of culture of UCBderived CD56+ cells with either cytokines alone (IL-7, IL-15, SCF, and FLT3L) or with cytokines and EL08.1D2 (middle and right, respectively). Results are representative of 3 or more experiments. (B) Sorting strategy to isolate CD56+CD94–CD117high cells from UCB. The shown population was first gated on CD56+ cells (not shown). (C) Average fold expansion (±SD) of freshly isolated UCB CD56+CD94–CD117high cells after culture for 1 week either in the presence or absence of the stromal cell line (EL08.1D2). (D) Phenotype of FACSsorted UCB CD56+CD94–CD117high cells after 1 week of culture either in the presence (left panel) or absence (right panel) of the stromal cell line (EL08.1D2). (E) Expression of granzyme B and perforin in cultured UCB cells after transition to the CD56+CD94+CD117low/– phenotype. CD56+CD94–CD117high cells from UCB were FACS sorted and cultured on the stromal cell line for 1 week. Expression of granzyme B and perforin in either bulk (left), CD56+CD94–CD117high cells (middle), or CD56+CD94+CD117low/– cells (right) was determined by FACS. Panels B to E are the representative results of 4 separate donors.
CD56+CD94–CD117high cells are present in UCB. (A) Small numbers of CD56+CD94–CD117high cells can be detected in UCB. FACS staining for CD117 and CD94 (after gating on CD56) of UCB-derived NK cells (left). FACS analysis after 1 week of culture of UCBderived CD56+ cells with either cytokines alone (IL-7, IL-15, SCF, and FLT3L) or with cytokines and EL08.1D2 (middle and right, respectively). Results are representative of 3 or more experiments. (B) Sorting strategy to isolate CD56+CD94–CD117high cells from UCB. The shown population was first gated on CD56+ cells (not shown). (C) Average fold expansion (±SD) of freshly isolated UCB CD56+CD94–CD117high cells after culture for 1 week either in the presence or absence of the stromal cell line (EL08.1D2). (D) Phenotype of FACSsorted UCB CD56+CD94–CD117high cells after 1 week of culture either in the presence (left panel) or absence (right panel) of the stromal cell line (EL08.1D2). (E) Expression of granzyme B and perforin in cultured UCB cells after transition to the CD56+CD94+CD117low/– phenotype. CD56+CD94–CD117high cells from UCB were FACS sorted and cultured on the stromal cell line for 1 week. Expression of granzyme B and perforin in either bulk (left), CD56+CD94–CD117high cells (middle), or CD56+CD94+CD117low/– cells (right) was determined by FACS. Panels B to E are the representative results of 4 separate donors.
To further test whether the UCB-derived CD56+CD117highCD94– cells share features with the in vitro–derived CD56+CD117highCD94– cells, they were FACS purified from freshly isolated UCB CD56+ cells (Figure 6B). These purified cells showed tremendous expansion after 1 week of culture in the presence of the stromal cell line compared with culture with cytokines alone (47.9vs 10.0-fold; P = .001; Figure 6C). Like the in vitro–generated CD56+CD117highCD94– cells, the UCB-derived CD56+CD117highCD94–cells differentiated into the CD117low/–CD94+ stage with a clear supportive role of the stromal cell line (Figure 6D) and acquired perforin and granzyme upon transition to the CD117low/–CD94+ stage (Figure 6E), and a subset of newly developed CD117low/–CD94+ cells acquired CD16 and KIR (not shown). These results show that the population of CD56+CD117highCD94– cells in UCB is the in vivo counterpart of those defined in the in vitro differentiation system.
Discussion
Here we describe 2 subpopulations of CD56+ cells in human NK-cell differentiation cultures defined by the SCF receptor (c-kit, CD117), with a CD117low/– and CD117high population. CD94 was expressed by the CD117low/– and not by the CD117high subset, thus defining mutually exclusive NK-cell subpopulations in these cultures (CD117highCD94– and CD117low/–CD94+). Further phenotypic analysis demonstrated that the CD117highCD94– subpopulation lacked expression of multiple NKRs found on the CD117low/–CD94+ cells. This correlated with functionality since only CD117low/–CD94+ cells were cytotoxic and able to secrete IFN-γ upon stimulation. Both were derived from the same, single primitive CD34+CD38–Lin– precursor. The ratios of these 2 subsets changed during differentiation, such that CD117highCD94– cells predominated early and progressively decreased, whereas there was a reciprocal increase of the CD117low/–CD94+ fraction. The above-mentioned observations suggested that these 2 subpopulations represent stages of NK-cell differentiation and that the CD117low/–CD94+ cells arise from the CD117highCD94– cells. This was directly demonstrated since FACS-purified CD117highCD94– cells gave rise to the CD117low/–CD94+ population.
The EL08.1D2 stromal cell line supported the early expansion of CD56– cells containing NK-cell progenitors over the first 2 weeks in culture. Importantly, we also determined that this stromal cell line supported the CD117highCD94– to CD117low/–CD94+ transition predominantly by a contact-dependent mechanism. After 1 week of culture on EL08.1D2, on average approximately 41% of purified CD117highCD94– cells acquired a CD117low/–CD94+ phenotype. FACS-sorted cells were cultured at 100, 1000, and 10 000 cells per 96-well plate and the rate of CD117highCD94– to CD117low/–CD94+ conversion was nearly identical after 1 week (not shown). This approach allowed us to conclude that insufficient contact with the stromal cell line is unlikely to explain the lack of transition by a significant fraction of CD117highCD94– cells after sorting. Thus, the transition from CD117highCD94– to CD117low/–CD94+ may be selective.
Interestingly, small percentages of sorted CD117highCD94– cells transitioned to a CD117low/–CD94+ phenotype in the absence of the stromal cell line. However, prior to FACS sorting, NK progenitor cells were in contact with EL08.1D2, so it is possible that some of these CD117highCD94– cells had already been triggered to transition. In fact, we identified a discrete population with an intermediate phenotype (CD117lowCD94low/–) that showed a reduced requirement for stromal cells to mature to the CD117low/–CD94+ cells. Others have shown that CD56+ cells derived from CD34+ HPCs (with IL-2 and SCF, without stromal cell support) have similar characteristics to the above described CD117highCD94– cells described here.33 Thus, the transition from the CD117highCD94– to the CD117low/–CD94+ stage may require signals provided by the cellular environment (such as EL08.1D2). The nature of these signals is unknown, but the EL08.1D2 stromal cells likely mimic the physiologic milieu of NK-cell development. Freud has shown that CD34dimCD45RA+Integrin α4β7+ cells can be identified in human lymph nodes (LNs) and give rise to CD56+ cells in vitro after stimulation with IL-2 or IL-15 or activated T cells.34 These CD56+ cells were mainly CD94– and had other features in common with the CD117highCD94– cells described here. However, these investigators were able to demonstrate cytotoxicity and cytokine production, which may be due to the minor fraction of CD56+CD94+ cells present in these cultures. Given that the LN can be a site of NK-cell differentiation34 and that the predominant fraction of CD56+ cells in LN shares features with the CD117low/–CD94+ population35 indicates that the transition described here may occur within the LN. A redundant role of bone marrow may also exist.
We also observed that a small fraction CD117low/–CD94+ cells reverted back to the intermediate phenotype (CD117lowCD94low/–). This phenomenon occurred in all donors tested (n = 6). The EL08.1D2 stromal cell line influenced this process since in its absence the rate of reversion to the intermediate phenotype (CD117low/–CD94+ to CD117lowCD94low/–) was significantly higher. Because the intensity of NKG2A staining varied on the CD117low/–CD94+ cells (not shown), we hypothesized that insufficient inhibitory NKG2A signaling may account for this process. However, reversion to the intermediate phenotype was not dependent on the level of NKG2A expression since it occurred in both FACS-sorted CD117low/–CD94+NKG2Ahigh and CD117low/– CD94+NKG2Alow/– cells (not shown). Collectively, the EL08.1D2 stromal cell line influences both the CD117highCD94– and CD117low/– CD94+ cell populations, supporting the transition to and the maintenance of the more mature differentiation stage (CD117low/–CD94+).
Here we describe a coordinated acquisition of NKRs and functional properties by developing NK cells that define stages of NK-cell differentiation following CD56 expression. In agreement with previous human studies,36 we found that CD161 was among the first receptors expressed by developing CD56+ cells. In fact, we could identify a CD161+ population prior to the emergence of CD56+ cells (not shown). Another receptor expressed at the immature CD56+CD117highCD94– stage was NKp44. However, we did not detect this receptor on CD56+CD117highCD94– cells freshly isolated from UCB. Nevertheless, NKp44 was up-regulated on these cells after culture with NK-cell differentiation media containing IL-7, IL-15, SCF, and FLT-3L (not shown). Thus, we interpret these results to suggest that NKp44 can be expressed already at this early developmental stage likely in response to cytokines, similar to the effect of IL-2 and IL-15 on mature NK cells.37,38 Considering the significance of NKp44 in NK-cell development, patients with an inherited deficiency of DAP12 (Nasu-Hakola disease) have normal numbers of functional NK cells, suggesting that signaling via NKp44-DAP12 pathway is not essential for NK-cell development.39 Moreover, the choriocarcinoma cell line, JEG, which has been shown to trigger NKp44,40 did not improve NK-cell development when added to this in vitro differentiation system (not shown).
Importantly, the acquisition of cytotoxic properties was correlated with the expression of self MHC-specific inhibitory receptors (CD94/NKG2A) in differentiating NK cells. Such an orderly pattern suggests a mechanism assuring self-tolerance so that each cytotoxic NK cell would be inhibited by self-HLA (ie, HLA-E). Our studies also show that the acquisition of CD94/NKG2A occurs prior to KIR receptors in this differentiation system. Acquisition of CD94/NKG2A, but not KIR receptors, by NK cells developing from human thymocytes has also been shown.41 A similar paradigm (CD94/NKG2A acquisition prior to Ly49) has been described in developing murine NK cells.42 According to current data, all human NK cells express at least one inhibitory receptor specific for self-HLA, either KIR or CD94/NKG2A.16,17 We demonstrate that in NK-cell differentiation KIRs are acquired following CD94/NKG2A acquisition, which marks an important transition in NK-cell development (ie, acquisition of cytotoxic capacity and IFN-γ secretion). However, NKG2A/CD94–KIR+ NK cells are present in human peripheral blood.16,17,43 This leads us to hypothesize that the final NK-cell repertoire is shaped by the loss of CD94/NKG2A by cells expressing inhibitory KIR for which there is an HLA-ligand in the host. Our hypothesis is based on the in vitro NK-cell development study presented in the current paper. Further support is found in a computational modeling study of NK-cell inhibitory receptor formation44 and by the increasing proportions of CD94/NKG2A–KIR+ NK cells with aging.45 Moreover, in the setting of immune reconstitution following allogeneic hematopoietic cell transplantation, newly generated donor NK cells mainly express CD94/NKG2A, whereas KIR receptors are acquired later.46-48 This may have important implications, as the ligand for NKG2A (HLA-E) shows limited polymorphism that does not affect target recognition.49
Here we also show that the immature stage of NK-cell differentiation defined in vitro (CD117highCD94–) can be found in the CD56+ fraction of UCB. These CD56+CD117highCD94– cells are distinct from CD56bright NK cells that are known to express CD117,50 since CD56bright NK cells are uniformly CD94+.51 CD56+CD117highCD94– cells isolated from UCB had characteristics similar to their in vitro–derived counterparts with regard to the status of differentiation, given their proliferative capacity and lack of cytotoxic machinery. Perforin and granzyme B were acquired upon transition to the next developmental stage (CD56+CD117low/– CD94+), supported by the stromal cell line. Some of these newly developed CD56+CD117low/–CD94+ cells acquire CD16 and KIR. Although the exact lineage relationship between CD56bright and CD56dim NK cells is not clear, our results suggest that the CD56+CD117highCD94– stage of NK-cell development can give rise to NK cells with features of both CD56bright and CD56dim subsets. Whether this is an exclusive pathway of NK-cell development remains to be determined. The expression of CD8 characterizes a subset of mature NK cells with enhanced lytic potential and resistance to activation-induced apoptosis.52 We show that CD8 is acquired by a minor fraction of CD56+CD117low/–CD94+ cells, suggesting that this is a late step in NK-cell differentiation. Recently, NK cells without self MHC-specific inhibitory receptors (ie, lacking CD94/NKG2A and Ly49 receptors) have been identified in mice and such cells have reduced natural cytotoxicity.18 The CD56+CD117highCD94– population defined herein, both in the in vitro culture system and in UCB, could be an analogous cell population. Collectively, using an in vitro model that mimics the ontogeny of human NK-cell development, we define the differentiation stages that mark the transition from noncytotoxic to cytotoxic CD56+ cells.
Authorship
B.G. designed, performed, and analyzed experiments and wrote the paper; N.K. performed and analyzed experiments; M.S. performed and analyzed experiments; R.A.J.O. and E.A.D. created essential reagent; B.R.B. and J.S.M. contributed to experimental design, data analysis, and writing of the paper; and M.R.V. designed and analyzed experiments and wrote the paper.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 10, 2006; DOI 10.1182/blood-2006-04-020198.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We would like to acknowledge Valerie McCullar for many helpful discussions and the assistance of the Flow Cytometry Core Facility of the University of Minnesota Cancer Center, a comprehensive cancer center designated by the National Cancer Institute.
This work was supported by grants from the National Institutes of Health (NIH), nos. K08 HL04 505-03 (M.R.V.), P01 CA65 493 and R01 HL55 417 (J.S.M.), and R01 CA72 669 (B.R.B.), and was supported in part by National Cancer Institute grant no. P30 CA77 598.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal