Abstract
The SH2 domain-containing leukocyte adaptor protein of 65 kDa (SLP-65) is the key effector for signaling downstream of the B-cell antigen receptor (BCR). SLP-65 controls not only B lymphopoiesis and humoral immunity but also possesses a yet poorly defined tumor suppressor activity that is lost in many cases of acute lymphoblastic leukemia. We found that the 2 isoforms of human SLP-65 are differentially involved in positive and negative B-cell signaling. Reconstitution experiments revealed that an atypical SH3 domain-binding motif, which is present in the long but not in the short SLP-65 isoform, mediates association to Grb2 and suppresses activation of mitogen-activated protein kinases p38 and JNK as well as up-regulation of c-Fos expression. In turn, the short isoform activates not only AP1-driven but also NF-κB–driven gene transcription more potently than the long isoform. Conversely, the long rather than the short SLP-65 isoform promotes BCR-induced B-cell apoptosis. Our data further delineate the structural requirements of positive and negative SLP-65 signal transduction in normal and neoplastic cells.
Introduction
Proper development and immune effector functions of B cells rely on the intracellular adaptor protein SLP-651 (also named BLNK2 or BASH3 ). SLP-65 is used by the B-cell antigen receptor (BCR) on mature B lymphocytes as well as by the pre-BCR on precursor cells for coupling to a variety of intracellular signaling pathways.4,5 Consequently, SLP-65 deficiency in engineered mouse mutants6-9 or human patients10 interferes with early B lymphopoiesis and causes severe defects in humoral immune responses. Moreover, the block of B-cell differentiation can lead to pre–B-cell acute lymphoblastic leukemia (pre-B ALL) due to continued pre-BCR signaling and perpetual V(D)J gene exon rearrangements.11-13
To achieve the diverse signaling functions in a coordinated manner, SLP-65 accommodates modular protein interaction domains that allow the formation of multiple molecular signaling complexes in a stimulation-independent and stimulation-dependent manner. Proteins with Src homology (SH) 3 domains, such as Grb2,1,2,14 can be constitutively recruited to the N-terminal half of SLP-65 by proline-rich binding motifs with the typical consensus sequence PxxP, where x can be any amino acid. Inducible protein associations of SLP-65 are mediated by phosphotyrosine/SH2-domain interactions in 2 ways. First, following BCR activation Syk-mediated phosphorylation of SLP-651,2 creates specific docking sites for SH2 domain-containing proteins such as the adaptor proteins Grb2,1,2,14 Nck1,2,14 and CrkL,15 the guanine nucleotide exchange factor Vav,1,2,14 the E3 ubiquitin ligase c-Cbl,16 and, importantly, components of the Ca2+ initiation complex, that is, Bruton tyrosine kinase (Btk)17-19 and phospholipase C (PLC)–γ2.2,19-21 Second, the C-terminally located SH2 domain of SLP-65 binds the phosphorylated hematopoietic progenitor kinase HPK-122,23 and a non-ITAM phosphotyrosine residue in the BCR signaling component Ig-α.24,25
Dissecting the mechanistic details of SLP-65 signaling pathways has been greatly facilitated by DT40 chicken B cells, which are easily susceptible to gene targeting and hence allow reconstitution of signaling cascades with wild-type or mutant elements.26 The analysis of SLP-65–deficient DT40 cells revealed that the Ca2+ initiation complex in concert with Vav3-regulated Rac1 activation triggers c-Jun NH2-terminal kinase JNK.20 Similarly, the response of p38 mitogen-activated protein kinase (MAPK) requires PLC-γ2 and Rac1 activation.20,27,28 The SLP-65/Grb2 complex was initially thought to couple BCR ligation via SOS to the Ras/Erk pathway, but it then turned out that Ras is activated predominantly by the PLC-γ2 product diacylglycerol, which recruits and activates Ras guanine nucleotide-releasing protein 3 (RasGRP3).29 However, in DT40 as well as in human B cells, SLP-65 seems to be the critical control element of all 3 MAPK families19 and is also upstream of cytosolic transcription factors NFAT and NF-κB.30-32
The multiple signaling roles of SLP-65 explain the importance of this adaptor for B-cell physiology in mouse and man. Signaling homeostasis in human B cells, however, is maintained by 2 SLP-65 isoforms of 68 and 70 kDa.2,14 They are expressed in equal amounts and generated by alternative splicing of exon 8 present in the primary slp-65 transcript. The additional exon 8-encoded 23 amino acids of full-length SLP-65 encompass an atypical SH3 domain-binding motif (PxxxRxxKP) that is also found in SLP-76,33-36 the paralog of SLP-65 in T cells.5 SLP-76 binds constitutively via this functionally essential motif to the Grb2-related adaptor downstream of Shc (Gads).37 On T-cell activation, the SH2 domain of Gads provides a plasma membrane anchor for SLP-76 by binding to the tyrosine-phosphorylated LAT (linker of activated T cells).5 Membrane recruitment of SLP-65 in B cells is not completely understood but clearly different from that of SLP-76 in T cells.38 Hence, the role of the atypical SH3 domain-binding site in SLP-65 and the existence of 2 equally expressed isoforms remained unclear. We show here that full-length SLP-65 and the exon 8-splice variant possess distinct biochemical and functional properties that determine cytoplasmic, nuclear, and cellular responses of activated B cells.
Materials and methods
Cells, antibodies, and reagents
Ramos and DT40 B cells as well as their derivatives were cultured and stimulated through their BCR with 10 μg/mL anti–chicken IgM monoclonal antibody (mAb; M4, Southern Biotechnology, BioMol, Hamburg, Germany) or 10 μg/mL F(ab′)2 fragments of polyclonal goat anti–human IgM antibodies (Dianova, Hamburg, Germany) as described.1,39-41 Cells were solubilized in NP-40 lysis buffer (10 mM Tris/HCl, pH 7.8, 137.0 mM NaCl, 0.5 mM EDTA, 1 mM Na3VO4, 1% NP-40, 1 mM NaF, 10% glycerol, and protease inhibitors P2714 [Sigma-Aldrich, Deisenhofen, Germany]) or in RIPA buffer (50 mM Tris/HCl, pH 7.4, 137.5 mM NaCl, 0.5 mM EDTA, 2 mM Na3VO4, 1% NP-40, 1% glycerol, 0.5% sodium desoxycholate, 0.1% SDS, 10 mM Na4P2O7 and protease inhibitors P2714 [Sigma-Aldrich]). Antibodies used for immunoblotting are specific for human SLP-65 (2C9, BAbCo, Berkeley, CA), Grb2 (3F2, BioMol), Flag peptide (M2, Sigma-Aldrich), Erk (Becton Dickinson, Heidelberg, Germany), p38, pJNK (rabbit mAbs, New England Biolabs, Frankfurt, Germany), phosphotyrosine (4G10, Upstate Biotechnology, Lake Placid, NY), c-Fos (K-25, Santa Cruz Biotechnology, Santa Cruz, CA), PLC-γ2 (Q-20, Santa Cruz Biotechnology), Vav3 (kindly provided by T. Kurosaki, RIKEN Research Center for Allergy and Immunobiology, Yokohama, Japan), and human actin (Sigma-Aldrich). Inhibitors for p38 (SB202190) and JNK (SP600125) were purchased from Calbiochem (Darmstadt, Germany) and added to the cell cultures at the described concentrations for 1 hour prior to BCR activation. The N-terminally biotinylated peptide encompassing amino acids 202-214 of human/mouse SLP-65 used for affinity purifications of Grb2 was purchased from Jerini (Berlin, Germany).
Expression constructs and protein purification
The cDNA encoding wild-type chicken SLP-65 harboring an N-terminal peptide flag was inserted into pCRII-TOPO vector (Invitrogen, Karlsruhe, Germany). Using the QuickChange method, codons for amino acids 261-269 were deleted to generate chicken s-SLP-65. Each of the 2 cDNAs was inserted into pAbes-puro and the resulting expression constructs were introduced into SLP-65–deficient DT40 cells by electroporation (250 V, 970 μF). Transfectants were selected in the presence of puromycin (1 μg/mL) and screened by Western blot analysis. Wild-type murine SLP-65 was equipped with an N-terminal His-tag by ligation of the cDNA into pET15b (Novagen, Darmstadt, Germany). Recombinant His-SLP-65, produced in BL21(DE3) bacteria (Novagen), was purified using Ni-NTA-Superflow (Qiagen, Hilden, Germany), eluted with thrombin (Sigma-Aldrich) to remove the His epitope, further purified by size exclusion chromatography (Sephadex-200; Amersham Biosciences, Freiburg, Germany), and finally concentrated by centrifugation (Amicons, Millipore, Eschborn, Germany). Polymerase chain reaction (PCR) fragments encoding wild-type murine Grb2 or its isolated domains were ligated into pGEX-4T-1 (Amersham Biosciences). Recombinant GST fusion proteins were expressed in BL21(DE3) bacteria, purified via GSH-Sepharose 4FastFlow (Amersham Biosciences) and size exclusion chromatography (Sephadex-75, Amersham Biosciences), and finally concentrated by centrifugation (Vivaspins, Vivascience, Hanover, Germany).
Flow cytometry
For monitoring BCR-induced Ca2+ mobilization, 106 DT40 cells were loaded with 1 μM Indo-1-AM (Molecular Probes, BioMol, Hamburg, Germany) in 700 μL RPMI 1640, 5% FCS, 0.0015% pluronic acid F127 (Molecular Probes, BioMol) at 30°C for 25 minutes. Cell suspensions were diluted 2-fold with RPMI 1640, 10% FCS and incubated for 10 minutes at 37°C. Cells were washed twice with Krebs Ringer solution and on BCR activation the ratios of fluorescence intensities at 440 and 510 nm wavelengths were recorded on an LSR II (Becton Dickinson). For monitoring induction of apoptosis, resting or M4-stimulated 5 × 105 DT40 cells were washed twice with ice-cold PBS, stained with 7-amino-actinomycin D (7-AAD) and annexin V–PE (Apoptosis Detection Kit I, Becton Dickinson) according to the manufacturer's instructions, and analyzed by fluorescence-activated cell sorting (FACS) on a FACSCalibur (Becton Dickinson).
Isothermal titration calorimetry
The thermodynamic parameters of SLP-65/Grb2 interactions were determined by using isothermal titration calorimetry (ITC; MCS-ITC, MicroCal, Northampton, MA) as described by Pierce et al.42 Briefly, purified SLP-65 (25 μM) was placed into a reaction chamber at 25°C in 20 mM Tris/HCl, pH 8.0, 150 mM NaCl, 2 mM DTE. Full-length Grb2 or Grb2 domains (250 μM) were titrated through a syringe, and the resulting deviations from the equilibrium for heating power were measured and integrated to yield the enthalpy change for each injection. Data were fitted using χ2 minimization on a model assuming a single set of sites to calculate the equilibrium dissociation constant Kd of the SLP-65/Grb2 complex. All steps of the data analysis were performed using ORIGIN (v5.0) software provided by the manufacturer (MicroCal, Northampton, MA).
Luciferase reporter assays
For the measurement of AP1 and NF-κB activation, 107 DT40 cells were transiently cotransfected by electroporation (300 V, 975 μF) with 10 μg of the β-galactosidase expression plasmid pCMV-β (Becton Dickinson-Clontech, Heidelberg, Germany) and 20 μg of the reporter gene construct 3xTRE/tk43 or pGL2/7xNFκB-tkpro,43 which contain either 3 AP1 or 7 NF-κB binding sites 5′ of the thymidine kinase promoter (kindly provided by E. Serfling, Institute of Pathology, University of Würzburg, Germany). Transfectants were cultured for 24 hours, starved for 12 hours in RPMI 1640 1% FCS, 0.1% chicken serum, and then stimulated for 6 hours with M4 mAb in the presence or absence of MAPK inhibitors. After lysis, luciferase and β-galactosidase activities were assayed and standardized as described by Brummer et al.44
Results
Only full-length SLP-65 associates constitutively with Grb2 via the atypical SH3 domain-binding motif
The atypical SH3 domain-binding motif, PxxxRxxKP, which is missing in the exon 8-splice variant of human SLP-65, is highly conserved during evolution (Figure 1A). A similar sequence in SLP-76 binds with high affinity to the C-terminal SH3 domain of Grb2-related Gads in T cells.35 The individual interaction domains of Grb2 were expressed as GST fusion proteins and used for affinity purifications of SLP-65 proteins from resting and BCR-activated human Ramos B cells (Figure 1B). Anti–SLP-65 immunoblotting confirmed the previously reported stimulation-dependent binding of the Grb2 SH2 domain to both SLP-65 isoforms (lanes 3-4, see also Wienands et al,1 Fu et al,2 and Fu and Chan14 ). No SLP-65 binding could be detected for the N-terminal Grb2 SH3 domain (lanes 1-2), although this GST-SH3 fusion protein is functional and affinity purifies c-Cbl (data not shown). In contrast, the GST fusion protein containing the C-terminal Grb2 SH3 domain purifies full-length SLP-65 from both resting and stimulated B cells to equal amounts but not the faster migrating exon 8-splice variant (lanes 5-6).
To confirm the specificity of the constitutive SLP-65/Grb2 interaction and to directly quantify its biochemical parameters, we used ITC (for details, see “Materials and methods”). Briefly, ITC directly measures the binding equilibrium by determining the heat evolved on association of a ligand with its binding partner.42 This provides a basis for calculating biophysical parameters such as the dissociation constant (Kd) or the stoichiometry of the complex. Here, the isolated Grb2 SH3 domains or full-length Grb2 were titrated into a sample chamber containing recombinantly expressed full-length SLP-65. In accordance with the data obtained by affinity purification, no binding was observed between SLP-65 and the N-terminal SH3 domain of Grb2 (Figure 1C, middle panel). The C-terminal SH3 domain binds SLP-65 with a Kd of 20 ± 6 μM at a 1:1 stoichiometry (right panel). The full-length proteins associate with a slightly higher affinity, that is, a Kd of 6 ± 2 μM (left panel). This Kd value is, however, within the accuracy of ITC and confirms the 1:1 stoichiometry of the complex. The data further confirm that constitutive binding to full-length SLP-65 is solely achieved by the Grb2 C-terminal SH3 domain, which appears to specifically recognize the atypical SH3 domain-binding motif. Indeed, an isolated peptide comprising that region (amino acids 202-214 of human SLP-65; Figure 1A) binds the C-terminal SH3 domain of Grb2 in ITC with a Kd of 9 ± 3 μM (data not shown). Moreover and as shown in Figure 1D, when immobilized on a Sepharose matrix the same SLP-65 peptide purifies wild-type Grb2 from B-cell lysates (lanes 1-2) but not a mutant Grb2 protein carrying an inactivated C-terminal SH3 domain41 in which a critical tryptophane residue has been replaced by lysine (W193K, lanes 3-4). These data demonstrate a highly specific interaction between the atypical SH3 domain-binding motif of SLP-65 and the C-terminal SH3 domain of Grb2. The resulting constitutive interaction between the 2 adaptor proteins is restricted to full-length SLP-65, which shows that the 2 SLP-65 isoforms possess unique biochemical properties.
The atypical SH3 domain-binding motif of SLP-65 allows constitutive association with Grb2. (A) Amino acid sequence comparison (single-letter code) of human (top) and chicken (bottom) SLP-65 proteins. The human SLP-65 Δexon 8-splice variant lacks 23 amino acids in the central proline-rich region encompassing an atypical SH3 domain-binding motif P-(x)3-R-(x)2-K-P (x can be any amino acid). This particular motif is missing in the 9 amino acid–shortened chicken SLP-65 variant used in this study (chicken s-SLP-65). The sequence of a synthetic peptide, SLP-65[pep], which represents the atypical SH3-domain binding motif and which was used for affinity purifications is indicated. (B) Affinity purification of human SLP-65 and SLP-65 Δexon 8 by individual Grb2 domains. Human Ramos B cells were left untreated (lanes 1, 3, and 5) or stimulated through their BCR (lanes 2, 4, and 6) and cleared cellular lysates were subjected to affinity purifications with GST fusion proteins harboring isolated Grb2 domains (N-terminal SH3 domain, lanes 1-2; central SH2 domain, lanes 3-4; C-terminal SH3 domain, lanes 5-6). Purified proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and SLP-65 isoforms were detected by immunoblotting with anti–SLP-65 antibodies. The relative molecular mass of marker protein is indicated on the left. (C) Binding parameters of the constitutive SLP-65/Grb2 complex. Recombinantly produced SLP-65 was mixed stepwise in an isothermal titration calorimeter with either full-length Grb2 (left panel) or the isolated N- and C-terminal Grb2 SH3 domains (middle and right panels, respectively). Resulting changes of the heating power (top panel each) on association between binding partners were integrated and plotted versus the molar ratio Grb2/SLP-65 in the bottom panels. Curves according to a single-site binding model were fitted to obtain the indicated dissociation constants (Kd). NBD indicates no binding detectable. (D) Affinity purification of Grb2 by the SLP-65 peptide. grb2–/– DT40 cells were reconstituted with either wild-type Grb2 (lanes 1-2) or a mutant version carrying an inactivated C-terminal SH3 domain (W193K, lanes 3-4). From the cleared lysates of these cells, Grb2 was precipitated using an immobilized biotinylated SLP-65 peptide (SLP-65[pep], described in panel A) and visualized by anti-Grb2 immunoblotting. The relative molecular mass of marker protein is indicated on the left.
The atypical SH3 domain-binding motif of SLP-65 allows constitutive association with Grb2. (A) Amino acid sequence comparison (single-letter code) of human (top) and chicken (bottom) SLP-65 proteins. The human SLP-65 Δexon 8-splice variant lacks 23 amino acids in the central proline-rich region encompassing an atypical SH3 domain-binding motif P-(x)3-R-(x)2-K-P (x can be any amino acid). This particular motif is missing in the 9 amino acid–shortened chicken SLP-65 variant used in this study (chicken s-SLP-65). The sequence of a synthetic peptide, SLP-65[pep], which represents the atypical SH3-domain binding motif and which was used for affinity purifications is indicated. (B) Affinity purification of human SLP-65 and SLP-65 Δexon 8 by individual Grb2 domains. Human Ramos B cells were left untreated (lanes 1, 3, and 5) or stimulated through their BCR (lanes 2, 4, and 6) and cleared cellular lysates were subjected to affinity purifications with GST fusion proteins harboring isolated Grb2 domains (N-terminal SH3 domain, lanes 1-2; central SH2 domain, lanes 3-4; C-terminal SH3 domain, lanes 5-6). Purified proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and SLP-65 isoforms were detected by immunoblotting with anti–SLP-65 antibodies. The relative molecular mass of marker protein is indicated on the left. (C) Binding parameters of the constitutive SLP-65/Grb2 complex. Recombinantly produced SLP-65 was mixed stepwise in an isothermal titration calorimeter with either full-length Grb2 (left panel) or the isolated N- and C-terminal Grb2 SH3 domains (middle and right panels, respectively). Resulting changes of the heating power (top panel each) on association between binding partners were integrated and plotted versus the molar ratio Grb2/SLP-65 in the bottom panels. Curves according to a single-site binding model were fitted to obtain the indicated dissociation constants (Kd). NBD indicates no binding detectable. (D) Affinity purification of Grb2 by the SLP-65 peptide. grb2–/– DT40 cells were reconstituted with either wild-type Grb2 (lanes 1-2) or a mutant version carrying an inactivated C-terminal SH3 domain (W193K, lanes 3-4). From the cleared lysates of these cells, Grb2 was precipitated using an immobilized biotinylated SLP-65 peptide (SLP-65[pep], described in panel A) and visualized by anti-Grb2 immunoblotting. The relative molecular mass of marker protein is indicated on the left.
SLP-65 isoforms differently couple BCR engagement to MAPK activation
To individually assess the biochemical and functional traits of SLP-65 isoforms in vivo, we used the chicken DT40 B-cell system, which has been used previously to elucidate many SLP-65 functions. SLP-65–deficient DT40 cells20 were reconstituted with Flag-tagged versions of either full-length chicken SLP-65 or a shortened SLP-65 deletion mutant (s-SLP-65) lacking the atypical SH3 domain-binding motif (Figure 1A). Both SLP-65 proteins are expressed in equal amounts by the transfectants and become inducibly tyrosine phosphorylated (Figure 2A). They associate equally well with known SLP-65–binding proteins such as PLC-γ2 and Vav3 (Figure 2B, left and right panels, respectively). Also the BCR-induced Ca2+ response is restored by both SLP-65 species, albeit slightly reduced in s-SLP-65–expressing cells (Figure 2C). Hence, BCR-proximal signaling appears to be independent of the atypical SH3 domain-binding motif present in full-length SLP-65.
We next analyzed the SLP-65 transfectants for BCR-distal activation of Erk, p38, and JNK by immunoblotting of B-cell lysates with antibodies recognizing the phosphorylated (ie, activated) forms of the different MAPKs (Figure 3). In the absence of any SLP-65 expression, phosphorylation of Erk is weakly detectable on 3 minutes of BCR activation (Figure 3A, lanes 1-4). Erk phosphorylation occurs with accelerated kinetics and is strongly enhanced on expression of either of the 2 SLP-65 forms (lanes 5-12). The situation is different for BCR-regulated p38 (Figure 3B) and JNK (Figure 3C) activation. First, inducible phosphorylation of p38 and JNK is totally dependent on SLP-65 (lanes 1-4). Second, full-length and s-SLP-65 promote p38 as well as JNK activation with strikingly different capacities (lanes 5-12). In the presence of full-length SLP-65, phosphorylation of p38 and JNK peaks at about 10 minutes following BCR stimulation and declines thereafter (lanes 5-8). Expression of s-SLP-65 leads to a more rapid and more robust activation (lanes 9-12). This is most evident 5 minutes after BCR ligation, where phosphorylation of the 2 MAPKs is barely detectable in cells expressing full-length SLP-65 but readily detectable in cells expressing s-SLP-65 (compare lanes 6 with 10 in the upper panels of Figure 3B-C). Moreover, at this time point phosphorylation of p38 and JNK in the latter cells has already reached a level of intensity that is much stronger than the maximum phosphorylation at any other time in cells expressing full-length SLP-65. Finally, the duration of p38/JNK activation is also dramatically different for the 2 types of SLP-65 transfectants. Even 20 minutes after BCR activation, p38 and JNK are still highly phosphorylated in cells expressing s-SLP-65, whereas in cells expressing full-length SLP-65, phosphorylation is nearly back to baseline levels especially in the case of JNK. Collectively, s-SLP-65 promotes a much stronger p38/JNK activation than full-length SLP-65 and the kinetics are both more rapid as well as sustained. The data reveal for the first time that the 2 SLP-65 isoforms possess distinct capabilities to activate cytoplasmic signaling pathways and suggest a negative regulatory role for the atypical SH3 domain-binding motif present in full-length SLP-65.
Functional reconstitution of early BCR signaling by full-length and s-SLP-65. (A) In vivo phosphorylation of SLP-65 proteins. SLP-65–deficient DT40 parental cells (slp-65–/–, lanes 1-4) or transfectants stably expressing Flag-tagged versions of either full-length chicken SLP-65 (SLP-65, lanes 5-8) or the shortened form s-SLP-65 (lanes 9-12) (as in Figure 1) were left untreated (lanes 1, 5, and 9) or stimulated for the indicated times in minutes through their BCR (lanes 2-4, 6-8, and 10-12). Cleared cellular lysates were analyzed by immunoblotting with antibodies recognizing phosphotyrosine or the engineered peptide Flag in SLP-65 (top and bottom panels, respectively). (B) Association of SLP-65 isoforms with PLC-γ2 and Vav3. SLP-65–deficient DT40 cells (lanes 1-2 and 9-10) and the SLP-65–positive transfectants described in panel A as well as PLC-γ2– and Vav3-deficient DT40 cells (lanes 7-8 and 15-16, respectively) were left untreated or stimulated through their BCR for the indicated times. Cleared cellular lysates were subjected to immunoprecipitation with antibodies to PLC-γ2 (lanes 1-8) or Vav3 (lanes 9-16). SLP-65 proteins were detected by immunoblotting with antibodies to phosphotyrosine (top panels) or SLP-65 (middle panels). To control for immunoprecipitation efficiency and loading, PLC-γ2 and Vav3 were visualized by probing the membranes with anti–PLC-γ2 or anti-Vav3 antibodies (bottom panels). The relative molecular masses of marker proteins are indicated on the left. (C) Analysis of SLP-65–regulated Ca2+ mobilization. Cells described in panel A were stimulated through their BCR, and elevation of intracellular Ca2+ concentrations [Ca2+]i were monitored by flow cytometry. Turquoise, blue, and red curves represent profiles of empty vector–transfected slp-65–/– cells and transfectants expressing full-length or s-SLP-65, respectively.
Functional reconstitution of early BCR signaling by full-length and s-SLP-65. (A) In vivo phosphorylation of SLP-65 proteins. SLP-65–deficient DT40 parental cells (slp-65–/–, lanes 1-4) or transfectants stably expressing Flag-tagged versions of either full-length chicken SLP-65 (SLP-65, lanes 5-8) or the shortened form s-SLP-65 (lanes 9-12) (as in Figure 1) were left untreated (lanes 1, 5, and 9) or stimulated for the indicated times in minutes through their BCR (lanes 2-4, 6-8, and 10-12). Cleared cellular lysates were analyzed by immunoblotting with antibodies recognizing phosphotyrosine or the engineered peptide Flag in SLP-65 (top and bottom panels, respectively). (B) Association of SLP-65 isoforms with PLC-γ2 and Vav3. SLP-65–deficient DT40 cells (lanes 1-2 and 9-10) and the SLP-65–positive transfectants described in panel A as well as PLC-γ2– and Vav3-deficient DT40 cells (lanes 7-8 and 15-16, respectively) were left untreated or stimulated through their BCR for the indicated times. Cleared cellular lysates were subjected to immunoprecipitation with antibodies to PLC-γ2 (lanes 1-8) or Vav3 (lanes 9-16). SLP-65 proteins were detected by immunoblotting with antibodies to phosphotyrosine (top panels) or SLP-65 (middle panels). To control for immunoprecipitation efficiency and loading, PLC-γ2 and Vav3 were visualized by probing the membranes with anti–PLC-γ2 or anti-Vav3 antibodies (bottom panels). The relative molecular masses of marker proteins are indicated on the left. (C) Analysis of SLP-65–regulated Ca2+ mobilization. Cells described in panel A were stimulated through their BCR, and elevation of intracellular Ca2+ concentrations [Ca2+]i were monitored by flow cytometry. Turquoise, blue, and red curves represent profiles of empty vector–transfected slp-65–/– cells and transfectants expressing full-length or s-SLP-65, respectively.
The presence of the atypical SH3 domain-binding motif reduces the ability of SLP-65 to activate p38 and JNK. The slp-65–/– B cells (lanes 1-4) and transfectants expressing equal amounts of either full-length SLP-65 (lanes 5-8) or s-SLP-65 (lanes 9-12) were analyzed for the ability to activate distinct MAPK families on BCR engagement. The activation of Erk (A), p38 (B), and JNK (C) was monitored by immunoblotting of cleared cellular lysates with antibodies to phospho-Erk, phospho-p38, and phospho-JNK, respectively (top panels). Equal loading was controlled by probing the membranes with anti-Erk, anti-p38, or antiactin antibodies, respectively (bottom panels). The time of BCR activation (in minutes) is denoted above each lane. Relative molecular masses of marker proteins are indicated on the left.
The presence of the atypical SH3 domain-binding motif reduces the ability of SLP-65 to activate p38 and JNK. The slp-65–/– B cells (lanes 1-4) and transfectants expressing equal amounts of either full-length SLP-65 (lanes 5-8) or s-SLP-65 (lanes 9-12) were analyzed for the ability to activate distinct MAPK families on BCR engagement. The activation of Erk (A), p38 (B), and JNK (C) was monitored by immunoblotting of cleared cellular lysates with antibodies to phospho-Erk, phospho-p38, and phospho-JNK, respectively (top panels). Equal loading was controlled by probing the membranes with anti-Erk, anti-p38, or antiactin antibodies, respectively (bottom panels). The time of BCR activation (in minutes) is denoted above each lane. Relative molecular masses of marker proteins are indicated on the left.
AP1-driven and NF-κB–driven gene transcription is supported predominantly by s-SLP-65
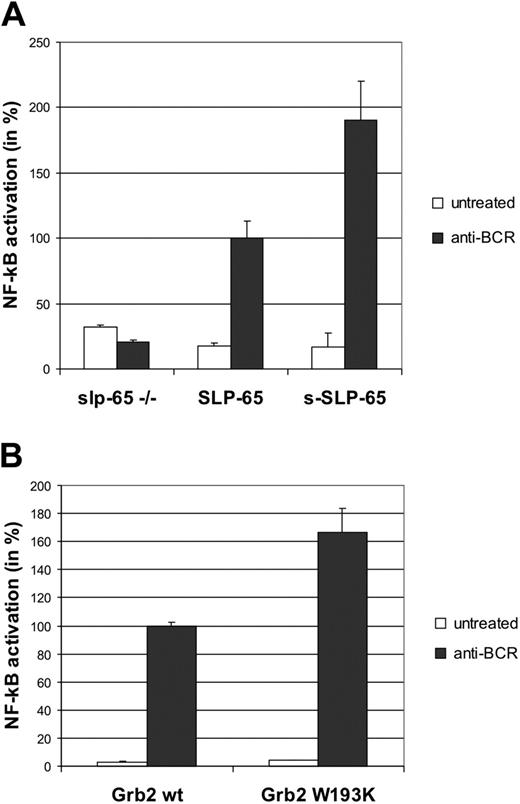
To test whether the observed differences in the activation of cytoplasmic effector proteins also affect nuclear responses, the different SLP-65 transfectants were equipped with a luciferase reporter gene construct under the transcriptional control of the AP1 complex comprising c-Jun and c-Fos. Empty vector-transfected cells served as control and cotransfection of a β-galactosidase expression plasmid allowed for the normalization of reporter gene activity according to the transfection efficiencies. As shown in Figure 4A, only SLP-65–positive cells show inducible AP1 activity (□, ▪). The magnitude of activation is approximately 3-fold higher in cells expressing s-SLP-65 than in cells expressing full-length SLP-65. Specific inhibition of the upstream regulator of c-Jun, that is, JNK, reduced the AP1 response in both SLP-65 transfectants by half (▦), whereas p38 inhibition does not (▨). Hence, AP1 is a downstream target of SLP-65, whose activation is, however, differentially regulated by the 2 SLP-65 isoforms. This conclusion is further supported by BCR-induced up-regulation of c-Fos expression, which is dramatically enhanced in cells expressing s-SLP-65 compared to those expressing full-length SLP-65 but not at all observed in SLP-65–deficient cells (Figure 4B). To assess the role of Grb2 for SLP-65–regulated AP1 activation, we performed the luciferase reporter assay with DT40 cells expressing either wild-type Grb2 or the W193K variant,41 which is unable to associate with the atypical SH3 domain-binding site in full-length SLP-65 (Figure 1). As shown in Figure 4C, expression of the Grb2 mutant supports AP1 activation significantly stronger than wild-type Grb2, which is consistent with the negative regulatory role of the constitutive SLP-65/Grb2 interaction. Similar results were obtained when we tested the BCR-induced NF-κB response of our SLP-65 and Grb2 transfectants (Figure 5). As previously reported,30 NF-κB activation is critically dependent on the presence of SLP-65 (Figure 5A). The short isoform conveys activation more potently than full-length SLP-65 (Figure 5A) and again, inactivation of the C-terminal SH3 domain in Grb2 leads to enhanced signaling (Figure 5B). Noteworthy, the NF-κB response, however, was not sensitive to inhibition of p38 or JNK (data not shown). Collectively, these findings show that the presence or absence of the atypical SH3 domain-binding site regulates BCR-induced signaling at the level of gene transcription and again demonstrate the functional differences between SLP-65 isoforms.
The atypical SH3 domain-binding motif and the C-terminal SH3 domain of Grb2 reduce the ability of SLP-65 to activate AP1. (A) SLP-65–regulated activation of an AP1-driven luciferase reporter gene. SLP-65–deficient cells were reconstituted with either full-length SLP-65 (middle bars) or s-SLP-65 (right bars) or, as control, were transfected with the empty vector (left bars). Subsequently, the AP1 reporter construct was transiently introduced by electroporation together with a β-galactosidase expression plasmid (for determination of transfection efficiency). Enzymatic activities of luciferase and β-galactosidase were determined for resting cells (□) and cells stimulated without further treatment through their BCR for 6 hours (▪) or cells that were pretreated for 1 hour prior to BCR activation with 10 μM of either SB202190 to inhibit p38 (p38-i, ▨) or SP600125 to inhibit JNK (JNK-i, ▦). At least 3 independent clones were measured in 4 independent experiments and data are normalized according to the transfection efficiency. Error bars indicate SD. (B) Immunoblot analysis of BCR-mediated synthesis of c-Fos. DT40 cell lines described in panel A were left untreated or BCR-stimulated for 1 hour and lysed and c-Fos expression was determined by immunoblotting with anti–c-Fos antibodies (top panel). Equal loading of cellular lysates was controlled by immunoblotting with antiactin antibodies (bottom panel). The relative molecular masses of marker proteins are indicated on the left. (C) Impact of the C-terminal Grb2 SH3 domain on AP1 activation. DT40 cells expressing equal amounts of either wild-type or W193K mutant Grb2 (left and right bars, respectively; also Figure 1D) were transfected with the AP1 reporter and β-galactosidase constructs, and resulting enzymatic activities in resting and BCR-stimulated cells (□, ▪, respectively) were determined as described in panel A. Error bars indicate SD of 3 independent measurements.
The atypical SH3 domain-binding motif and the C-terminal SH3 domain of Grb2 reduce the ability of SLP-65 to activate AP1. (A) SLP-65–regulated activation of an AP1-driven luciferase reporter gene. SLP-65–deficient cells were reconstituted with either full-length SLP-65 (middle bars) or s-SLP-65 (right bars) or, as control, were transfected with the empty vector (left bars). Subsequently, the AP1 reporter construct was transiently introduced by electroporation together with a β-galactosidase expression plasmid (for determination of transfection efficiency). Enzymatic activities of luciferase and β-galactosidase were determined for resting cells (□) and cells stimulated without further treatment through their BCR for 6 hours (▪) or cells that were pretreated for 1 hour prior to BCR activation with 10 μM of either SB202190 to inhibit p38 (p38-i, ▨) or SP600125 to inhibit JNK (JNK-i, ▦). At least 3 independent clones were measured in 4 independent experiments and data are normalized according to the transfection efficiency. Error bars indicate SD. (B) Immunoblot analysis of BCR-mediated synthesis of c-Fos. DT40 cell lines described in panel A were left untreated or BCR-stimulated for 1 hour and lysed and c-Fos expression was determined by immunoblotting with anti–c-Fos antibodies (top panel). Equal loading of cellular lysates was controlled by immunoblotting with antiactin antibodies (bottom panel). The relative molecular masses of marker proteins are indicated on the left. (C) Impact of the C-terminal Grb2 SH3 domain on AP1 activation. DT40 cells expressing equal amounts of either wild-type or W193K mutant Grb2 (left and right bars, respectively; also Figure 1D) were transfected with the AP1 reporter and β-galactosidase constructs, and resulting enzymatic activities in resting and BCR-stimulated cells (□, ▪, respectively) were determined as described in panel A. Error bars indicate SD of 3 independent measurements.
BCR-induced apoptosis is supported predominantly by full-length SLP-65
Activation of the proto-oncogenes c-Jun and c-Fos has been implicated in different and, in part, inverse cellular responses such as induction of apoptosis or stimulation of proliferation.45 We therefore monitored early and late apoptotic features of the SLP-65–deficient parental line and the different SLP-65 transfectants by staining the cells with annexin V and 7-AAD in the absence of and 24 hours after BCR engagement (Figure 6). A representative result of a 2-color flow cytometric density blot analysis is shown in panel A and the statistical calculation of the final stages of apoptosis is depicted in panel B. Without BCR activation, a small but similar percentage of all cell types stains positively for both apoptotic marker dyes (Figure 6A, left panels). BCR engagement of SLP-65–deficient cells has no effect (upper right plot). In marked contrast, both types of SLP-65 transfectants undergo BCR-induced cell death but the number of apoptotic cells that express full-length SLP-65 is increased about 2-fold compared to cells expressing s-SLP-65 (Figure 6A, middle and lower right panels). This difference holds true for early and late phases of apoptosis. The level of BCR surface expression is the same on SLP-65–deficient parental cells and both types of SLP-65 transfectants (data not shown). Pharmacologic inhibition of p38 or JNK slightly attenuates induction of apoptosis in both SLP-65 transfectants to a similar extent (Figure 6B). In conclusion, BCR-induced apoptosis is critically dependent on SLP-65 but expression of distinct SLP-65 isoforms differently affects this cellular response. It remains to be seen whether full-length SLP-65 couples more efficiently to apoptotic pathways than s-SLP-65 or whether expression of s-SLP-65 protects from apoptosis, for example, by increased activation of AP1 or NF-κB target genes. The fact that induction of apoptosis and AP1 activation are differently and distinctively affected by MAPK inhibition strongly suggests that these responses are separately regulated by SLP-65 signaling.
Inducible activation of NF-κB is inhibited by the atypical SH3-binding motif in SLP-65 and the C-terminal SH3 domain of Grb2. (A) SLP-65–regulated activation of NF-κB. SLP-65–deficient DT40 cells (left bars) and the 2 transfectants expressing either full-length or s-SLP-65 (middle and right bars, respectively) were transfected with an NF-κB reporter construct together with the β-galactosidase expression plasmid. Resulting enzymatic activities of resting (□) and BCR-stimulated cells (▪) were determined as described in Figure 4A. Error bars indicate SD of 6 independent measurements. (B) Impact of the C-terminal Grb2 SH3 domain on NF-κB activation. DT40 cells expressing equal amounts of either wild-type or W193K mutant Grb2 (left and right bars, respectively; also Figure 1D) were transfected with the NF-κB reporter and β-galactosidase constructs, and resulting enzymatic activities in resting (□) and BCR-stimulated cells (▪) were determined as described in Figure 4A. Error bars indicate SD of 3 independent measurements.
Inducible activation of NF-κB is inhibited by the atypical SH3-binding motif in SLP-65 and the C-terminal SH3 domain of Grb2. (A) SLP-65–regulated activation of NF-κB. SLP-65–deficient DT40 cells (left bars) and the 2 transfectants expressing either full-length or s-SLP-65 (middle and right bars, respectively) were transfected with an NF-κB reporter construct together with the β-galactosidase expression plasmid. Resulting enzymatic activities of resting (□) and BCR-stimulated cells (▪) were determined as described in Figure 4A. Error bars indicate SD of 6 independent measurements. (B) Impact of the C-terminal Grb2 SH3 domain on NF-κB activation. DT40 cells expressing equal amounts of either wild-type or W193K mutant Grb2 (left and right bars, respectively; also Figure 1D) were transfected with the NF-κB reporter and β-galactosidase constructs, and resulting enzymatic activities in resting (□) and BCR-stimulated cells (▪) were determined as described in Figure 4A. Error bars indicate SD of 3 independent measurements.
s-SLP-65–mediated apoptosis is diminished after BCR stimulation.(A) BCR-induced apoptosis mediated by SLP-65 and s-SLP-65. SLP-65–deficient cells (top row) or transfectants expressing either full-length or s-SLP-65 (middle and bottom rows, respectively) were left untreated or stimulated through their BCR for 24 hours (left and right panels, respectively). Following staining of the cells with 7-AAD and annexin V–PE, early and late apoptotic cells were identified by flow cytometry. (B) Statistical evaluation and influence of MAPK inhibition on SLP-65–regulated cell death. Apoptotic rates of resting and BCR-stimulated cells were calculated for 3 independent clones analyzed at least 2 times as described in panel A and for cells pretreated prior to BCR activation with 2.5 μM p38-i (SB202190, ▨) or 5 μM JNK-i (SP600125, ▦) to inhibit p38 and JNK, respectively. Error bars indicate SD of 9 independent experiments.
s-SLP-65–mediated apoptosis is diminished after BCR stimulation.(A) BCR-induced apoptosis mediated by SLP-65 and s-SLP-65. SLP-65–deficient cells (top row) or transfectants expressing either full-length or s-SLP-65 (middle and bottom rows, respectively) were left untreated or stimulated through their BCR for 24 hours (left and right panels, respectively). Following staining of the cells with 7-AAD and annexin V–PE, early and late apoptotic cells were identified by flow cytometry. (B) Statistical evaluation and influence of MAPK inhibition on SLP-65–regulated cell death. Apoptotic rates of resting and BCR-stimulated cells were calculated for 3 independent clones analyzed at least 2 times as described in panel A and for cells pretreated prior to BCR activation with 2.5 μM p38-i (SB202190, ▨) or 5 μM JNK-i (SP600125, ▦) to inhibit p38 and JNK, respectively. Error bars indicate SD of 9 independent experiments.
Discussion
It is now well established that the fate of developing and mature B cells is under the surveillance of SLP-65. Here we showed that the 2 isoforms of human SLP-65 contribute differently to that task. First, we elucidated biochemical differences between full-length SLP-65 and the Δexon 8-splice variant, in that only the former binds constitutively Grb2 via its C-terminal SH3 domain. We then showed that this does not affect early BCR signaling events such as Syk-mediated SLP-65 phosphorylation and subsequent mobilization of Ca2+ ions. Also Erk activation is equally well promoted by both SLP-65 isoforms. In marked contrast, activation of p38 and JNK is mediated predominantly through s-SLP-65, suggesting a negative signaling role for the atypical SH3 domain-binding site, which is present only in full-length SLP-65. The different signaling efficiencies of SLP-65 isoforms for the activation of cytoplasmic effectors also affect nuclear responses. A specifically high activation of AP1 and NF-κB transcription factor complexes is observed downstream of s-SLP-65. It appears that both AP1 components are targeted because we observe up-regulation of c-Fos protein expression, and the increased JNK activation may lead to enhanced phosphorylation of c-Jun. The general necessity of SLP-65 for BCR-triggered NF-κB activation can be explained by the observation that this pathway requires the SLP-65 effector PLC-γ2 and its enzymatic product diacylglycerol.46,47 Both SLP-65 isoforms couple, however, to PLC-γ2 with similar efficiency. Reduced NF-κB activation in the presence of full-length SLP-65 thus suggests a regulatory role for the atypical SH3 domain-binding motif on further downstream events such as IKK activation or degradation of IκB. On the cellular level, BCR-induced entry into early and late stages of apoptosis is enhanced by full-length SLP-65 up to more than 200% compared with s-SLP-65. In summary, the efficiency of SLP-65–mediated signal transduction for BCR-triggered cytoplasmic, nuclear, and cellular responses is regulated by the presence or absence of the atypical SH3 domain-binding site. We conclude that the 2 human SLP-65 isoforms exert different signaling functions for normal and probably also neoplastic B-cell growth and that the exon 8-encoded Grb2-binding site plays a key role in these processes.
Based on the documented function of Grb2 in other receptor systems48 and the reported role of Gads for SLP-76 signaling in T cells,5,37,49,50 it is tempting to speculate that Grb2 regulates the subcellular localization of SLP-65. Constitutive binding to Grb2 may target full-length SLP-65 to a cellular compartment that is different from the one where s-SLP-65 or the Δexon 8-splice variant resides. This compartmentalization does not affect the coupling of SLP-65 isoforms to upstream regulators such as Syk and thus the availability of signaling-competent SLP-65 pools because full-length and s-SLP-65 become equally well phosphorylated on BCR activation. Distinct localizations may, however, affect the efficiency with which SLP-65 pools communicate to downstream effector cascades. Alternatively to the spatial model of Grb2-regulated SLP-65 signal efficiency, Grb2 may more directly modulate SLP-65 activity by recruiting negative effector enzymes into its vicinity. The 2 possibilities are, of course, not mutually exclusive. It is also important to note that we cannot rule out the existence of additional ligands for the atypical SH3 domain-binding motif and hence the possibility that other modulators than Grb2 are responsible for the different signaling capacities of SLP-65 isoforms.
Direct evidence for a targeting function of Grb2 in SLP-65–regulated signaling is provided by the finding that both molecules act in concert to localize Vav3 into signaling-competent membrane microdomains, that is, lipid rafts.28 As in the case of SLP-65, the C-terminal SH3 domain of Grb2 mediates constitutive association with Vav3.51-53 This complex formation promotes initial membrane translocation of Vav3, whereas SLP-65 is reported to sustain this residency.28 Moreover, the cooperation between Grb2 and SLP-65 for Vav3 localization is required for optimal Rac1 activation, which is upstream of JNK and p38.20,27,28 Nonetheless, we do not observe striking differences of Vav3 phosphorylation in our SLP-65 transfectants or a preferred association of Vav3 to one of the 2 SLP-65 isoforms. Likewise, Rac1 activation is similarly supported by both SLP-65 isoforms (data not shown). Thus, the mechanism by which the lack of the SH3 domain-binding site in SLP-65 improves JNK/p38 activation remains to be elucidated.
The functional significance of distinct JNK/p38 activation by SLP-65 isoforms is demonstrated by our AP1 reporter gene assay showing a particularly high transcriptional activity in cells expressing s-SLP-65. How exactly this translates into cellular responses is difficult to predict because the different members of JNK and p38 MAPK families are implicated in both proapoptotic and antiapoptotic signaling.54 This holds also true for c-Jun and c-Fos family members constituting a diverse array of AP1 transcription factor complexes, which have been described as a “double-edged sword.”55 Depending on the composition of the AP1 complex in a given cell type or at a certain developmental stage, its activation can have opposing effects on cell growth and differentiation and therefore tumor development. It is thus possible that a high level of signaling by the JNK/p38-AP1 pathway protects from BCR-induced apoptosis, explaining our finding that cells expressing s-SLP-65 undergo diminished apoptosis compared with cells expressing full-length SLP-65. In line with this interpretation, JNK activity has been implicated in survival of transformed B lymphoblasts.56 Alternatively, by binding Grb2 (or other SH3 domain-containing effectors) full-length SLP-65 may couple more efficiently to apoptotic pathways than s-SLP-65. We favor the latter possibility for 2 reasons. First, we find that BCR-induced apoptosis is dependent on SLP-65 expression and, thus, SLP-65 acts as a positive rather than a negative regulator of this response. Second and most importantly, pharmacologic inhibition of the 2 MAPKs has different and distinct effects on AP1 activation versus induction of apoptosis. Therefore, these SLP-65–regulated responses appear to be separate signaling pathways. It is, however, conceivable that increased NF-κB signaling downstream of s-SLP-65 protects from cell death, which is consistent with the well-documented role of this transcription factor in cell proliferation, survival, and transformation.57,58 Although studies in the DT40 system have elucidated several key mechanisms of BCR signal transduction, possible differences between this cell line and primary human B cells, which normally express the 2 SLP-65 isoforms, cannot be excluded.
Collectively, our findings suggest that the 2 isoforms of human SLP-65 act in concert to fine-tune intracellular signaling events that, in turn, trigger appropriate cellular responses dependent on the developmental stage or nature of the antigen, that is, B-cell activation or death by apoptosis. This delicate signaling balance requires a precise regulation of the splicing machinery that includes or excludes exon 8-encoded sequences into the slp-65 mRNA and hence determines relative expression levels of SLP-65 isoforms. Any failure to maintain this equilibrium may interfere with proper B-cell function or contribute to tumorigenesis.
Authorship
A.G. designed and performed the experiments, and J.W. provided the project's concept.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 15, 2006; DOI 10.1182/blood-2006-02-005397.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We want to thank Dr Christian Herrmann for his expert help with ITC measurements and Drs Tomohiro Kurosaki, Edgar Serfling, and Stefan Klein-Heßling for kindly providing valuable reagents.
This work was supported by the Deutsche Forschungsgemeinschaft through grant FOR521.

![Figure 1. The atypical SH3 domain-binding motif of SLP-65 allows constitutive association with Grb2. (A) Amino acid sequence comparison (single-letter code) of human (top) and chicken (bottom) SLP-65 proteins. The human SLP-65 Δexon 8-splice variant lacks 23 amino acids in the central proline-rich region encompassing an atypical SH3 domain-binding motif P-(x)3-R-(x)2-K-P (x can be any amino acid). This particular motif is missing in the 9 amino acid–shortened chicken SLP-65 variant used in this study (chicken s-SLP-65). The sequence of a synthetic peptide, SLP-65[pep], which represents the atypical SH3-domain binding motif and which was used for affinity purifications is indicated. (B) Affinity purification of human SLP-65 and SLP-65 Δexon 8 by individual Grb2 domains. Human Ramos B cells were left untreated (lanes 1, 3, and 5) or stimulated through their BCR (lanes 2, 4, and 6) and cleared cellular lysates were subjected to affinity purifications with GST fusion proteins harboring isolated Grb2 domains (N-terminal SH3 domain, lanes 1-2; central SH2 domain, lanes 3-4; C-terminal SH3 domain, lanes 5-6). Purified proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and SLP-65 isoforms were detected by immunoblotting with anti–SLP-65 antibodies. The relative molecular mass of marker protein is indicated on the left. (C) Binding parameters of the constitutive SLP-65/Grb2 complex. Recombinantly produced SLP-65 was mixed stepwise in an isothermal titration calorimeter with either full-length Grb2 (left panel) or the isolated N- and C-terminal Grb2 SH3 domains (middle and right panels, respectively). Resulting changes of the heating power (top panel each) on association between binding partners were integrated and plotted versus the molar ratio Grb2/SLP-65 in the bottom panels. Curves according to a single-site binding model were fitted to obtain the indicated dissociation constants (Kd). NBD indicates no binding detectable. (D) Affinity purification of Grb2 by the SLP-65 peptide. grb2–/– DT40 cells were reconstituted with either wild-type Grb2 (lanes 1-2) or a mutant version carrying an inactivated C-terminal SH3 domain (W193K, lanes 3-4). From the cleared lysates of these cells, Grb2 was precipitated using an immobilized biotinylated SLP-65 peptide (SLP-65[pep], described in panel A) and visualized by anti-Grb2 immunoblotting. The relative molecular mass of marker protein is indicated on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/12/10.1182_blood-2006-02-005397/4/m_zh80230604480001.jpeg?Expires=1769168772&Signature=JVFwZgF6oMcHkx2MhKEgzCwD5lxEfcd5LRLAQgvg2Qwvj3kLNT-YF5T7QCdnGQJfABoJfeJzjzWN~HUc2B81BmhcG8wdmNz-Y8vV09NWU7hcuPsg7LzIG0zCgNPhCvPOvNd87eybtQPDR6Z40RsdDPB96Obmyg-oK5wvhKAk8h~PL6y4hTQ5Z6BsIu4c3e762d5HB7Bnt33VsrvurY~Ox2xRRpXPCZr1SQ72HzjGXpclOtGAYlmGjS47hUesb5LB4rduC8pr8TbQGPgBmZ9dCvcs0unuxvB5dBAaX3LKiNlat1bi5HbbPwMBquYKd23cVfCR4GLqzN31x09rD15zUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Functional reconstitution of early BCR signaling by full-length and s-SLP-65. (A) In vivo phosphorylation of SLP-65 proteins. SLP-65–deficient DT40 parental cells (slp-65–/–, lanes 1-4) or transfectants stably expressing Flag-tagged versions of either full-length chicken SLP-65 (SLP-65, lanes 5-8) or the shortened form s-SLP-65 (lanes 9-12) (as in Figure 1) were left untreated (lanes 1, 5, and 9) or stimulated for the indicated times in minutes through their BCR (lanes 2-4, 6-8, and 10-12). Cleared cellular lysates were analyzed by immunoblotting with antibodies recognizing phosphotyrosine or the engineered peptide Flag in SLP-65 (top and bottom panels, respectively). (B) Association of SLP-65 isoforms with PLC-γ2 and Vav3. SLP-65–deficient DT40 cells (lanes 1-2 and 9-10) and the SLP-65–positive transfectants described in panel A as well as PLC-γ2– and Vav3-deficient DT40 cells (lanes 7-8 and 15-16, respectively) were left untreated or stimulated through their BCR for the indicated times. Cleared cellular lysates were subjected to immunoprecipitation with antibodies to PLC-γ2 (lanes 1-8) or Vav3 (lanes 9-16). SLP-65 proteins were detected by immunoblotting with antibodies to phosphotyrosine (top panels) or SLP-65 (middle panels). To control for immunoprecipitation efficiency and loading, PLC-γ2 and Vav3 were visualized by probing the membranes with anti–PLC-γ2 or anti-Vav3 antibodies (bottom panels). The relative molecular masses of marker proteins are indicated on the left. (C) Analysis of SLP-65–regulated Ca2+ mobilization. Cells described in panel A were stimulated through their BCR, and elevation of intracellular Ca2+ concentrations [Ca2+]i were monitored by flow cytometry. Turquoise, blue, and red curves represent profiles of empty vector–transfected slp-65–/– cells and transfectants expressing full-length or s-SLP-65, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/12/10.1182_blood-2006-02-005397/4/m_zh80230604480002.jpeg?Expires=1769168772&Signature=CR2QTm8-Xb4EJ4Viw40A148AL6cUKSSO-24wG9pl6WOYmqNVPLo1XIzHyW5J3eZmxt3NjUh1wURYeJl3AZeutXxz8vtaolb2uIECig-afAxNE9Ju~ywNh~vrc5voZcYXQS7YT6jTQKPQYeEDVY~L0Fg~Y~h3gEEUy8rhT32AIhv8kd052qUs7LMtUfW8RaTGlzHHfq9NQAZhUai2EmKeiU3vtxBTZ1KkbTKm5Tv1E5WMk7~bStcbK1AKweqilmhOWTVgQoqFqjiX8hqDS0P7zoDPaJ-X2ASdhVbkMcFVfwpgE2svLt52mwrnP-UEx-2zsRjXMfqXno6Ek5bfN0qa3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal