Abstract
Prophylactic treatment for hemophilia A involves infusion of factor VIII (FVIII) concentrates every 2 to 3 days. Liposomes can be efficacious vehicles for medicines, and surface modification by PEGylation can prolong liposome circulation time. When reconstituted with PEGylated liposomes (PEGLip's), recombinant FVIII binds noncovalently but with high affinity to the external liposome surface. This preparation showed prolongation of FVIII half-life and increased protection from bleeding in preclinical models. Here we report a blinded, controlled, crossover, multicenter clinical study that evaluated the time free from bleeding episodes in patients with hemophilia A during prophylaxis with standard rFVIII (no liposomes) or PEGLip rFVIII (PEGLip reconstituted) at 25 and 35 IU/kg doses. Of 24 enrolled patients, 23 were eligible for efficacy analysis. Mean number of days without bleeds was 7.2 ± 1.7 with standard rFVIII compared with 13.3 ± 4.8 with PEGLip rFVIII at the 35 IU/kg dose and 5.9 ± 1.7 with standard rFVIII versus 10.9 ± 2.9 with PEGLip rFVIII at the 25 IU/kg dose (P < .05 between treatment groups for each dose). PEGLip rFVIII was well tolerated. These data suggest that reconstitution of rFVIII with PEGLip's may reduce the frequency of treatment during prophylaxis.
Introduction
Prophylactic replacement therapy for hemophilia A is based on intravenous infusions of purified factor VIII (FVIII) concentrates.1-10 Since the half-life of human FVIII is about 10 to 12 hours,11 infusions typically need to be repeated every 2 to 3 days to maintain a FVIII level above 1% in patients treated according to the pharmacokinetic (PK) dosing model.1,12-15 The prevention of bleeding episodes, especially in young patients, is of vital importance, as it reduces the occurrence of hemophilic arthropathy, which usually develops secondary to repeated intraarticular bleeding episodes.3,4,6,7,16-21 Prophylactic infusions performed in order to prevent bleeding episodes can delay the time of onset of hemophilic arthropathy and reduce the severity of pain and sequelae. Patients dosed along PK parameters usually demonstrate very few bleeding episodes and low morbidity.22
Repeated regular intravenous infusions performed at home every 2 to 3 days over the course of decades places a great burden on patients to be compliant with their therapy. Compliance with treatment directives has been directly linked to the number of infusions given, with compliance increasing as the number of infusions decreased.23 A FVIII molecule or product formulation that demonstrates increased time in circulation could greatly improve the efficacy and quality of life associated with prophylactic treatment for hemophilia A.
The method most commonly used for prolongation of half-life of recombinant proteins, the covalent incorporation of polyethylene glycol (PEG; PEGylation),24 is not yet available for FVIII. An alternative approach is to incorporate the FVIII protein with a carrier molecule that can be modified by PEGylation, resulting in a prolongation of the time the molecule provides hemostatic efficacy. The advantage with such an approach is that the FVIII molecule would not need to be altered and, provided that the carrier did not confer any conformational changes to the FVIII or act as an adjuvant for the immune system, the risk for FVIII inhibitor development would not be increased compared with standard treatment. Furthermore, since low levels of FVIII have been shown to confer prophylactic efficacy, the goal of maintaining FVIII levels above 1% as in the PK model may not be necessary.25-28
Baru et al29 reported the use of PEGylated liposomes as carriers for recombinant FVIII (Kogenate FS). Synthetic PEGy-lated liposomes, composed of 90% (wt/wt) palmitoyl-oleoylphosphatidylcholine (POPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanol-amine-N-[poly-(ethyleneglycol)-2000] (DSPE-PEG 2000), 97:3 molar ratio, respectively, were suspended in 50-mM sodium citrate buffer (9% wt/vol solution). FVIII binds noncovalently and in a specific sequence-dependent fashion to the outer surface of the PEGylated liposomes and is available for clotting at all times. The half-life of FVIII associated with PEGylated liposomes is significantly prolonged in mice,29 although half-life has not yet been determined in humans. Moreover, results from a hemophilia mouse tail-cut model demonstrated that prophylactic infusion of FVIII reconstituted with PEGylated liposomes extended the hemostatic efficacy of FVIII compared with infusions of standard FVIII.29 Toxicology experiments support a good tolerability profile for PEGylated liposomes.45 Here we report a clinical study that was performed to evaluate the clinical efficacy and safety of rFVIII reconstituted with PEGylated liposomes when used with the goal of prolonging the bleeding-free period following an infusion administered as part of a prophylactic treatment regimen.
Patients, materials, and methods
Study design
The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines. Approval was obtained from the appropriate ethics committees as well as by the Ministry of Health. All patients gave written informed consent prior to participation. The study was designed as a controlled, patient-blinded (for prophylactic infusions only), crossover trial.
After fulfilling all eligibility criteria, each patient received treatment for 3 consecutive bleeding episodes with standard rFVIII (Kogenate FS; Bayer HealthCare Pharmaceuticals, Berkeley, CA). Directly following this wash-in period, each patient was treated in 3 identically designed study segments. In each segment the patient received 1 prophylactic infusion followed by treatment for 3 spontaneous bleeding episodes. Each prophylactic infusion was administered during a nonbleeding state after a 4-day wash-out period with no factor replacement treatment. The first prophylactic infusion (study segment 1) was given with standard rFVIII, whereas the prophylactic infusion in segments 2 and 3 was given with PEGylated liposome–reconstituted rFVIII (PEGLip rFVIII). All infusions were administered with the same dose of rFVIII within each dosing cohort (ie, 35 IU/kg or 25 IU/kg). The higher-dose group used approximately 150 IU FVIII/mL of liposome and the lower-dose group used 100 IU/mL of liposome.
The infusions were administered using speed-controlled infusion pumps. Flow rates were 22 mL/h for the first 5 mL, 44 mL/h for the second 5 mL, and 60 mL/h for the remaining volume. Each infusion required approximately 20 to 30 minutes, depending on the volume to be infused. All infusions (both prophylactic and on-demand) were given in the hemophilia center. In vivo recovery was assessed after each prophylactic injection. Samples were obtained before injection and at 10, 30, and 60 minutes after the infusion. Blood samples for clinical chemistry and complete blood count (CBC) were collected prior to and 1 hour after each prophylactic infusion. Vital signs (blood pressure and heart rate) were monitored before, during, and up to 1 hour after each prophylactic infusion. The primary outcome parameter was the number of days between the prophylactic infusion and the first spontaneous bleed/treatment episode. In order to not interfere with activities of daily life, there were no scheduled visits between the prophylactic infusion and spontaneous bleeding episodes. FVIII measurements were performed in the central laboratory using the chromogenic method.30,31 Inhibitors to FVIII were assessed using the Bethesda method (Nijmegen modification32 ) using plasma samples collected from all patients prior to study start and at study end.
Liposomes
Liposomes composed of POPC and DSPE-PEG 2000 (Genzyme Pharmaceuticals, Liestal, Switzerland; 97:3 molar ratio, respectively) were prepared as follows. Lipids were dissolved to 15% wt/vol in tert-buthanol (Reidel-de Haen, Seelze, Germany) and lyophilized. The resulting dry lipid powder was resuspended to 100 mM phospholipids (PLs) in a 50-mM sodium citrate buffer (pH 7.0) to form liposomes. The liposomes were downsized by sequential extrusion using polycarbonate filters (Whatman, Florham Park, NJ) to a size of 80 to 110 nm.
Dosing
For the 35 IU/kg cohort, 7 mL of liposomes per 1000 IU of rFVIII was used; for the 25 IU/kg cohort, 10 mL of liposomes per 1000 IU of rFVIII was used. The 35 IU rFVIII/kg dose group received rFVIII reconstituted with PEGLip's at a concentration of approximately 150 IU rFVIII per mL of liposome solution. The 25 IU rFVIII/kg group received rFVIII reconstituted at a concentration of approximately 100 IU FVIII per mL of liposome aqueous dispersion. Thus, both FVIII dose groups (25 and 35 IU/kg) received about the same total amount of liposomes (approximately 22 mg per kg of body weight).
Patient inclusion and exclusion criteria
Included patients were adult males (18-60 years of age) with a weight of 50 to 100 kg and severe hemophilia A (FVIII:C ≤ 1%). Patients were to have received on-demand factor replacement therapy and have accumulated at least 150 treatment cumulative exposure days (CEDs) to previous FVIII products or at least 25 CEDs to previous products during the year prior to study initiation; patients had to have received at least 15 infusions during the previous year. Patients were to have a minimum bleeding/treatment pattern of a mean of 4 episodes per month, evenly distributed within each month, during the 3 months preceding study inclusion. HIV-positive patients were eligible if their CD4 lymphocyte counts were at least 0.4 × 109 cells/L (400 cells/μL). Patients were excluded if they had current evidence or a history of inhibitors, a history of adverse reactions related to FVIII or known sensitivity to blood products, a platelet count less than 90 × 109 cells/L (90 000 cells/μL), or a concomitant debilitating disease or ongoing symptomatic infection. Patients were also excluded if they had a positive complement activation test in the screening sample. The complement activation test measured serum levels of protein S (vitronectin)–bound complement terminal complex (SC5b-9) using an enzyme-linked immunosorbent assay (ELISA) kit (Quidel, San Diego, CA). Patients could not have participated in another ethics committee–approved clinical trial (including medical device studies) within the past 30 days. Patients had to understand and be willing to comply with the requirements of the study protocol. All patients provided written informed consent.
Results
Twenty-four patients with severe hemophilia A were enrolled and treated with PEGLip-reconstituted rFVIII in 2 different dose groups. The first 12 patients were treated with 35 IU rFVIII/kg reconstituted with PEGLip's. The second group of 12 patients were treated with 25 IU rFVIII/kg reconstituted with PEGLip's.
The demographics of the 2 cohorts are shown in Table 1. There were no statistically significant differences between the 2 cohorts. The 35-IU/kg group had a higher mean dose per bleed in the historic treatment period, which was likely due to the small number of patients who received treatment, with about 1000 IU per bleeding episode compared with most patients having received 500 to 600 IU per bleeding episode (mainly hemarthroses). All patients had at least 1 joint with severe arthropathy. Prior to study initiation, all patients were receiving on-demand treatment for bleeding episodes. All patients were HIV negative.
Patient demographics and characteristics
. | Dosing cohort, mean (SD) . | . | |
|---|---|---|---|
| Characteristic . | 35 IU/kg . | 25 IU/kg . | |
| No. of patients | 12 | 12 | |
| Age, y | 25.8 (7.3) | 26.9 (8.1) | |
| Weight, kg | 65.8 (12.1) | 65.7 (10.3) | |
| Height, cm | 177.3 (6.1) | 175.2 (6.3) | |
| Infusions during previous 90 days, n | 29.0 (5.4) | 31.7 (6.1) | |
| Period between bleeds (treatments), d | 3.7 (1.0) | 3.4 (0.9) | |
| Dose per bleed, IU rFVIII | 714 (303) | 530 (38) | |
. | Dosing cohort, mean (SD) . | . | |
|---|---|---|---|
| Characteristic . | 35 IU/kg . | 25 IU/kg . | |
| No. of patients | 12 | 12 | |
| Age, y | 25.8 (7.3) | 26.9 (8.1) | |
| Weight, kg | 65.8 (12.1) | 65.7 (10.3) | |
| Height, cm | 177.3 (6.1) | 175.2 (6.3) | |
| Infusions during previous 90 days, n | 29.0 (5.4) | 31.7 (6.1) | |
| Period between bleeds (treatments), d | 3.7 (1.0) | 3.4 (0.9) | |
| Dose per bleed, IU rFVIII | 714 (303) | 530 (38) | |
One of the 24 patients used another FVIII replacement therapy concomitant with the study product and was therefore excluded from the efficacy analysis. The patient did not receive any PEGLip rFVIII. Hence there were 11 patients available for efficacy analysis in the 35 IU/kg group and 12 in the 25 IU/kg group. In the 25 IU/kg group, 3 of the patients received a single PEGLip rFVIII infusion due to lack of liposomes.
In vivo recovery
In vivo recovery was determined for all 3 prophylactic infusions (Table 2). In the 35 IU/kg cohort, in vivo recovery for the standard rFVIII infusion was 2.7 ± 0.6 IU/dL per IU/kg and recoveries for the first and second PEGLip rFVIII infusions were 3.1 ± 0.7 and 3.0 ± 0.6 IU/dL per IU/kg, respectively. The 25 IU/kg cohort had a mean recovery of 2.1 ± 0.7 IU/dL per IU/kg for the standard rFVIII infusion and 2.1 ± 0.3 and 2.2 ± 0.6 IU/dL per IU/kg for the 2 PEGLip rFVIII prophylactic infusions, respectively. The difference between recovery values for the standard rFVIII preparation and the rFVIII liposomal infusions was not statistically significant in any group.
In vivo recovery (chromogenic method)
. | In vivo recovery for each dosing cohort . | . | |
|---|---|---|---|
| Treatment . | 35 IU/kg . | 25 IU/kg . | |
| Segment 1, rFVIII | |||
| n | 11 | 12 | |
| Mean, IU/dL per IU/kg | 2.7 | 2.1 | |
| Standard error, IU/dL per IU/kg | 0.6 | 0.7 | |
| Median, IU/dL per IU/kg | 2.7 | 2.1 | |
| Segment 2, PEGLip rFVIII, infusion 1 | |||
| n | 11 | 12 | |
| Estimate, IU/dL per IU/kg | 3.1 | 2.1 | |
| Standard error, IU/dL per IU/kg | 0.7 | 0.3 | |
| Median, IU/dL per IU/kg | 2.8 | 2.1 | |
| Segment 3, PEGLip rFVIII, infusion 2 | |||
| n | 10 | 10 | |
| Estimate, IU/dL per IU/kg | 3.0 | 2.2 | |
| Standard error, IU/dL per IU/kg | 0.6 | 0.6 | |
| Median, IU/dL per IU/kg | 2.9 | 2.2 | |
. | In vivo recovery for each dosing cohort . | . | |
|---|---|---|---|
| Treatment . | 35 IU/kg . | 25 IU/kg . | |
| Segment 1, rFVIII | |||
| n | 11 | 12 | |
| Mean, IU/dL per IU/kg | 2.7 | 2.1 | |
| Standard error, IU/dL per IU/kg | 0.6 | 0.7 | |
| Median, IU/dL per IU/kg | 2.7 | 2.1 | |
| Segment 2, PEGLip rFVIII, infusion 1 | |||
| n | 11 | 12 | |
| Estimate, IU/dL per IU/kg | 3.1 | 2.1 | |
| Standard error, IU/dL per IU/kg | 0.7 | 0.3 | |
| Median, IU/dL per IU/kg | 2.8 | 2.1 | |
| Segment 3, PEGLip rFVIII, infusion 2 | |||
| n | 10 | 10 | |
| Estimate, IU/dL per IU/kg | 3.0 | 2.2 | |
| Standard error, IU/dL per IU/kg | 0.6 | 0.6 | |
| Median, IU/dL per IU/kg | 2.9 | 2.2 | |
Bleeding-free time following a prophylactic infusion
A total of 279 bleeding episodes occurred during the study, of which 255 (91.4%) were spontaneous and 24 (8.6%) were trauma related. Of the spontaneous events, 245 (96.1%) occurred in joints, with knee and elbow bleeds being the most common (30% and 27%, respectively). No inflammatory target joints were observed during the study. There were no changes in the pattern of bleeding severity observed in patients during the trial.
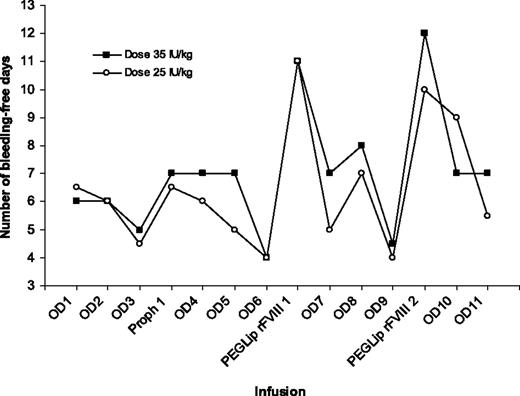
For patients in the 35 IU/kg cohort, the mean number of bleeding-free days following a standard prophylactic infusion with rFVIII was 7.2 ± 1.7 days compared with 13.3 ± 4.8 days following the PEGLip rFVIII infusions (Table 3; Figure 1). The difference between treatments was statistically significant (P < .001, paired t test). The mean difference between rFVIII and PEGLip rFVIII infusions was 6.1 days (median 5.0; 95% confidence interval [CI] 3.2-9.1). Eighteen (82%) of 22 of the PEGLip rFVIII infusions had a bleeding-free period of 10 days or more compared with 1 (9%) of 11 control rFVIII infusions.
Days without a bleeding episode following prophylactic infusions
. | No. of bleeding-free days . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment . | Mean . | Median . | Minimum . | Maximum . | SD . | 95% CI . | |||||
| rFVIII 35 IU/kg | |||||||||||
| Standard | 7.2 | 7.0 | 5 | 11 | 1.7 | 6.1-8.3 | |||||
| PEGLip | 13.3 | 13.5 | 6.0 | 20.5* | 4.8 | 10.1-16.5 | |||||
| rFVIII 25 IU/kg | |||||||||||
| Standard | 5.9 | 6.5 | 3 | 8 | 1.7 | 4.8-7.0 | |||||
| PEGLip | 10.9 | 11.0 | 5.5* | 16 | 2.9 | 9.0-12.7 | |||||
. | No. of bleeding-free days . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment . | Mean . | Median . | Minimum . | Maximum . | SD . | 95% CI . | |||||
| rFVIII 35 IU/kg | |||||||||||
| Standard | 7.2 | 7.0 | 5 | 11 | 1.7 | 6.1-8.3 | |||||
| PEGLip | 13.3 | 13.5 | 6.0 | 20.5* | 4.8 | 10.1-16.5 | |||||
| rFVIII 25 IU/kg | |||||||||||
| Standard | 5.9 | 6.5 | 3 | 8 | 1.7 | 4.8-7.0 | |||||
| PEGLip | 10.9 | 11.0 | 5.5* | 16 | 2.9 | 9.0-12.7 | |||||
For patients treated with 35 IU/kg rFVIII, n = 11 for each group; with 25 IU/kg rFVIII, n = 12 for each group.
Mean of 2 infusions.
The mean number of days following a standard prophylactic infusion with 25 IU/kg rFVIII was 5.9 ± 1.7 days compared with 10.9 ± 2.9 days following the PEGLip rFVIII infusions (Table 3; Figure 1). The difference was statistically significant (P = .002, paired t test). The mean difference between rFVIII and PEGLip rFVIII infusions was 5.0 days (median 3.8, 95% CI 2.3-7.6). Fourteen (67%) of 21 of the PEGLip rFVIII infusions had a bleeding-free period of 10 days or more compared with 0 of 12 control rFVIII infusions. The prolongation of the bleeding-free period was highly specific for the PEGLip rFVIII infusion and occurred following both infusions of PEGLip rFVIII and in both dose groups (Figure 1).
The standard rFVIII infusion resulted in a bleeding-free period that was generally similar to the bleeding-free period following on-demand treatment for a bleeding episode. To test for a possible carryover effect (most likely that the infusion following immediately after a PEGLip rFVIII infusion would have a prolonged bleeding-free period), the bleeding-free period following the first on-demand treatment after the prophylactic treatments was examined (Table 4). The data indicate a trend toward a slightly prolonged bleeding-free period; however, the difference between the first on-demand infusion following the PEGLip rFVIII prophylactic infusion and the first on-demand infusions following standard rFVIII prophylactic infusion was not statistically significant (t test). The slight upward trend in bleeding-free days may represent improved joint status following a period with higher than usual dosing (ie, more IU of FVIII per bleeding episode). It should also be noted that this trend is only observed for the first on-demand infusion following a prophylactic infusion and not with subsequent infusions.
Carryover effect analysis
Treatment . | 35 IU/kg . | 25 IU/kg . |
|---|---|---|
| Segment 1, rFVIII | ||
| Mean bleeding-free time,* d (median) | 7.2 (7.0) | 5.7 (6.0) |
| SD, d | 2.2 | 2.6 |
| No. of patients | 11 | 12 |
| Segment 2, PEGLip rFVIII, infusion 1 | ||
| Mean bleeding-free time,* d (median) | 8.7 (7.0) | 6.0 (5.0) |
| SD, d | 5.8 | 4.0 |
| No. of patients | 11 | 12 |
| Segment 3, PEGLip rFVIII, infusion 2 | ||
| Mean bleeding-free time,* d (median) | 10 (7.0) | 7.7 (9.0) |
| SD | 7.6 | 2.7 |
| No. of patients | 11 | 9 |
Treatment . | 35 IU/kg . | 25 IU/kg . |
|---|---|---|
| Segment 1, rFVIII | ||
| Mean bleeding-free time,* d (median) | 7.2 (7.0) | 5.7 (6.0) |
| SD, d | 2.2 | 2.6 |
| No. of patients | 11 | 12 |
| Segment 2, PEGLip rFVIII, infusion 1 | ||
| Mean bleeding-free time,* d (median) | 8.7 (7.0) | 6.0 (5.0) |
| SD, d | 5.8 | 4.0 |
| No. of patients | 11 | 12 |
| Segment 3, PEGLip rFVIII, infusion 2 | ||
| Mean bleeding-free time,* d (median) | 10 (7.0) | 7.7 (9.0) |
| SD | 7.6 | 2.7 |
| No. of patients | 11 | 9 |
Bleeding-free days following first on-demand treatment for each dosing cohort.
Median bleeding-free days following infusion of rFVIII or PEGLip rFVIII. OD indicates bleeding episode followed by on demand treatment; Proph 1, standard rFVIII prophylaxis infusion; and PEGLip rFVIII, PEGylated liposome–reconstituted Kogenate FS prophylaxis infusion. OD6 and OD9 were shorter than prior intervals, since patients were scheduled for the prophylactic infusion in a nonbleeding state following a 4-day wash-out period.
Median bleeding-free days following infusion of rFVIII or PEGLip rFVIII. OD indicates bleeding episode followed by on demand treatment; Proph 1, standard rFVIII prophylaxis infusion; and PEGLip rFVIII, PEGylated liposome–reconstituted Kogenate FS prophylaxis infusion. OD6 and OD9 were shorter than prior intervals, since patients were scheduled for the prophylactic infusion in a nonbleeding state following a 4-day wash-out period.
Safety
Patients were regularly monitored for clinical adverse events. No clinically significant laboratory adverse event related to the study drug was reported. Blood samples were obtained immediately prior to and 1 hour after each prophylactic infusion. Small but significant decreases in hemoglobin values were detected following the second liposome infusion compared with preinfusion values. This is most likely due to blood samples (in vivo recovery and safety samples) being drawn, as similar findings occurred after the standard rFVIII infusion. There were no signs of hemolysis and no drop in platelet count associated with any of the infusions. Following the second PEGLip rFVIII infusion there was a small increase in total cholesterol (from 4.13 to 4.29 mM) that was not statistically significant; and a change in low-density lipoprotein (LDL) cholesterol (from 2.64 to 3.03 mM) that was statistically significant. Since the liposomes are lacking cholesterol, they become a sink for cholesterol from red blood cells, lipoproteins, and tissues33 ; this increase represents a redistribution of tissue cholesterol, which is a known effect of liposome infusion.34,35 Apart from these expected changes in cholesterol levels there were no other clinically significant changes in any laboratory parameters.
There were no differences in vital signs between infusions of standard rFVIII or PEGLip rFVIII. One clinical adverse event was reported as possibly related to the study drug. The event was a feeling described as “warm in throat” that occurred during the first PEGLip rFVIII infusion. This event was mild and transient and did not recur during the second PEGLip rFVIII infusion. FVIII inhibitor assays (Bethesda assay, Nijmegen modified) were performed on plasma samples collected from all patients prior to study start and at study end. All inhibitor assays were negative (< 0.6 BU).
Discussion
This study was designed to evaluate the clinical impact of treatment by assessing the time period protected from bleedings following a prophylactic infusion instead of using a PK approach to achieve bleeding protection (ie, maintain a plasma concentration of FVIII < 1%). The results showed that prophylactic treatment of severe hemophilia patients with rFVIII reconstituted with PEGylated liposomes nearly doubled the time period free from bleedings compared with standard rFVIII treatment. These results support the finding in animal studies that prolonged protection from bleeding was available in spite of a low plasma concentration of FVIII.29
The approach used in this study is principally similar to 1 of 2 methods used to determine the interval between infusions given to prevent bleedings in the prophylactic treatment of patients with hemophilia A in the clinical setting. The PK method aims at keeping the FVIII level above 1% to 2% at all times.1,12-15 A dosing scheme is derived by titrating individual patient's treatment according to FVIII half-life or using repeated measurements of FVIII trough levels. Usually this requires frequent infusions of FVIII, 2 to 4 times per week. An alternative method is to clinically “titrate” the patient by using a standard dose of 30 to 50 IU/kg of FVIII and adapting the interval between treatments until no spontaneous bleeding episodes appear.7 As the ultimate goal of treatment is to prevent bleeding episodes, the latter approach is clinically more attractive, since it accounts for the large variation present in the patient population and does not require as frequent blood sampling as the PK method. As bleeds can be prevented even at a FVIII concentration below the minimum target trough level of 1% used in the PK titration method,25-27,36 the “clinical” approach was used in this study of rFVIII reconstituted with PEGylated liposomes.
This is the first human clinical trial using a comparative controlled approach to show a statistically significant prolongation of the efficacy of a FVIII product. Although many studies have reported successful animal experiments using FVIII gene therapy approaches, validity in humans has not yet been demonstrated.37-43 The approach used here avoids many of the obstacles with gene therapy and would serve as an intermediate step in hemophilia care toward the ultimate goal of a cure. As the liposomes do not specifically bind only FVIII, this technology, if proven effective for hemophilia, could be useful in other medical areas as well. It is likely that there are several mechanisms responsible for the prolonged bleeding-free period observed in the current study. One possible mechanism, suggested by the animal data, is that measurable circulating FVIII levels are present for a longer period of time following infusion with PEGLip rFVIII compared with standard rFVIII. Alternatively, PEGylated liposomes are known to be more prevalent in highly vascularized tissues,44 so it is possible that higher FVIII concentrations are achieved in certain localized areas, such as joints, and convey a protective effect. A third possibility is that the liposomes, in conjunction with FVIII, enhance the coagulation process by associating with platelets.
The 25 IU/kg dose consistently gave a bleeding-free time period that was shorter compared with that in the 35 IU/kg dose cohort. A similar difference was observed for the liposome infusion with a clear increase in number of days without bleedings in both dose groups, although the increase was not as great with the 25 IU/kg dose. It seems unlikely that the 2 cohorts should both respond in a similar way and also show such a regular dose response by chance.
The patients in this study received 7 infusions with standard rFVIII prior to the first infusion with the PEGLip rFVIII (3 wash-in doses, 1 prophylactic dose, and 3 additional doses for on-demand bleedings). In order to minimize bias due to interventions required by the study protocol, no study visits were scheduled between the prophylactic infusions until the next bleeding episode, and all prophylactic infusions were patient blinded. The prophylactic infusions were not randomized due to uncertainty of a possible carryover effect of the PEGLip rFVIII and, if such an effect did occur, how long such an effect would last. While it could be speculated that there was a “cooling off” effect that was responsible for the positive response, this is unlikely, since the bleeding-free period following the infusion given immediately after the first PEGLip rFVIII infusion returned to the 6- to 7-day range and remained within that range after additional infusions until the next PEGLip rFVIII infusion (Figure 1). This suggests that the infusion order was the cause of the effect.
Treatment for patients with hemophilia is life-long, and long-term safety is of critical importance. Pegylated molecules have been approved for chronic administration for other indications, and lipids have been used in various therapeutic formulations approved for both short-term and long-term therapy. The results of this trial demonstrate that liposomes are well tolerated in a short-term study. The half-life or circulation time of PEGLip rFVIII is not currently known; however, a PK study is ongoing.45 Further studies are needed to fully explore the use of liposomes in association with FVIII, including the optimization of treatment intervals and doses to ensure that there is no accumulation of liposomes or other adverse effects. This study was performed in a population of hemophilic patients that had advanced hemophilic joint disease. Future studies in other patient populations, with different characteristics (age, severity, joint damage, etc), as well as long-term studies will be needed to fully evaluate the clinical benefits and safety aspects of the PEGylated liposomes.
These data show the possibility of a prophylactic FVIII regimen with replacement infusions only once weekly. Such a treatment would represent a major improvement in convenience, which could increase compliance, could reduce the number of bleeding episodes, and may also reduce the morbidity29 and improve the quality of life for the hemophilia population.
Authorship
J.S. designed research, analyzed data, and wrote the paper; and O.P.P., T.A.A., and Y.A. performed research.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 3, 2006; DOI 10.1182/blood-2006-03-008276.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by a grant from Bayer HealthCare Pharmaceuticals (Berkeley, CA) and by OMRI Laboratories (Rehovot, Israel).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal