Abstract
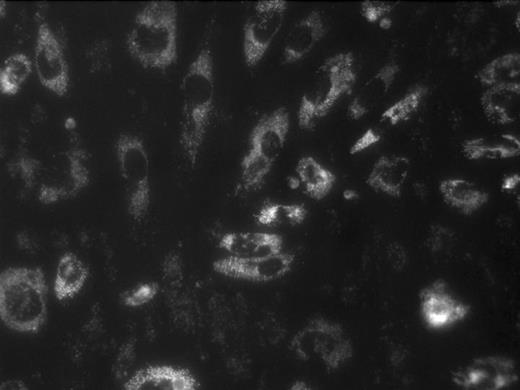
Human blood flowing over monolayers of endothelial cells (ECs) in vitro under controlled flow conditions constitutes a promising model of blood-EC interactions, especially in a system permitting real-time imaging of single platelets (1 μm resolution or better) and platelet aggregates. We have developed such a model and have used it to study platelet adhesion/aggregation on monolayers of human renal microvascular ECs grown on optically clear vinyl slides and activated with TNFα (20 ng/ml; hr 0 to 22) and Shiga toxin-1 (Stx; 10 pM; hr 18 to 22), in simulation of probable events in the childhood hemolytic uremic syndrome (HUS). Normal donor blood, collected into 4U/ml low MW heparin (dalteparin) and 10 μM mepacrine (platelet, EC, and granulocyte label), was drawn at shear rates of 270–650 sec−1 through a parallel-plate flow chamber for which one wall was one of the above slides. Such shear rates give rise to shear stresses which approximate the 20–25 dynes/cm2 estimated to exist in glomerular arterioles. Platelets, ECs, and granulocytes were imaged in real time using epifluorescence digital videomicroscopy. With activated, but not with control ECs, clusters of platelets deposit on the monolayers in strings (Fig.1, upper right, with flow from top to bottom) superposed upon ECs, for which the cytoplasm is fluorescent. The strings are tethered at the upstream end, as has been observed by others using platelet-rich plasma. Granulocytes, identifiable by size (Fig. 1, upper middle), also adhere to activated ECs. In five paired experiments, monolayer preincubation × 30 min with 50 nM active-site inactivated recombinant factor VIIa (ASIS; courtesy of Dr. Ulla Hedner, Novo Nordisk Pharmaceuticals) largely eliminated the platelet strings, while reducing the number of adherent granulocytes to zero. In control experiments, preincubation of monolayers with 100 nM of a monoclonal antibody directed against human tissue factor similarly largely eliminated both platelet strings and adherent granulocytes. In other control experiments, the use of the above concentration of low MW heparin did not by itself block fibrin formation (by immunostaining) in the boundary region near the slide-blood interface. These findings may be explained on the basis that ASIS both interrupts the tissue factor pathway of coagulation and blocks tissue factor-associated upregulation of endothelial cell E-selectin. ASIS therefore may have promise as a therapy for the prothrombotic and proinflammatory effects of childhood HUS. Studies with the TAB monoclonal antibody and ALEXA 555 are in progress to permit platelet-specific labeling and quantitation of platelet adhesion/aggregation with and without ASIS.
Disclosures: Novo Nordisk Pharmaceuticals has partially supported this work through a laboratory research grant.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal