Abstract
A control of clopidogrel response is one proposed strategy for an improvement of anti-platelet therapy. Different methods have been evaluated for this indication. We assessed ADP induced aggregation in whole blood in healthy blood donors and patients treated with clopidogrel 75 mg qd using a new monitoring method.
Methods: Platelet function was determined using multiple electrode aggregometry (MEA) on the Multiplate analyzer (Dynabyte, Munich, Germany). This device uses a single use test cell with two separate impedance sensors, consisting of a total of 4 electrodes and has 5 channels for parallel tests. Aggregation was triggered using ADP (6.4 μM, ADPtest, Dynabyte) and using a combination of ADP (6.4 μM) and prostaglandin E1 (20 nM) (ADPtest high sensitivity = ADPtest HS, Dynabyte). The combination of ADP + prostaglandin E1 (PGE1) is used to enhance sensitivity for the effects of clopidogrel onto ADP induced platelet activation. PGE1 reduces intracellular calcium mobilisation and therefore platelet activation and acts thus synergistical to the effect of clopidogrel. For the analysis 300 μl of blood are analyzed with the addition of 300 μl of saline. Aggregation was quantified by the area under the curve in arbitrary U (1 U corresponds to 10 AU*min). Following IRB approval venous blood was collected from 120 blood donors without anamnestic intake of platelet inhibitors in the 2 weeks before the analysis and 160 patients taking clopidogrel 75 mg qd using the direct thrombin inhibitor Melagatran in a final concentration of 15 μg/ml as the anticoagulant.
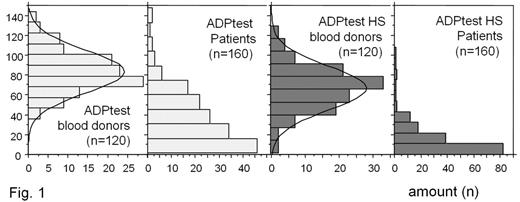
Results: The distribution of the aggregation values is shown in Fig. 1. The median (min-max) was 83 (36–143) for the blood donors in the ADPtest and 68 (1–130) in the ADPtest HS. In the patient group the median (mix–max) was 30 (2–147) in the ADPtest and 10 (0–108) in the ADPtest HS. If a non-response rate of 25% is assumed, the cut-off between clopidogrel responders and non-responders would be 51 for the ADPtest and 21 for ADPtest HS.
Discussion: The comparison of the results of clopidogrel-treated patients and healthy controls (blood donors) reveals a higher sensitivity of ADPtest HS vs. ADPtest for the effects of clopidogrel onto ADP induced aggregation in whole blood. Due to the use of a direct thrombin inhibitor as the anticoagulant in our study platelet function was determined under physiological levels of ionized calcium.
For both test methods a significant proportion of patients does not show adequate reductions in their vitro platelet aggregation. Prospective trials are required to show whether a clopidogrel-non-response in this particular method is associated with an increased occurrence of arterial thromboembolism.
Disclosures: Andreas Calatzis is a coninventor of the presented method and a coowner of the manufacturer of this device (Dynabyte medical, Munich).; The study was supported by the manufacturer of the Multiplate device (Dynabyte medical).
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal