Abstract
Introduction: Hemophilia patients with inhibitors experience frequent bleeds and reduced quality of life (QoL). This trial evaluated whether once−daily prophylaxis therapy with activated recombinant factor VII (rFVIIa; 90 or 270 μg/kg) for 3 months can effectively reduce bleed frequency compared with on−demand therapy, without compromising safety.
Methods: Thirty−eight hemophilia inhibitor patients with frequent bleeds entered a 3−month observation period where on−demand therapy was continued according to local practice. Twenty−two patients with a stable bleed frequency (≥4 bleeds/month) were randomized 1:1 to once−daily dosing with 90 or 270 μg/kg rFVIIa in a double−blind, parallel−group design. To maintain blinding, the same volume was administered in both groups. Patients then entered a 3−month post−treatment observation period, where on−demand therapy was resumed.
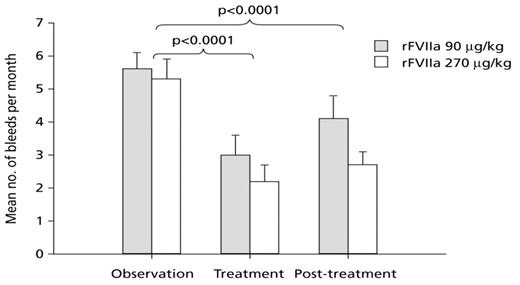
Results: The number of bleeds per month was significantly reduced by 45% and 59% with 90 or 270 μg/kg rFVIIa prophylaxis, respectively, compared with the observation period (Fig.). Similar reductions were seen for all bleeds, irrespective of site or cause. The majority of this reduced bleeding frequency was maintained in the 3 month post−treatment period (27% and 50% reductions, respectively). There were no treatment− or dose−dependent patterns in the number or type of adverse events. No thromboembolic events or withdrawals due to adverse events or ineffective treatment were reported. The benefits of reducing bleeding frequency in this trial translated into improvements in QoL.
Conclusions: These results support the concept of secondary rFVIIa prophylaxis in inhibitor patients with frequent bleeds. Clinically relevant reductions in number of bleeds and improvements in quality of life were observed during prophylaxis compared with conventional on−demand therapy without raising any safety concerns.
Disclosures: Consulting fees or other remuneration from sponsor of study, NovoNordisk.; Research grants from sponsor of study, NovoNordisk.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal