Abstract
Background: We have previously shown that CLL B cells secrete vascular endothelial growth factor (VEGF) and express VEGF receptors: VEGFR-1 and VEGFR-2. Secreted VEGF protects CLL B cells from spontaneous and drug induced apoptosis via increased levels of Mcl-1 and XIAP; however, the exact mechanism of this process is unknown. In solid tumors, there is increasing evidence that signaling through VEGF receptor known as neuropillin-1 (NRP-1) is critical for VEGF induced resistance to apoptosis. Hypothesis: NRP-1 is expressed by CLL B cells and is critical for VEGF mediated protection from apoptosis.
Methods: To demonstrate the presence of NRP-1 on CLL B cells, we conducted flow cytometry and immunoblotting. We then evaluated the ability of NRP-1 blocking antibodies to induce apoptosis of primary CLL B cells. To do this, circulating CLL B cells were isolated by density gradient centrifugation. Patient samples with greater than 80% of CD5+ CD19+ cells in a mononuclear cell population, as assessed by flow cytometry, were cultured in AIM-V media in 24-well plates at 1.5 x 106 cells/mL. To occupy NRP-1, we added NRP-1 blocking antibodies (Calbiochem, Darmstadt, Germany) at increasing concentrations (0.5 μg/mL -10 μg/mL) to cultured CLL B cells. Cell death was assessed using an annexin and propidium iodide flow assay after 24 h of in vitro culture. CLL cells cultured without antibodies and isotype nonspecific monoclonal antibodies were used as controls.
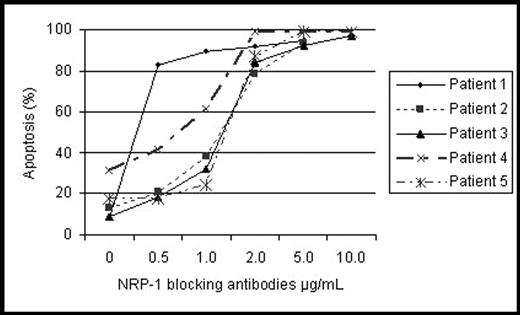
Results: CLL B cells were found to express NRP-1 but not uniformly. Most patients had flow positive evidence for NRP-1 but a distinct percentage (35%) was very low (≤5%) or negative. However, immunoblot analysis revealed moderate to low levels of NRP-1 protein with evidence of tyrosine phosphorylation in all tested CLL patients (N=9). NRP-1 blocking antibodies, but not VEGF-R1 and -R2 blocking antibodies, induced apoptosis in a dose dependent manner in primary CLL cells (n=5, FIG.) at antibody concentrations starting at 1 μg/mL (p=0.003). The effect of the NRP-1 blockade varied between patients, with a median IC50, 1.5 μg/mL (range 0.5–2 μ/mL). Importantly, concentrations of 5 μg/mL and higher induced apoptosis in more than 90% of the CLL B cells. We also found that the sensitivity of CLL B cells to NRP-1 blocking antibody, in terms of apoptosis induction, was correlated with the number of NRP-1 receptors as assessed by flow cytometry. CLL B cell clones with no detectable NRP-1 had no induction of cell death when exposed to the NRP-1 blocking antibody. Finally, immunoprecipitation and immunoblot assays indicated that NRP-1 physically interacted with VEGF-R2 on CLL B cells. This suggests that NRP-1 could be enhancing VEGF binding affinity on VEGF-R2 to further increase the ability of VEGF to generate signals that lead to apoptosis modulation.
Conclusion: We have found that NRP-1 blocking antibodies induce cell death in NRP-1 positive CLL B cells. Similar results using NRP-1 blocking peptides rather than blocking antibodies have been observed in breast cancer (
Disclosures: NIH-NCI CA 95241.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal